Abstract
The opioid analgesic tapentadol was the first pain medication to be developed for the treatment of pain in children under a formal process established by the regulatory authorities. This article summarizes the outcomes of the pediatric development program for tapentadol across the entire age range from birth (including neonates) to adolescents <18 years of age. In addition, the challenges experienced when designing and conducting the pediatric tapentadol clinical trials as well as the interactions with the regulatory authorities are discussed. As a first outcome, the oral solution of tapentadol was authorized in the EU in 2018 as a new treatment option in the hospital setting for moderate to severe acute pain in children from 2 to <18 years of age.
Keywords:
Introduction
Very few pain medications are authorized for pediatric use, in particular for the neonatal and infant population.Citation1 The treatment of acute or long-term pediatric pain often relies on data extrapolated from adult trials and best practice guidelines recommended by global experts.Citation2 However, children are not simply small adults; this vulnerable population requires medications thoroughly assessed for their pharmacokinetic (PK) and pharmacodynamic (ie safety and efficacy) properties across a wide age and developmental range. In order to support the development of medicines for children, both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) established a regulatory frameworkCitation3,Citation4 including the Pediatric Study Plan (US) and the Pediatric Investigation Plan (EU). These are mandatory unless a waiver or deferral has been granted, and incentives are available if particular conditions are fulfilled. Currently, incentives for the pharmaceutical industry to conduct these trials include exclusive marketing for 6 months in the US and a 6-month extension of the supplementary protection certificate in the EU.
Not all medications in development for adults may be suitable for use in the pediatric population. Specific conditions for which some medications are developed may not occur in children, or a product may not offer significant therapeutic benefits or may be considered ineffective or unsafe. Moreover, for pain medications with an opioid mechanism of action, their potential for developing an opioid use disorder needs to be considered.Citation5 For tapentadol, an opioid analgesic with two mechanisms of action, it was clear that a development program in the pediatric population was appropriate as pain is present in children and the treatment may be a suitable option for this population. Tapentadol was shown to provide effective pain relief in multiple acute and chronic pain indications in adult patientsCitation6–Citation8 with a more favorable gastrointestinal tolerability profile compared to typical opioids acting through µ-receptors only. This profile can be explained by the combination of two synergistic mechanisms of action in one molecule ie µ-opioid receptor agonism (MOR) and noradrenaline reuptake inhibition (NRI)Citation9 resulting in a reduced µ-load.Citation9,Citation10 The investigation of tapentadol for the relief of moderate to severe pediatric pain was not only supported by its benefit/risk profile in adults but also by its specific pharmacokinetic and pharmacogenomic profileCitation5 resulting in a predictable PK profileCitation11 and the absence of active metabolites contributing to the analgesic effect.Citation12 With Phase II glucuronidation as the main metabolic pathway,Citation13 there is a low potential for drug–drug interactions.Citation14,Citation15
These considerations have led to an agreed pediatric investigational plan with the Pediatric Committee of the EMA (PDCO) in the EU and an agreed pediatric study plan with the US FDA for the development of various tapentadol formulations.
Efficacy and Safety of Tapentadol in the Pediatric Population
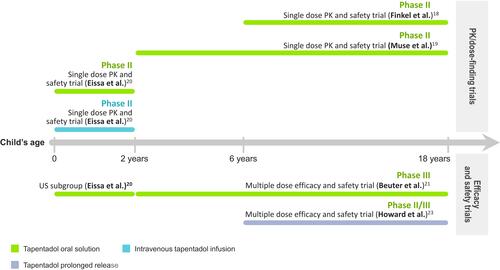
An overview of the trials included in the tapentadol pediatric program is shown in ; provides a summary of the trials and main trial outcomes. The guiding principle for the pediatric tapentadol program was that similar exposure in children as observed in adults at effective dose levels would result in an effective and safe profile in children. Therefore, extensive PK profiling supported by modelling and simulation techniques took place to address all age groups and all tapentadol formulations used in this program and to determine the doses used in the efficacy trials.Citation16,Citation17
Table 1 Summary of Tapentadol Trials for Moderate to Severe Acute or Long-Term Pediatric Pain
Efficacy
Treatment of Acute Pain in the Pediatric Population
The single-dose PK trials were powered to assess the PK profile of tapentadol oral solution (OS) and tapentadol intravenous infusion (IV) in the pediatric population in order to determine appropriate tapentadol doses for the treatment of acute pain in children. Efficacy evaluations were thus only exploratory but permitted a first indication about the effectiveness of the medication. Pain intensity was measured using age-appropriate rating scales. In older children (≥2 years), mean pain intensity improved during the first hours after tapentadol OS administration.Citation18,Citation19 Supplemental analgesic medications were given on average at least 5 h after tapentadol administration which coincided with a decrease in tapentadol serum concentrations. In children <2 years, mean pain intensity reductions were already observed 15 min after the start of tapentadol OS or IV treatment.Citation20
