Abstract
Postamputation pain (PAP) is highly prevalent after limb amputation but remains an extremely challenging pain condition to treat. A large part of its intractability stems from the myriad pathophysiological mechanisms. A state-of-art understanding of the pathophysiologic basis underlying postamputation phenomena can be broadly categorized in terms of supraspinal, spinal, and peripheral mechanisms. Supraspinal mechanisms involve somatosensory cortical reorganization of the area representing the deafferentated limb and are predominant in phantom limb pain and phantom sensations. Spinal reorganization in the dorsal horn occurs after deafferentataion from a peripheral nerve injury. Peripherally, axonal nerve damage initiates inflammation, regenerative sprouting, and increased “ectopic” afferent input which is thought by many to be the predominant mechanism involved in residual limb pain or neuroma pain, but may also contribute to phantom phenomena. To optimize treatment outcomes, therapy should be individually tailored and mechanism based. Treatment modalities include injection therapy, pharmacotherapy, complementary and alternative therapy, surgical therapy, and interventions aimed at prevention. Unfortunately, there is a lack of high quality clinical trials to support most of these treatments. Most of the randomized controlled trials in PAP have evaluated medications, with a trend for short-term Efficacy noted for ketamine and opioids. Evidence for peripheral injection therapy with botulinum toxin and pulsed radiofrequency for residual limb pain is limited to very small trials and case series. Mirror therapy is a safe and cost-effective alternative treatment modality for PAP. Neuromodulation using implanted motor cortex stimulation has shown a trend toward effectiveness for refractory phantom limb pain, though the evidence is largely anecdotal. Studies that aim to prevent PA P using epidural and perineural catheters have yielded inconsistent results, though there may be some benefit for epidural prevention when the infusions are started more than 24 hours preoperatively and compared with nonoptimized alternatives. Further investigation into the mechanisms responsible for and the factors associated with the development of PAP is needed to provide an evidence-based foundation to guide current and future treatment approaches.
Keywords:
Historical aspects
The word amputation can trace its origin to the Latin term “amputatio,” meaning “to cut around.” Yet, amputations have been practiced since the dawn of mankind. Historical and archaeological records demonstrate that purposeful amputations have been performed since Neolithic times, dating back at least 45,000 years.Citation1 This evidence consists of stone knives and saws found with the skeletal remains of amputated stumps.
It is likely that postamputation pain (PAP) has plagued humans for countless millennia. However, our understanding of PAP has significantly evolved over the centuries, with the full impact beginning to unravel only recently. Perhaps the major advances in amputation care and our understanding of their sequelae have occurred during war. For hundreds of years, horrific limb injuries have been the result of man’s fascination with armed conflict. Reporting on 86 civil war amputees, the renowned physician Weir Mitchell coined the term “phantom pain,” recording an incidence as high as 90%.Citation2
But for the most part, the concept of PAP was largely ignored by the mainstream medical establishment, with post-World War II prevalence rates consistently estimated at less than 5%.Citation3,Citation4 Moreover, many of these patients were ostracized, and their symptoms attributed to either psychopathology or secondary gain.Citation4
Today, the management of amputations engenders public attention and research dollars far in excess of its epidemiological burden. PAP is widely considered to be one of the most challenging among all pain conditions to treat, as is evidenced by the plethora of trials that continue to be conducted. A large part of its intractability stems from the myriad pathophysiological mechanisms that can result in PAP. Whereas mechanism-based pain treatment is generally considered to be superior to etiologic-based therapy,Citation5,Citation6 the obstacles involved in identifying the predominant mechanism(s) – which are prodigious under the best of circumstances – can become nearly insurmountable for a condition as phenotypically and pathogenetically disparate as PAP. The purpose of this review is therefore to provide an evidence-based framework from which to evaluate therapies and guide treatment for PAP.
