Abstract
Objectives
Adherence to medication for the treatment of fibromyalgia (FM) is predictive of lower overall health-care costs, and thus a lower burden on both patients and providers. The objectives of this study were to examine the predictors of adherence to and persistence with duloxetine therapy among commercially insured FM patients, and to identify subgroups of patients with high duloxetine persistence and adherence.
Study design
This cross-sectional, retrospective study analyzed medical and pharmacy records over 1 year for patients in the US aged 18–64 years with FM who initiated (no prior 90-day use) duloxetine treatment in 2008.
Methods
Adherence to duloxetine was measured by medication possession ratio (MPR), with high adherence defined as MPR ≥ 0.8. Persistence was defined as the duration of therapy from the index date to the earliest of: the ending date of the last prescription, the date of the first gap of >15 days between prescriptions, or the end of the study period (12 months). Demographic and clinical predictors of adherence were examined via multiple logistic regression (MLR), and subgroups of duloxetine-persistent and -adherent patients were identified using classification and regression trees (CART).
Results
Among 4660 duloxetine patients, 33% achieved high adherence. Factors associated with high adherence from MLR included older age, North Central and Northeast regions, prior venlafaxine, pregabalin, selective serotonin reuptake inhibitor (SSRI), or other antidepressant use, or comorbid dyslipidemia or osteoarthritis (all P < 0.05). CART analysis revealed that patients with prior antidepressant use, aged ≥46, or prior osteoarthritis had higher MPR (all P < 0.05), and patients aged ≥45 with a history of SSRI, venlafaxine, or anticonvulsant use had longer duration of therapy (all P < 0.05).
Conclusions
Patients with high adherence to and persistence with duloxetine were significantly older and had prior antidepressant use.
Introduction
Fibromyalgia (FM) is a chronic and often debilitating syndrome characterized by chronic widespread musculoskeletal pain and stiffness, nonrestorative sleep, and fatigue.Citation1 Given its many and diverse symptoms, some of which may present with other disorders, FM can be difficult to diagnose.Citation2 Common comorbidities in FM patients include gastrointestinal disorders, migraines, respiratory or circulatory conditions, and mental and mood disorders.Citation3 In the United States, the prevalence of FM is estimated at 2%–4%, predominantly in women, and it increases with age.Citation4 After osteoarthritis, FM represents the second most common disorder treated by rheumatologists.Citation5 It is estimated that FM treatment translates into an estimated economic burden in excess of US $20 billion annually.Citation6
The predominant theory underlying the pathophysiology responsible for the increased pain sensitivity (ie, allodynia and hyperalgesia) in those with FM is central sensitization due to dysregulation of pain pathways.Citation7 Both serotonin (5-HT) and norepinephrine (NE) are key modulators of endogenous pain inhibitory mechanisms via the descending pain inhibitory pathways in the brain and spinal cord.Citation8 It has been postulated that in FM, the net inhibitory effect of critical monoamines, such as 5-HT and NE, is reduced or lost, consequently shifting the descending pain modulatory system from a state of inhibition toward a state of pain facilitation.Citation9,Citation10 This idea is supported by the clinical observation that FM patients have lower levels of 5-HT and NE metabolites in their cerebrospinal fluid compared to controls.Citation11,Citation12
Duloxetine is a potent and selective dual 5-HT and NE reuptake inhibitor that has been shown to be effective in treating patients with FM.Citation13–Citation16 As a consequence, duloxetine was approved in the US for the treatment of FM in 2008. Given its recent approval, there has not been ample opportunity to study the real-world use of duloxetine in managing FM. In this vein, only recently have observational studies been published examining adherence to duloxetine in FM patients.Citation17–Citation20 Previous studies have shown that FM patients on higher doses (ie, >30 mg/day) of duloxetine were more likely to adhere to treatment and that higher adherence was associated with lower commercial health-care costs.Citation19,Citation20
Given the importance of medication adherence and persistence to treatment outcomes, including costs, the present study was designed to examine the predictors of adherence to and persistence with duloxetine therapy among commercially insured FM patients who initiated duloxetine in 2008. Although previous reports have focused on adherence to duloxetine treatment and its predictors,Citation17–Citation20 we are unaware of any study that has examined either duloxetine persistence or its predictors in FM patients. A second study goal was to identify subgroups of FM patients who were persistent with or adherent to duloxetine using classification and regression tree (CART) analysis.
Methods
Study design
This was a cross-sectional, retrospective claims database analysis assessing 1-year adherence and persistence to duloxetine.