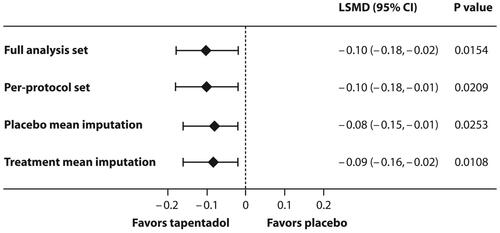
The tapentadol OS doses established by PK analyses and subsequent population PK (popPK) modelling were investigated in a randomized, double-blind, placebo-controlled, multiple-dose confirmatory trial. This trial employed an immediate rescue design to ensure that all patients had access to pain medication (supplemental opioid analgesic medication) irrespective of their allocated treatment group. Efficacy in the treatment of moderate to severe acute pain was determined by comparing the amount of supplemental opioid analgesic medication used within the first 24 h after the first dose of trial medication in the tapentadol and the placebo groups. In children aged 2 to <18 years, tapentadol OS showed significantly better efficacy compared to placebo ().Citation21 The result was confirmed by sensitivity analyses and supported by secondary efficacy analyses. In the subgroup of children aged <2 years, the sample size was too low to allow for a comprehensive comparison of the treatment groups.Citation20
Figure 2 Statistically significant treatment differences in favor of tapentadol oral solution for the primary trial endpoint (amount of supplemental opioid analgesic medication used within the first 24 h after intake of trial medication) in children aged 2 to <18 years with moderate to severe acute pain. Main analysis (full analysis set) and sensitivity analyses.
The findings led to the approval of tapentadol OS in the EU for children 2 to <18 years of age for the treatment of moderate to severe acute pain which can be adequately managed only with opioid analgesics (dose recommendation of 1.25 mg/kg bodyweight every 4 h).Citation22 In line with the design of the clinical trials conducted in a hospital setting and with a total duration of 72 hours as agreed with the PDCO, the use of tapentadol OS is currently limited to a hospital setting and for up to 3 days only. Moreover, because in children below the age of 2 years only sparse efficacy and safety data and no PK data following multiple dosing are available, no tapentadol formulation is currently authorized for the treatment of pain in children <2 years of age.
Treatment of Long-Term Pain in the Pediatric Population
An open-label, randomized, active-controlled tapentadol prolonged release (PR) trial with subsequent tapentadol extension phase investigated the efficacy of tapentadol PR in children aged 6 to <18 years suffering from a wide range of painful conditions expected to require opioid treatment for more than 14 days.Citation23 Pain intensity was measured using age-appropriate rating scales. The primary efficacy endpoint was the number of treatment responders.
Tapentadol PR reduced pain intensity from moderate to mild pain and showed non-inferiority to the active comparator morphine PR over the 14-day randomized treatment period. Sensitivity analyses supported the primary endpoint result. None of the patients discontinued treatment due to lack of efficacy. For patients requiring long-term treatment pain intensity scores remained stable during the subsequent 12-month tapentadol extension phase ().
Figure 3 Stable pain intensity scores during up to 12 months treatment with tapentadol PR in children 6 to <18 years of age using two pain rating scales (“as observed” data).
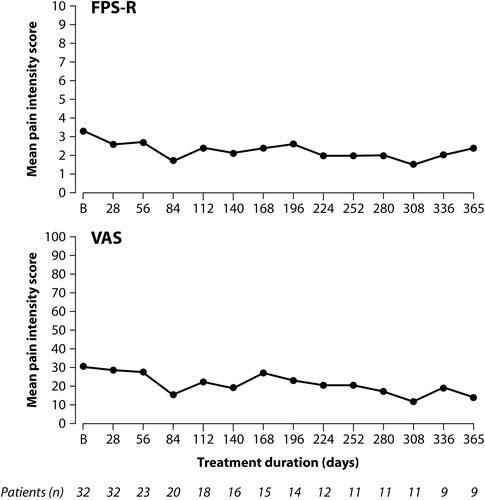
In contrast to adults with chronic pain conditions who would generally only receive PR opioids after experiencing pain for at least 3 months and who usually continue on these medications for long periods, pediatric patients had a shorter history of pain and in most cases did not require pain treatment with a strong analgesic for a long period of time: nearly half of the patients entering the extension phase (44.4%) discontinued treatment during the first 3 months, mainly because opioid treatment was no longer required. Although usually administered for a short period of time, a twice-daily dosing with a PR formulation might be a useful alternative to immediate release (IR) opioids in patients ≥6 years as IR formulations require more frequent dosing (eg every 4–6 h) which may impact on patient compliance and may be difficult and time-consuming for caregivers as acknowledged by the FDA.Citation24
Safety
The tapentadol safety profile was as expected for an analgesic which includes an opioid mode of action. The adverse event profile also reflected specific characteristics of the pediatric trial population, its underlying diseases, and concomitant treatments. Compared to adults, there was no higher susceptibility of the pediatric trial population to side-effects. There were no new safety issues or tapentadol-related adverse events identified in pediatric trials.