Definitions and epidemiology
In the United States, the prevalence of limb loss was 1.6 million in 2005, which is projected to increase to 3.6 million by 2050.Citation7 Approximately 185,000 upper- or lower-limb amputations are performed annually. According to a study by Dillingham and colleagues examining data from the Healthcare Cost and Utilization Project from 1988 to 1996, vascular pathology is the most common etiology, accounting for 82% of limb loss discharges followed, in descending order, by trauma (16.4%), cancer (0.9%), and congenital anomalies (0.8%).Citation8 The loss of a body part can lead to painful and nonpainful neurologic sequelae that fall into three distinct descriptive categories: phantom limb pain (PLP), residual limb pain (RLP), and phantom sensations (PSs). Although these categories will be described independently, one cross-sectional study by Ephraim and colleagues performed in 914 individuals with limb loss found that up to 95% experienced at least one of these categories.Citation9 Furthermore, patients surveyed about their postamputation sensations often have a difficult time distinguishing one category from another.Citation10
PLP is a painful or unpleasant sensation in the distribution of the lost or deafferentated body part. PLP varies in character from neuropathic-type descriptors such as sharp, shooting, or electrical-like, to more nociceptive-specific adjectives such as dull, squeezing, and cramping. It can be localized to the entire limb or just one region of the missing limb. PLP typically occurs within the first 6 months after loss of a limb, but its prevalence several years after surgery has been reported to be as high as 85%, and it can persist for years after surgical amputation.Citation11,Citation12 In a prospective study evaluating 58 patients who underwent limb amputation, Jensen et al found that PLP changed over time from an exteroceptive-like pain (ie, knife-like or sticking) localized to the entire limb or a proximal region, to a more proprioceptive-like pain (burning or squeezing) localized to the distal areas of the amputated limb.Citation13
PLP should be distinguished from RLP, also known as “stump” pain, which is localized to the remaining body part after amputation. Stump pain is typically described as a sharp, burning, electrical-like, or “skin-sensitive” pain which can be localized to a superficial incision, be perceived deep in the residual limb, or sometimes encompass the whole residual limb. The reported incidence of stump pain can be as high as 74%, and similar to phantom pain can persist for years.Citation14 Stump pain can be further subdivided into postsurgical nociceptive, neurogenic, prosthogenic, arthrogenic, ischemic, sympathetically maintained, pain referred from the spine or joints, or pain secondary to abnormal stump tissue such as adhesive scar tissue or heterotopic ossification.Citation12 Although PLP and RLP often coexist, RLP is usually more bothersome immediately after amputation, whereas PLP may predominate 1–12 months after the amputation event.Citation15 Studies have found a significant correlation between the magnitude of RLP and PLP.Citation16
PSs are defined as nonpainful perceptions emanating from the lost body part after deafferentation or amputation. PSs are common in the postoperative period, with one-third of patients experiencing PSs within 24 hours, three-quarters of patients within 4 days, and 90% of patients within 6 months after surgery.Citation13 Unlike PLP and RLP, amputation of a body part is not essential prior to the development of PSs. PSs have been reported after avulsion of the brachial plexus without amputation of the limb,Citation17 and following spinal cord injury.Citation18 PSs can be subdivided into kinetic, kinesthetic, and exteroceptive perceptions. Kinetic sensations are perceived movements of the amputated body part that can be willed or spontaneous, such as the movement of toes in an amputated foot. Kinesthetic sensations refer to the size, shape or position of the amputated body part, such as feeling that a hand is twisted. Exteroceptive perceptions can include touch, pressure, tingling, temperature, itch, and vibratory sensations.Citation18 PSs are typically experienced in regions with disproportionately large cortical representation, such as the hands and feet. PSs can result not just from amputation of an extremity but also excision of other body parts such as the breast after mastectomy, which is estimated to occur in approximately 25% of individuals.Citation19 Telescoping refers to the perception of progressive shortening of the amputated limb, which results in the sensation that the distal part of the limb is becoming more proximal.Citation13 For example, a patient with an above the elbow amputation may initially feel phantom pain or sensations in the entire forearm and hand. Over time, the same patient may perceive his or her hand to be close to the stump, but not feel the proximal forearm. This phenomenon occurs in one-quarter to two-thirds of major limb amputees.Citation20