Data sources
The study sample was constructed from the Thompson Reuters MarketScan Commercial Claims and Encounters Database (January 1, 2007 to December 31, 2009). A summary of how the study sample was generated is listed in . The MarketScan Database is a de-identified, nationwide medical claims database licensed by Thompson Medstat (Ann Arbor, MI), which is compliant with the Health Insurance Portability and Accountability Act. The MarketScan Database includes insurance claims of inpatient, outpatient, emergency room, pharmacy, behavioral healthcare, and enrollment data for employees and their dependents from large employer-sponsored health insurance in the US. Historically, MarketScan databases have >500 million claim records, and the databases have claims data from approximately 100 payers annually. All records are linked via unique patient identifiers, which allow for comprehensive tracking of an individual’s health-care utilization. Demographic and health insurance plan characteristics including age, gender, geographic region, health plan type, and enrollment status are available. Detailed health-care records included dates of service, provider type, payments, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes. Pharmacy claim files contain National Drug Code, dispense date, quantity dispensed, and plan and patient costs.
Table 1 Cohort selection
Study procedures
The study cohort selection criteria are summarized in . As shown, the target population of this study was adult FM patients aged 18–64 years who were initiated on duloxetine therapy. The inclusion criteria for this study were as follows. (1) Patients were initiated on duloxetine therapy between January 1, 2008 and December 31, 2008. The first filled duloxetine prescription was defined as the index date. (2) Patients had no record of use of the study medication in the prior 90 days. (3) Patients had at least one medical claim with an associated inpatient or outpatient FM diagnosis (as defined using ICD-9-CM: 729.1x). The diagnosis had to occur from 1 year prior to the index date to 1 month after the index date. (4) Patients had continuous enrollment from 12 months prior to the index date to 12 months after the index date. (5) Patients were in the age range of 18–64 years as of the index date. (6) Patients had at least 30 days cumulative duloxetine supply over the 12-month period following the index date. Duloxetine patients with a diagnosis of diabetic peripheral neuropathic pain (ICD-9-CM: 250.6× or 357.2) or depression (ICD-9-CM: 296.2x, 296.3x, 300.4x, 309.1x, or 311.xx) in the prior 12 months were excluded.
Measures of adherence and persistence
Patient adherence to duloxetine was reported based on medication possession ratio (MPR). The MPR was defined as the number of days that duloxetine was supplied from all claims during the 1-year study period after the index date divided by 365 days. High and low adherence to duloxetine were defined as MPRs of ≥0.80 and <0.80, respectively.Citation21 Thus a patient who filled prescriptions adequate to supply the drug for at least 80% (292 days) of the 1-year period was considered to have high adherence.
Patient persistence was measured using duration of therapy, defined as the duration of therapy from the index date to the earliest of either (1) the ending date of the last prescription; (2) the date of the first gap of >15 days between prescriptions; or (3) the end of the study period (12 months).Citation21 High and low persistence with duloxetine were defined as durations of ≥180 and <180 days, respectively.Citation22
Statistical analysis
Patients were classified into two cohorts based on high or low adherence. Differences in demographic and clinical characteristics between the two adherence cohorts were examined via chi-square test (categorical variables) or t-test (continuous variables). A stepwise multiple logistic regression (MLR) analysis with 0.1 as the entry and 0.05 as the exit significance levels was used to determine predictors of adherence, adjusting for the following patient and prescription characteristics: patient age; gender; geographical region (Northeast, North Central, South, and West); health plan type (consumer-driven health plans; comprehensive, exclusive provider organization; health maintenance organization; high-deductible health plan; point of service; preferred provider organization; and other plans); comorbidities in the prior year; select psychiatric, chronic pain, and sleep disorders; and use of medications in the prior year (psychotropic, sleep, and pain).
In addition to comparisons between the dichotomous high- and low-adherence and -persistence cohorts, factors associated with the best adherence and persistence outcomes were independently identified using CART analysis. Patients with FM were recursively partitioned into more homogeneous subgroups with respect to adherence or persistence based on the optimal factors (ie, demographic characteristics, comorbidities, or prior medications) at each split. Partitioning was stopped when adherence or persistence for the two subgroups resulting from a partition was not statistically different using a χ2 test (for binary adherence) or F-test (for MPR or duration of therapy) (α = 0.05) or the node size was less than 250 patients (about 5% of the whole population).