Tapentadol OS/IV
Treatment-emergent adverse events (TEAEs) were reported for 51.2% of patients in single-dose tapentadol OS trialsCitation18–Citation20 and for 28.9% of patients in the single-dose tapentadol IV trial;Citation20 TEAEs occurred in 57.1% of patients receiving multiple doses of tapentadol OS for the treatment of acute painCitation20,Citation21 (). None of the TEAEs in the single-dose trials were serious; two patients in the multidose trial, however, experienced serious TEAEs (abdominal abscess and seizure)Citation21 but they were deemed unlikely related to tapentadol treatment by the investigators. The main TEAEs were vomiting (21.4% [53/248]) and nausea (14.5% [36/248]) across the tapentadol OS trials and vomiting (10.5%) in the tapentadol IV trial. Of note is that pyrexia was reported in 5.2% (15/286) of all patients dosed with tapentadol. This is not a known side-effect of tapentadol treatment and none of these events were deemed related to trial medication by the investigators. The occurrence of pyrexia can probably be explained by confounding factors reported for these patients such as inflammation reactions due to surgery or an orthopedic implant, pre-existing fever, peri-splenitis, skin inflammation, or fever due to extensive blood loss. Incidences of pyrexia have also been reported under placebo, tapentadol, and oxycodone treatment for acute pain following major surgery in adult patients.Citation7
Table 2 Main Treatment-Emergent Adverse Events under Tapentadol Treatment for Acute Pain (Including Events Suggestive of Respiratory Depression)
Respiratory depression is a major clinical concern when administering centrally acting opioid analgesic medications. Respiratory depression was not documented as a TEAE in any of the trials. Furthermore, the incidence of TEAEs suggestive of respiratory depression was low: oxygen saturation decreased (3.2% of patients [9/286]), hypoxia (1.4% [4/286]), and PO2 decreased (0.4% [1/286]). Most of these TEAEs were considered not related to tapentadol treatment by the investigators; three cases of hypoxia were deemed at least possibly related.
Multiple doses of tapentadol OS were well tolerated across the age range. The proportions of patients with TEAEs were 54.5% (birth to <2 years), 39.1% (2 to <6 years), 46.9% (6 to <12 years), and 71.7% (12 to <18 years). Patients in the oldest age group received a higher amount of supplemental opioid analgesic medication than the other groups which might have contributed to the higher TEAE incidences.
There was no indication for a higher susceptibility to side-effects of the youngest patients (<2 years of age) compared to the older groups aged 2 years and above. In all age groups, vomiting was the most common TEAE: 2 patients (18.2%) in the group <2 years, 4 patients (17.4%) in the 2 to <6 years group, 8 patients (25%) in the group from 6 to <12 years, and 13 patients (24.5%) aged from 12 to <18 years.
Overall, there were no clinically relevant changes in hematology and clinical chemistry parameters under multiple-dose tapentadol OS treatment.
Tapentadol PR
Tapentadol PR treatment was well tolerated. The most frequently reported TEAEs were nausea (22.2% of patients), constipation (15.6%), and abdominal pain, vomiting and headache (each 13.3%) during the 14-day randomized period and nausea (30.6%) and headache (27.8%) during the extension phase. The degree of constipation remained stable during the first 14 days; a slight decrease was observed under long-term treatment.
Small fluctuations in clinical chemistry and hematology laboratory parameters and in vital signs were not considered clinically relevant. There was one TEAE indicative of respiratory depression (bradypnea of moderate intensity) which was considered by the investigator to be certainly related to tapentadol PR treatment and led to treatment withdrawal. There was no indication of clinically relevant opioid withdrawal symptoms.
The adverse event profile was overall in line with the safety profile known from adult tapentadol PR trials;Citation8 no new safety issues or tapentadol-related side-effects were identified. None of the adverse events occurring after the final tapentadol dose were considered to be long-term consequences of the trial medication.
Inclusion of Results from the Development Plan into the Product Label
In the US, the pediatric study plan is still ongoing. In the EU, the findings of the clinical trial program led to the approval of tapentadol OS for children 2 to <18 years of age for the treatment of moderate to severe acute pain which can be adequately managed only with opioid analgesics (dose recommendation of 1.25 mg/kg bodyweight every 4 h).Citation22 The clinical program had been agreed with PDCO assuming that it would allow extension of the label from adults to children. This assumption turned out to be invalid. In contrast to the adult program, regulatory authorities approved a more restricted label in the pediatric population by mimicking the clinical trial conditions in the product label. As a result, tapentadol OS use is restricted to a hospital setting, limited to a total duration of use of 72 hours and use in children ≥2 years of age as the available data from the agreed program with PDCO were deemed insufficient in the younger age group.
From this experience, it is clear that the requirements for approving a pediatric formulation might differ from the agreed development plan. The sufficiency of the data acquired for the PR formulation in children/adolescents is currently under re-evaluation prior to submission of a request for label extension of tapentadol PR to children ≥6 years.
New Insights into the Pediatric Development of Drugs Initially Targeted for Adults
The challenges of conducting pediatric trials for the treatment of pain have been discussed in detail in the introductory article of the thematic tapentadol series.Citation1 This chapter describes how some of these challenges were handled in the tapentadol program.
Formulations Developed for Adult Patients May Not Be Suitable for Dosing Across the Entire Pediatric Age Range
Tapentadol is available for adult patients as immediate releaseCitation25 and prolonged releaseCitation26 tablets and as an oral solution.Citation22,Citation27 Oral solutions are a common, generally well-accepted formulation even for very young infants. However, to accommodate for weight-adjusted dosing, a lower OS dose strength had to be developed for younger children. In addition, an intravenous drug formulation for neonates and children unable to take oral medications was developed for the treatment of acute pain in the pediatric development program. Palatability and acceptability of the oral solution were investigated in a multiple-dose efficacy and safety trial in children aged 2 to <18 yearsCitation21 and were considered sufficient to ensure intake compliance in this age range. Conditions of chronic pain are rare in children and even more so in the very young; therefore, the PR formulation of tapentadol already approved for adult pain treatment was assessed for the treatment of pediatric pain in children aged 6 years and older requiring longer treatment.
Determination of Age-Appropriate Analgesic Doses is Possible by Using popPK Modeling
The determination of age-appropriate doses for the pediatric population can be based on a thorough understanding of the drug PK profile and its age-related changes. However, this thorough understanding might be difficult to achieve given the limitations in conducting pediatric trials. These limitations include the small sample size of pediatric clinical trials to minimize medication exposure in this vulnerable population and a restricted number of blood samples for PK analysis.
In order to maximize the information gain from the available samples, modelling and simulation techniques were extensively used in the tapentadol program.
In the development of tapentadol for the treatment of acute pain in pediatrics, a similar exposure–response relationship in children and adults was assumed, and the aim was therefore to identify tapentadol doses resulting in exposures in children which would fall into the adult exposure range using the approved therapeutic tapentadol IR dose range (50–100 mg).
Throughout the tapentadol pediatric program, a staggered approach was adopted starting with exposing the oldest patients first even within the same trial. To best inform the dose selection, a popPK model that was developed on pooled data from adult trials of tapentadol IR was extended with allometric scaling to create the initial pediatric popPK model. This model was used to perform simulations and recommend doses to be investigated in the very first pediatric trials in subjects 2 years of age and above.Citation18,Citation19 As a result, a dose of 1.0 mg/kg was selected in order to match the targeted adult exposure.
For the very young children <2 years of ageCitation20 where allometric scaling alone might not be sufficient, a physiologically based pharmacokinetic model was utilized to recommend the starting dosesCitation28 taking into account the rapid changes in organ maturation and development in this very young population. The PK data collected from the pediatric trials were then integrated into the pediatric popPK model which was subsequently updated as PK data became available. shows the pathway of trials leading to the final popPK model for tapentadol OS with dose recommendations for the entire age range from birth to <18 years. Efficacy and safety of the thus established doses were then confirmed in a randomized placebo-controlled trial across the entire pediatric age range.Citation20,Citation21
Figure 4 Dose determination pathway resulting in the final popPK model for tapentadol oral solution with dose recommendations for the entire pediatric age range from birth to <18 years.
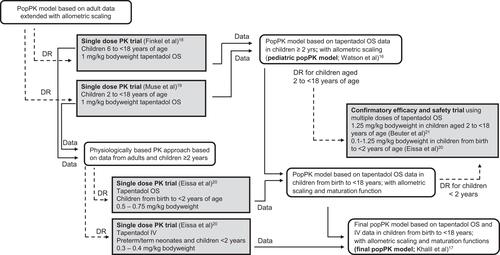
For pediatric patients requiring long-term treatment, the selection of tapentadol PR doses was based on a popPK model which integrated data from adult tapentadol PR trials and the available knowledge from pediatric tapentadol oral solution trials. Similarly, simulations with tapentadol PR aimed to identify the required doses that would result in steady-state exposures in pediatric patients similar to those reported for the therapeutic dose range of tapentadol PR in adults (50 to 250 mg b.i.d.). The established doses were investigated in a randomized, active-controlled trial with a tapentadol PR extension phase.Citation23
Limiting Exposure in the Pediatric Population During Development is Key for Ethical and Feasibility Purposes
The pediatric population is vulnerable and the inclusion of children in clinical trials is always a matter of great concern for parents, researchers, and ethic committees. In order to address the research questions about the effectiveness and safety of a new medicinal agent, the optimal trial design needs to be selected to ensure that medication exposure of children is limited to the absolute minimum rendering the trial feasible while ensuring that a reliable answer to the research question can still be obtained.