Analyses were conducted using SAS version 9.1 (SAS, Cary, NC) or Enterprise Miner version 5.2 (SAS), and the significance level was set a priori at P < 0.05.
Results
Demographic and clinical characteristics
Of the final sample of 4660 patients, approximately 88.6% were female (n = 4130, vs 530 males), and average age was 48 years. Almost 77% of the patients lived in the North Central or Southern regions of the US, and 61% were enrolled in a preferred provider organization ().
Table 2 Demographic characteristics among patients with fibromyalgia grouped by adherence to duloxetine over prior year
Based on MPR, the “low” adherence cohort (MPR < 0.80) comprised 3122 of the 4660 patients (67.0%), while the “high” adherence cohort (MPR ≥ 0.80) comprised 1538 patients (33.0%) (). The average MPRs for the low and high duloxetine-adherence cohorts were 0.33 and 0.94, respectively. Regarding duloxetine persistence, the low-adherence cohort had an average duration of therapy of 76.3 days, whereas the high-adherence cohort had an average duration of therapy of 279.4 days. Overall, the most prevalent comorbid medical conditions observed in addition to FM were low back pain, dyslipidemia, and hypertension (). Rates of low back pain (43.0% vs 38.0%; P < 0.001) and anxiety (10.2% vs 7.8%; P = 0.01) were significantly higher in the low (compared with the high) duloxetine-adherence cohort. Rates of dyslipidemia (33.2% vs 27.2%; P < 0.001), osteoarthritis (22.7% vs 17.9%; P < 0.001), and psoriatic arthropathy (2.1% vs 1.2%; P = 0.017) were significantly higher in the high (compared with the low) duloxetine-adherence cohort. In the low duloxetine-adherence cohort, >64% of patients used antidepressants and approximately 50% of patients used anticonvulsants prior to duloxetine initiation. In addition, approximately 75% of patients used narcotics and about 50% used nonsteroidal anti-inflammatory drugs (NSAIDs) in the prior 12 months. Rates of antidepressant (71.4% vs 64.4%; P < 0.001), anticonvulsant (53.5% vs 49.3%; P = 0.006), selective serotonin reuptake inhibitor (SSRI) (40.0% vs 30.9%; P < 0.001), pregabalin (33.0% vs 28.7%; P = 0.003), and venlafaxine (a dual 5-HT and NE reuptake inhibitor) (10.2% vs 6.7%; P < 0.001) use were significantly higher in the high compared with the low duloxetine-adherence cohort.
Table 3 Selected comorbid conditions and use of psychotropic and pain medications among patients with fibromyalgia grouped by adherence to duloxetine over prior year
Predictors of duloxetine adherence
Summarized in are the factors associated with high adherence to duloxetine therapy identified using a stepwise MLR approach. Among all the variables used in the model (ie, patient demographics, comorbidities, and prior medication use), the strongest association identified was that individuals >35 years old were more likely to adhere to duloxetine therapy compared to patients 18–35 years old (odds ratios = 1.42, 1.80, and 2.04 for 36–45, 46–55, and 56–64-year age-groups, respectively; all P < 0.05). In addition, patients living in the North Central or Northeast regions of the US were more likely to be in the high-adherence cohort compared with the Southern region (odds ratios = 1.22 and 1.38 for North Central and Northeast regions, respectively; both P < 0.05). Other significant factors associated with high adherence to duloxetine therapy included dyslipidemia and osteoarthritis (odds ratios = 1.19 and 1.21, respectively; both P < 0.05). Finally, prior use of pregabalin, antidepressants, SSRIs, or venlafaxine was associated with high duloxetine adherence (odds ratios = 1.23, 1.27, 1.32, and 1.39, respectively; all P < 0.05).
Table 4 Demographic and pretreatment clinical predictors of patient cohorts with fibromyalgia: multiple logistic regression
Predictors of duloxetine persistence
Summarized in are the factors associated with high persistence with duloxetine therapy, identified using MLR. As with the comparable analysis for adherence, the strongest association identified was that individuals >35 years old were more likely to persist with duloxetine therapy compared with patients 18–35 years old (odds ratios = 1.43, 1.85, and 1.89 for 36–45, 46–55, and 56–64-year age-groups, respectively; all P < 0.05). Geographically, patients living in the North Central region of the US were more likely to be in the high-persistence cohort compared with the Southern region reference group (odds ratio = 1.18; P < 0.05). Hypothyroidism was a significant factor associated with high persistence with duloxetine therapy (odds ratio = 1.24; P < 0.05), whereas chronic pulmonary disease and interstitial cystitis were associated with low persistence with duloxetine therapy (odds ratios = 0.79 and 0.33, respectively; both P < 0.05). Finally, prior use of SSRIs, venlafaxine, cyclooxygenase-2 selective inhibitors, anticonvulsants, or antidepressants was associated with high persistence with duloxetine therapy (odds ratios = 1.28, 1.51, 1.26, 1.36, and 1.27, respectively; all P < 0.05), whereas prior use of narcotics was associated with low persistence with duloxetine therapy (odds ratio = 0.86; P < 0.05).