In order to address the effectiveness and safety of tapentadol PR for the treatment of long-term pain in the pediatric development program, a new innovative approach developed by Hlavin et al was used for the sample size determination of the pediatric long-term pain trial. This methodology makes use of prior information and of extrapolation from a larger population to a small target population as defined in the EMA reflection paper on extrapolation.Citation30
Specifically, for the pediatric long-term pain trial, the original sample size calculation did not consider prior information and the trial was therefore planned with a sample size of 129 children. During the trial, more information became available from the pediatric program in acute pain. Consequently, the final sample size was then recalculated making use of the pre-existing evidence from clinical trials evaluating the efficacy of tapentadol IR and PR in adult acute and chronic pain and using data from tapentadol IR in the pediatric population. By utilizing all this prior knowledge and extrapolations from adults to pediatrics and from IR to PR, the body of evidence required from the pediatric long-term pain trial could be reduced. This was reflected in a more liberal one-sided statistical significance level of 0.1 for the pediatric trial as determined by the Hlavin methodology, while still maintaining confidence in the effectiveness of the drug in children after successful pediatric trials. With this adjusted one-sided type I error of 0.1, a reduced sample size of 69 children was derived, compared to 129 children required with the usual type I error of 0.025. With this new approach, medication exposure in this vulnerable population could be limited, feasibility improved, and completion of the clinical trial accelerated.
Safety is of Great Concern in Pediatric Trials and Requires Constant Monitoring
In the tapentadol PK trials, enrollment was staggered starting with the oldest patient group under constant surveillance of safety and tapentadol exposure before the next younger group received any trial medication. In addition, external independent data monitoring committees were established for trials in children <2 years and for the multiple-dose efficacy and safety trial to monitor the safety of this vulnerable population.
It is Challenging to Investigate a Pain Medication with a Broad Label in Adults in the Pediatric Population
Unlike medications indicated for a specific disorder, eg upper respiratory tract infection which can have a narrowly focused pediatric development, tapentadol with its broad authorization for acute and chronic pain indications required an extensive investigational plan in the pediatric population. In order to investigate if tapentadol could also provide effective pain relief in a broad range of pediatric pain conditions, appropriate pain conditions representative for acute and chronic pain in the pediatric population had to be identified. After extensive research, acute pain following surgery was assessed as an area of unmet need in the pediatric population with a sufficient number of patients that can be accessed to make a clinical trial feasible and as a representative model of acute pain. Two commonly used postsurgical pain models appropriate for the age group investigated (2 to <18 years) and associated with significant pain, namely tonsillectomyCitation31 and dental surgeryCitation32 were employed in one single dose PK trialCitation19 but all other trials in the program permitted a wider selection of surgeries to achieve sufficient sample sizes and to ensure broad applicability of the results.
Typical chronic pain indications as observed in adults are rare in children. Although chronic daily pain in children might be present due to chronic headaches, neurodegenerative disorders, inflammatory disorders, posttraumatic neuropathic pain conditions, or advanced cancer and other diseases, it is less prevalent than in adults.Citation33 Moreover, it is rare that one of these indications requires a long-term treatment with opioids in young children. In light of the low occurrence of pain requiring long-term treatment with opioids especially in young children and owing to the limitations of the tapentadol PR tablet formulations available (lowest dose available is 25 mg), the development program focused on patients above the age of six. Even when focusing on patients 6 years or older, it soon became clear that recruitment of a sufficient trial sample size when using the adult definition of chronic pain (ie, pain lasting for at least 3 months) would be very challenging. Moreover, discussion with experts revealed that the real need in this population is for a prolonged release formulation that can ensure adequate pain relief when use is anticipated to be longer than would be usual for the treatment of acute pain. The use of a PR formulation would allow in these cases for less frequent dosing facilitating compliance and improving quality of life for patients and caregivers. These considerations with expert input resulted in the following definition of pain for entry into the tapentadol PR trial: ‘pain expected to require twice-daily PR opioid treatment for at least 14 days’. This definition was agreed by the PDCO prior to trial initiation. It permitted trial sites to recruit patients with more diverse indications (cancer pain, painful burns, postsurgical pain) in addition to patients with traditional chronic pain conditions such as juvenile rheumatoid arthritis or congenital neuropathy.Citation23 The high proportion of patients completing 14 days of tapentadol treatment in that trial suggested that the entry criteria for severe long-term pain enabled recruitment of the appropriate patients requiring long-term treatment with a strong analgesic.
Experience Gained from Interactions with the Regulatory Authorities in the EU
Iterative Learning in the Course of Development
Tapentadol was one of the first compounds to go through the formal EU process of the Pediatric Investigation Plan (PIP) and as such had a forerunner role at the start of the learning curve for both the regulators and the pharmaceutical industry. The PIP for acute pain was agreed with PDCO in 2008 and was completed in 2018. In the course of the program, 14 modifications of this PIP were agreed. The second PIP concerning long-term pain was agreed in 2009 and is still ongoing. To date, this PIP has been modified 10 times. All modifications required the expenditure of considerable time and effort on both sides (ie PDCO and pharmaceutical company) until the modifications were agreed to and adopted. The PIP is intended to be a living document but too much detail early on necessitated this large number of changes. From the publication describing the EMA’s experience with pediatric programs and the development of an ongoing action plan, it is clear that improvements have in the meantime been made and are continuing to be made in the process with less detail required initially and more flexibility to permit alteration based on emerging data;Citation34,Citation35 this is clearly a positive development.
Gap Between PDCO/EMA and Assessing Competent Authorities
According to the PDCO website, “the PDCO’s main role is to assess the content of paediatric investigation plans (PIPs), which determine the studies that companies must carry out in children when developing a medicine” and “is not responsible for marketing authorisation applications for medicines for use in children”. This is problematic as successfully completing a PIP as agreed with the PDCO does not guarantee approval by the responsible authorities of the medicinal product for the pediatric population as intended by the PIP. The competent authorities for marketing authorization can, for example, restrict the use of the product if the program is deemed to be insufficient for the intended and unrestricted use. This can render effective use of the product in the pediatric population difficult or impossible by, eg limiting the use to single dose only or restricting use to certain settings. The ultimate goal of a pediatric development should be to make drugs available for the pediatric population. This can only be achieved when the development is in line with the valid regulatory requirements and the obtained data are reflected in the product label. In our opinion, this requires stronger involvement of regulatory experts from competent authorities in the assessment of PIPs and their modifications.
Different Requirements Imposed by Different Authorities
For tapentadol both a Pediatric Study Plan (US) and a Pediatric Investigation Plan (EU) were agreed with the respective regulatory body, FDA (US) and PDCO (EU) and subsequently executed. Unfortunately, the two plans, although largely overlapping, had some specific requirements leading to sometimes subtle or overt differences in plans between the two regions. An overt difference was the requirement in the US to conduct a placebo-controlled efficacy trial to support a pediatric long-term pain indication, whereas in the EU, a non-inferiority design was accepted. Some examples of more subtle differences include differing requirements with respect to the age ranges to be studied, eg birth to <17 years (not including preterm neonates) for US and birth to <18 years (including preterm neonates) for EU for the acute pain program. Such differences lead to increased complexity in trial design, trial conduct, and analysis and interpretation of data. This could be addressed by a closer alignment on authority requirements for pediatric programs across regions. Moreover, the lack of harmonization of national rules for conducting pediatric clinical trials across the European Union led to a further significant increase of trial duration. Some clinical trial applications for trials included in the program as agreed upon with the PDCO did not receive approval in certain EU countries due to specific local requirements. A closer upfront alignment on authority requirements for pediatric clinical trials across the EU would help to resolve such issues.
Recommendations for sponsors of future pediatric programs are provided ().
Box 1 Recommendations for Sponsors of Future Pediatric Programs
Conclusions
Although tapentadol is one of the latest approved opioid analgesics, it is the most thoroughly and systematically investigated strong analgesic across all age groups. Its positive benefit/risk profile that was already established in the adult population is now also supported in acute and long-term use in the pediatric population by data from its structured pediatric development program. A pediatric development is challenging and even if executed in compliance with all preconditions from, eg a PIP, and if positive results are obtained, it may not automatically result in accessibility of the medication for the targeted population. To date and as a tangible outcome of the pediatric development plans, tapentadol OS for children has been authorized in the EU in 2018, as a new treatment option in the hospital for moderate to severe acute pain in children from 2 to <18 years of age.
Abbreviations
EMA, European Medicines Agency; FDA, Food and Drug Administration; IR, immediate release; IV, intravenous; MOR, µ-opioid receptor agonism; NRI, noradrenaline reuptake inhibition; OS, oral solution; PDCO, Pediatric Committee; PIP, Pediatric Investigation Plan; PK, pharmacokinetic; popPK, population PK; PR, prolonged release; TEAE, treatment-emergent adverse event.