Examination of subgroups using CART analysis
Across the whole FM patient cohort, the average MPR was 0.53. The CART model identified prior use of antidepressants, patient age, and history of osteoarthritis as the most important factors defining the high-MPR subgroups. Patients who used antidepressants, were 45 years of age or older, and had osteoarthritis were more likely to possess duloxetine (). The subgroup meeting these criteria accounted for 515 (11.1%) of the 4660 patients with an elevated MPR (0.62).
Figure 1 (A–C) Classification and regression tree analysis for subgroups of adherent and persistent patients with fibromyalgia. (A) Medication possession ratio (MPR): total duloxetine supply days/365. Node size (N) >250. Partition testing: F-test, α = 0.05. (B) Adherence (low, high): adherence = high if MPR ≥ 0.80; adherence = low if MPR < 0.80. Node size (N) >250. Split testing: Pearson χ2, α = 0.05. (C) Duration of therapy (Dur): duration of therapy from the index date to the earliest of either the ending date of the last prescription, the date of the first gap of >15 days between prescriptions, or the end of the study period (12 months). Node size (N) >250. Partition testing: F-test, α = 0.05.
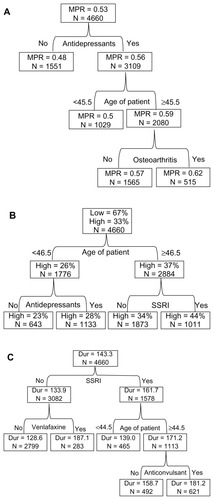
Highly adherent patients accounted for 33% of the whole FM patient cohort. The CART model identified patient age and prior use of SSRIs as the most important factors defining duloxetine-adherent subgroups in patients with FM (). Patients who were 46.5 years of age or older and used SSRIs were more likely to adhere to duloxetine. The subgroup meeting these criteria accounted for 1011 (21.7%) of the 4660 patients with an elevated adherence rate (44%).
On average, the duration of therapy was 143 days across the whole FM patient cohort. The CART model identified prior use of SSRIs or venlafaxine, patient age, and use of anticonvulsants as the most important factors defining subgroups with longer duration of therapy. Patients who used SSRIs and anticonvulsants and were 44.5 years of age or older were more likely to persist with duloxetine treatment (). The subgroup of 621 patients (13.3%) meeting these criteria had an average duration of therapy of 181 days. Similarly, patients who did not use SSRIs, but used venlafaxine, were also more likely to persist with duloxetine treatment (). The 283 patients (6.1%) who met these criteria had an average duration of therapy of 187 days.
Discussion
The present retrospective database study analyzed a large US-based commercial claim and encounters database to examine the predictors of adherence to and persistence with duloxetine therapy in FM patients over a 1-year period. To our knowledge, this is the first study to examine persistence with duloxetine therapy or its predictors. Overall, this cohort of FM patients had a combined average duration of therapy of 279.4 days. Comparing patients with high and low persistence using multiple logistic regression, higher persistence was associated with age > 35 years, geographic region, and prior use of antidepressants, anticonvulsant, cyclooxygenase-2 inhibitors, SSRIs, or venlafaxine. Greater age, geographic region, and prior use of antidepressants, and SSRIs or venlafaxine in particular, were also significantly associated with high adherence. Using CART analysis, which automatically detects key interactions between covariates, we independently identified prior use of SSRIs or venlafaxine and patient age as the most important predictors of persistence with duloxetine therapy (ie, longer duration of therapy) in this cohort of FM patients.