Acknowledgments
Conception and realization of the tapentadol pediatric development required the involvement of many dedicated individuals over many years. The authors would like to acknowledge everyone who contributed, in particular Maria Soledad Berdaguer, Christoph Beuter, Martin Brett, Roberta Bursi, Kevin Canales, Siak Leng Choi, Luis de la Fuente, Wieslaw Degorski, Jacqueline Delfgaauw, Jörg Diekmann, Henk Dieteren, Jens Donath, Kerry Drozda-Müller, Cecile Dubois, Alice Ebel, Jan Freijer, Jennifer Gilbride, Jutta Goldberg, Anne Gyllensvärd, Thomas Häufel, Gwyn Hopkins, Joachim Hoppmann, Uta Hössl, Andras Kasa, Lieven Kennes, Ayaz Khan, Akash Khandelwal, Tanja Küpper-Drost, Claudia Lefeber, Jiri Letal, Blanca Linares-Rivas Rico, Constanze List, Brigitte Lücking, Vishakha Oza, Corinne Pala, Wolfgang Prange, Klaus Pusecker, Jérôme Rapion, Ronald Rosenburg, Jasmina Savic, Carsten Schmidt, Achim Steup, Ariane Stollenwerk, Maria Stupar, Eva Tarau, Rolf Terlinden, Julia Tilken, Mayke van Cauteren, Gisela Volkers, Silvia von Pistor, Andrea Waßmuth, Estelle Watson, Paloma Weinrich, and Matthias Winkel. Writing and editorial assistance was provided by Elke Grosselindemann and Martin and Birgit Brett and was paid for by Grünenthal GmbH.
Disclosure
All authors are employees of Grünenthal GmbH. The authors report no other conflicts of interest in this work.
References
- Eerdekens M, Beuter C, Lefeber C, van den Anker J.The challenge of developing pain medications for children: therapeutic needs and future perspectives. J Pain Res. 2019;12:1649–1664. doi:10.2147/JPR.S195788
- Grégoire MC, Finley GA. Drugs for chronic pain in children: a commentary on clinical practice and the absence of evidence. Pain Res Manag. 2013;18(1):47–50. doi:10.1155/2013/402863
- Califf RM Best Pharmaceuticals for Children Act and Pediatric Research Equity Act Status Report to Congress; 2016. Available from: https://www.fda.gov/downloads/scienceresearch/specialtopics/pediatrictherapeuticsresearch/ucm509815.pdf. Accessed September 8, 2020.
- European Union. Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use, OJ L 378, 27.12;.2006. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed September 8, 2020.
- Nafziger AN, Barkin RL. Opioid therapy in acute and chronic pain. J Clin Pharmacol. 2018;58(9):1111–1122. doi:10.1002/jcph.1276
- Xiao JP, Li AL, Feng BM, Ye Y, Wang GJ. Efficacy and safety of tapentadol immediate release assessment in treatment of moderate to severe pain: a systematic review and meta-analysis. Pain Med. 2017;18(1):14–24. doi:10.1093/pm/pnw154
- Viscusi E, Allard R, Sohns M, Eerdekens M. Tapentadol immediate release for moderate to severe acute post-surgery pain. J Opioid Manag. 2019;15:51–67. doi:10.5055/jom.2019.0486
- Baron R, Eberhart L, Kern KU, et al. Tapentadol prolonged release for chronic pain: a review of clinical trials and 5 years of routine clinical practice data. Pain Pract. 2017;17:678–700. doi:10.1111/papr.12515
- Tzschentke TM, Christoph T, Kögel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs. 2014;28:319–329. doi:10.1007/s40263-014-0151-9
- Raffa RB, Elling C, Tzschentke TM. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘µ-load’. Adv Ther. 2018;35:1471–1484. doi:10.1007/s12325-018-0778-x
- Göhler K, Brett M, Smit JW, Rengelshausen J, Terlinden R. Comparative pharmacokinetics and bioavailability of tapentadol following oral administration of immediate- and prolonged-release formulations. Int J Clin Pharmacol Ther. 2013;51(4):338–348. doi:10.5414/CP201722
- Terlinden R, Kogel BY, Englberger W, Tzschentke TM. In vitro and in vivo characterization of tapentadol metabolites. Methods Find Exp Clin Pharmacol. 2010;32:31–38. doi:10.1358/mf.2010.32.1.1434165
- Tzschentke TM, De Vry J, Terlinden R, et al. Tapentadol hydrochloride. Analgesic, mu-opioid receptor agonist, noradrenaline reuptake inhibitor. Drugs Future. 2006;31:1053–1061.