With respect to adherence to duloxetine treatment, using a criterion of MPR ≥ 0.80, approximately 33% of the FM patients included in this study were classified as having high adherence. Predictors of high adherence to duloxetine treatment included a prior history of antidepressant use and greater age. These findings were consistent irrespective of the statistical analysis method employed (ie, MLR or CART analyses). It is interesting to note, however, that had a 6-month follow-up period been employed instead of a 1-year follow-up period, MPRs would have been considerably different across the FM patients (data not shown), which could potentially impact the factors identified as well as the predictive value of those factors. Therefore, the duration of periods assessed should be considered when comparing results across different analyses of adherence or persistence, and longer periods may be important to consider when assessing treatments for chronic conditions such as FM.
Similar to a previous study,Citation5 approximately 90% of the FM patients were female, and most of the FM patients had many comorbid medical conditions. In general, the two adherence cohorts had similar medical history profiles; however, there were some differences with respect to prevalence: rates of anxiety and low back pain were significantly higher in the low duloxetine-adherence cohort, whereas rates of osteoarthritis, psoriatic arthropathy, dyslipidemia, and hypertension were significantly higher in the high duloxetine-adherence cohort. Given that osteoarthritis, dyslipidemia, and hypertension may be expected to be more common in older patients, it is perhaps not surprising that these were associated with the older, more adherent cohort. Notably, however, these comorbidities did not significantly associate with high persistence. This finding suggests that the relationship between comorbid disease and persistence may be different from that between comorbid disease and adherence.
As previously mentioned, FM is a complex medical condition, with patients often taking multiple medications concomitantly.Citation19,Citation20 Similar trends were observed in the present study, with the vast majority of FM patients taking numerous medications in the year prior to study initiation, with narcotics, antidepressants, and anticonvulsants among the most prevalent. The concomitant use of narcotics and antidepressants was common in the FM patients and concordant with the literature.Citation5 Although not unexpected, opioid use among the FM patients was frequent despite clinical guidelines suggesting that this drug class (other than tramadol) should be prescribed only after other alternatives have been fully exhausted.Citation23 In addition, although concomitant medication use was not systematically analyzed, the possibility of combined duloxetine and pregabalin therapy has been proposed previously.Citation24 In a post hoc analysis of our data, at least one pregabalin prescription during the 1-year study period was recorded for 27% of patients with low adherence and for 30% of patients with high adherence, suggesting little or no correlation with adherence to duloxetine. Nonetheless, an in-depth analysis of concomitant medication usage could be a valuable addition to future studies.
Almost half of FM patients also used NSAIDs despite their lack of efficacy in treating FM,Citation25–Citation27 although this may be underestimated given that over-the-counter medication use was not captured. The widespread use of NSAIDs in this patient population may reflect the management of other painrelated comorbidities, such as low back pain and arthritis. Future studies assessing the use of NSAIDs in this patient population is certainly warranted.
This study has several limitations related to being a retrospective study based on a claims database. First, as a consequence of the study design, findings can only be interpreted as association and not causality. Second, as this was a claims database analysis, there exists potential for selection bias, miscoding of information, and consequent biases in estimation. Third, all medical conditions were identified solely based on ICD-9-CM diagnosis codes recorded in the administrative claims database, which may be subject to inaccuracies as no validation with patient medical records was performed. Fourth, prescription records were used to quantify patient adherence to therapy, which may not adequately reflect actual consumption. Finally, an important limitation was that no data pertaining to severity or duration of FM, pain levels, or socioeconomic data on education, ethnicity, employment status, income, and family environment were available. Such variables may be associated with adherence and persistence outcomes, and their inclusion might have resulted in improved identification of key patient subgroups. Thus, caution must be used when interpreting these results, particularly for some of the general factors associated with adherence and persistence, such as geographic region, which might reflect identifiable social or demographic factors that were not captured in the data sets analyzed here. Given the difficulty of reliably assessing pain levels in the absence of validated rating instruments, as well as the difficulty in accurately assessing time of onset for FM, data on the impact of pain levels and duration of FM from prospective studies may be needed to understand the impact of these factors on adherence and persistence. If such data were strongly associated with adherence or persistence, their availability would be expected to allow greater specificity in identifying those patients most likely to continue and benefit from further therapy.
In conclusion, the findings from this study indicate that one-third of FM patients were highly adherent to duloxetine therapy over a 1-year period. Patients who achieved high adherence to duloxetine therapy were significantly older and had a prior history of antidepressant use. In addition, in this cohort of FM patients, the CART model identified prior use of SSRIs or venlafaxine and patient age as the most important predictors of longer duloxetine therapy. These findings may be of use in identifying patient subgroups most likely to adhere to and persist with treatment of their FM with duloxetine, thus resulting in reduced overall health-care costs and burden for these patients and their health-care providers.
Acknowledgments
The authors would like to thank Yi Chen and Yun Fang from in Ventiv Clinical Services for helping with the preparation of the data sets and data analysis, respectively. We are also grateful to Steve Able, Baojing Zhu, and Emily Zhu from Eli Lilly for reviewing this manuscript. This study was funded by Eli Lilly and Company.
Disclosure
Drs. Cui, Zhao, Novick, and Faries are full-time employees of Eli Lilly and Company and are minor stockholders of Eli Lilly and Company. This project was sponsored by Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA. Employees of Lilly were involved in the study design, analysis of data, interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
References
- WrightCLMistSDRossRLJonesKDDuloxetine for the treatment of fibromyalgiaExpert Rev Clin Immunol20106574575620828282
- Schmidt-WilckeTClauwDJPharmacotherapy in f ibromyalgia (FM) – implications for the underlying pathophysiologyPharmacol Ther2010127328329420388527
- BergerADukesEMartinSEdelsbergJOsterGCharacteristics and healthcare costs of patients with fibromyalgia syndromeInt J Clin Pract20076191498150817655684
- NeumannLBuskilaDEpidemiology of fibromyalgiaCurr Pain Headache Rep20037536236812946289
- NavarroRPContemporary management strategies for fibromyalgiaAm J Manag Care200915Suppl 7S197S21819601687
- AnnemansLLe LayKTaiebCSocietal and patient burden of fibromyalgia syndromePharmacoeconomics200927754755919663526
- StaudRVierckCJCannonRLMauderliAPPriceDDAbnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndromePain2001911–216517511240089
- BasbaumAIFieldsHLEndogenous pain control systems: brainstem spinal pathways and endorphin circuitryAnnu Rev Neurosci198473093386143527
- BannisterKBeeLADickensonAHPreclinical and early clinical investigations related to monoaminergic pain modulationNeurotherapeutics20096470371219789074
- BellinghamGAPengPWDuloxetine: a review of its pharmacology and use in chronic pain managementReg Anesth Pain Med201035329430320921842
- LegangneuxEMoraJJSpreux-VaroquauxOCerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H] imipramine reuptake in the primary fibromyalgia syndromeRheumatology (Oxford)200140329029611285376
- RussellIJVaeroyHJavorsMNybergFCerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritisArthritis Rheum19923555505561374252
- ArnoldLMRosenAPritchettYLA randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorderPain20051191–351516298061
- ArnoldLMLuYCroffordLJA double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorderArthritis Rheum20045092974298415457467
- RussellIJMeasePJSmithTREfficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trialPain2008136343244418395345
- ChappellASBradleyLAWiltseCDetkeMJD’SouzaDNSpaethMA six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgiaInt J Gen Med200919110220428412
- KleinmanNLSanchezRJLynchWDCappelleriJCBerenIAJoshiAVHealth outcomes and costs among employees with fibromyalgia treated with pregabalin vs standard of carePain Pract201111654055121392253
- ZhaoYSunPWatsonPMitchellBSwindleRComparison of medication adherence and healthcare costs between duloxetine and pregabalin initiators among patients with fibromyalgiaPain Pract201111320421620807351
- ZhaoYChenSYWuNFraserKABoulangerLMedication adherence and healthcare costs among fibromyalgia patients treated with duloxetinePain Pract201111438139121199311
- WuNChenSBoulangerLRaoPZhaoYAverage daily dose, medication adherence, and healthcare costs among commercially-insured patients with fibromyalgia treated with duloxetineCurr Med Res Opin20112761131113921456939
- CramerJARoyABurrellAMedication compliance and persistence: terminology and definitionsValue Health2008111444718237359
- American Psychiatric AssociationPractice Guideline for Treatment of Patients with Major Depressive Disorder2nd edWashingtonAmerican Psychiatric Association Press2000
- FitzcharlesMASte-MariePAGamsaAWareMAShirYOpioid use, misuse, and abuse in patients labeled as fibromyalgiaAm J Med20111241095596021962316
- SerraEDuloxetine and pregabalin: safe and effective for the long-term treatment of fibromyalgia?Nat Clin Pract Neurol2008459459518852724
- MeasePFibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatmentJ Rheumatol Suppl20057562116078356
- YunusMBMasiATAldagJCShort term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trialJ Rheumatol19891645275322664173
- SolitarBMFibromyalgia: knowns, unknowns, and current treatmentBull NYU Hosp Jt Dis201068315716120969544