- Kneip C, Terlinden R, Beier H, Chen G. Investigations into the drug-drug interaction potential of tapentadol in human liver microsomes and fresh human hepatocytes. Drug Metab Lett. 2008;2:67–75. doi:10.2174/187231208783478434
- Smit JW, Oh C, Rengelshausen J, et al. Effects of acetaminophen, naproxen, and acetylsalicylic acid on tapentadol pharmacokinetics: results of two randomized, open-label, crossover, drug-drug interaction studies. Pharmacotherapy. 2010;30:25–34. doi:10.1592/phco.30.1.25
- Watson E, Khandelwal A, Freijer J, van den Anker J, Lefeber C, Eerdekens M. Population pharmacokinetic modeling to facilitate dose selection of tapentadol in the pediatric population. J Pain Res. 2019;12:2835–2850. doi:10.2147/JPR.S208454
- Khalil F, Choi SL, Watson E, et al. Population pharmacokinetics of tapentadol in children from birth to <18 years old. J Pain Res. 2020;13:3107–3123. doi:10.2147/JPR.S269549
- Finkel J, Goldberg J, Rosenburg R, et al. First evaluation of tapentadol oral solution for the treatment of moderate to severe acute pain in children aged 6 to <18. J Pain Res. 2019;12:1925–1936. doi:10.2147/JPR.S197348
- Muse D, Tarau E, Lefeber C, et al. Pharmacokinetics, safety, and efficacy of tapentadol oral solution for treating moderate to severe pain in pediatric patients. J Pain Res. 2019;12:1777–1790. doi:10.2147/JPR.S197039
- Eissa A, Tarau E, Beuter C, et al. Tapentadol for the treatment of moderate-to-severe acute pain in children under the age of two years. J Pain Res. 2021;14:229-248.
- Beuter C, Volkers G, Radic T, Goldberg J, van den Anker J. Efficacy and safety of multiple doses of tapentadol oral solution in the treatment of moderate to severe acute pain in children aged 2 to <18 years - a randomized, double-blind, placebo-controlled trial. J Pain Res. 2019;12:3099–3112. doi:10.2147/JPR.S207010
- Electronic Medicines Compendium. Palexia oral solution 20 mg/mL. Available from: https://www.medicines.org.uk/emc/product/5346/smpc. Accessed September 4, 2020.
- Howard R, Radic T, Sohns M, Eerdekens M, Waßmuth A. Tapentadol prolonged release for long-term treatment of pain in children. J Pain Res. 2020;13:3157–3170. doi:10.2147/JPR.S272751
- Food and Drug Administration. CDER conversations: pediatric pain management options. Available from: https://www.fda.gov/drugs/news-events-human-drugs/cder-conversation-pediatric-pain-management-options. Accessed December 30, 2019.
- Electronic Medicines Compendium. Palexia 50 mg film-coated tablets. Available from: https://www.medicines.org.uk/emc/medicine/28375. Accessed September 7, 2020.
- Electronic Medicines Compendium. Palexia SR prolonged release tablets. Summary of product characteristics. Available from: https://www.medicines.org.uk/emc/medicine/28373. Accessed September 7, 2020.
- Health Products Regulatory Authority. Palexia 4 mg/mL oral solution. Available from: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA2242-012-010_04032020131343.pdf. Accessed September 7, 2020.
- Khandelwal A, Bolger M, Brett M, Rosenburg R, Bursi R Physiologically-based pharmacokinetic (PBPK) model of tapentadol and tapentadol-O-glucuronide in adult and pediatric population. Available from: http://www.pkuk.org.uk/Contentimages/PKUK_2014_Programme_and_Abstractsnew.pdf. Accessed January 04, 2021.
- Hlavin G, Koenig F, Male C, Posch M, Bauer P. Evidence, eminence and extrapolation. Stat Med. 2016;35(13):2117–2132. doi:10.1002/sim.6865
- European Medicines Agency. Reflection paper on the use of extrapolation in the development of medicines for paediatrics. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-reflection-paper-use-extrapolation-development-medicines-paediatrics-revision-1_en.pdf. Accessed September 7, 2020.
- Hosseini Jahromi SA, Hosseini Valami SM, Hatamian S. Comparison between effect of lidocaine, morphine and ketamine spray on post-tonsillectomy pain in children. Anesth Pain Med. 2012;2:17–21. doi:10.5812/aapm.4092
- Urquhart E. Analgesic agents and strategies in the dental pain model. J Dent. 1994;22:336–341. doi:10.1016/0300-5712(94)90084-1
- Berde CB, Walco GA, Krane EJ, et al. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics. 2012;129(2):354–364. doi:10.1542/peds.2010-3591
- European Medicines Agency. 10-year report to the European Commission - general report on the experience acquired as a result of the application of the Paediatric Regulation. Available from: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/paediatrics_10_years_ema_technical_report.pdf. Accessed September 7, 2020.
- European Medicines Agency. European Medicines Agency and European Commission (DG Health and Food Safety) action plan on paediatrics. Available from: https://www.ema.europa.eu/en/documents/report/european-medicines-agency-european-commission-dg-health-food-safety-action-plan-paediatrics_en.pdf. Accessed September 8, 2020.
- Zannikos PN, Smit JW, Stahlberg HJ, Wenge B, Hillewaert VM, Etropolski MS. Pharmacokinetic evaluation of tapentadol extended-release tablets in healthy subjects. J Opioid Manag. 2013;9(4):291–300. doi:10.5055/jom.2013.0171