PAP is primarily a clinical diagnosis based on history and physical examination, though certain tests can help rule out alternative and often remediable diagnoses such as referred back pain, residual ischemia, prosthesis-related pain, neuromas, pressure-related wounds, and infection. A study by Smith and colleagues performed in 92 patients undergoing amputation found that back pain was more prevalent (71%) compared with that of the general population (45%).Citation21 Patients with chronic mechanical lower-back pain may have referred pain to the leg from such sources as the lumbar zygapophysial and sacroiliac joints, which can be mistaken for PLP.Citation22 Ischemic injury must be ruled out in patients presenting with PAP, especially since a large proportion of patients who undergo amputation have vascular insufficiency as the underlying etiology. Tests of distal perfusion such as transcutaneous oxygen tension (positive for ischemia if less than 20 mmHg) at the level of the residual limb can be useful in ruling out an ischemic etiology. Diagnosing a neuroma as a source of RLP may be useful when formulating a treatment plan. A positive Tinel’s sign (tapping on the injured nerve or neuroma elicits pain in the phantom limb or stump) represents a classic feature for neuroma. Prosthesis-related pain is often mistaken for classical RLP or PLP, and in some cases can be easily remedied. Occasionally, residual limbs may atrophy over time leading to stump shape changes relative to the original mold obtained during the casting process. This results in load-bearing and other forces inside the socket to shift from weight-tolerant to intolerant areas, which can cause erosion of the skin around contact points and overlying bony tissue. A careful skin and soft tissue examination of the stump can effectively rule out pressure wounds or frank infections that may be developing. Pressure points that develop over bone spurs or pathologic bone formation (ie, heterotopic ossification) can be a source of localized pain, which can be identified on plain radiographs. Infections such as osteomyelitis and residual graft infection can also be a source of chronic PAP.
Although primarily used in pain research, quantitative sensory testing (QST) may be a valuable tool for the diagnosis and management of PAP. QST entails the determination of pain thresholds or stimulus response curves for sensory processing under normal and pathophysiological conditions. For example, QST has been used to provide quantitative objective measures of neuropathic pain via the conductance of thermal testing in regions of heat allodynia, which can improve diagnosis and inform treatment.Citation23–Citation27 In short, QST may allow clinicians to better phenotype patients, which in turn may improve treatment outcomes.
Mechanisms
Treatment of PAP is very challenging because the underlying mechanisms are multifactorial in nature. Since mechanisticbased pain treatment is generally acknowledged to be superior to etiologic-based treatment, the difficulty in identifying a discrete mechanism(s) which can be directly addressed results in corresponding barriers to treatment.Citation5 The pathophysiology underlying phantom phenomena can be broadly categorized in terms of supraspinal, spinal, and peripheral mechanisms (see ). Supraspinal mechanisms related to phantom limb phenomena primarily involve reorganization of the somatosensory cortex surrounding the area representing the deafferentated limb. Ramachandran and colleagues demonstrated that brushing the face of upper limb amputees could elicit PSs.Citation28 They hypothesized that somatosensory cortical reorganization could explain why afferent nociceptive stimulation of a body part (eg, proximal stump or face for upperlimb amputees) whose cortical representation is adjacent to that of the phantom can produce sensations in the phantom. Specifically, tactile, proprioceptive, and nociceptive input from the face and tissues near the residual limb take over regions of the brain that no longer receive afferent input. Functional magnetic resonance imaging (MRI) studies after amputation of the hand have demonstrated that the cortical area corresponding to the hand is activated during proximal limb movements, and that cortical stimulation of this region evokes contraction of proximal upper-limb muscles.Citation29–Citation32 The greater the size of the deafferentated area and extent of cortical reorganization, the more intense the PSs.Citation33,Citation34
Figure 1 Mechanism-based treatment modalities for postamputation pain.
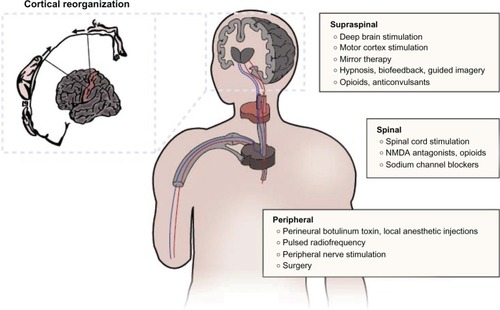
The evidence for peripheral mechanisms playing a role in PAP include the demonstration of spontaneous neuronal activity in the proximal end of cut nerves, the presence of stump pathology in some patients with phantom pain and the strong correlation between RLP and PLP, and the relief of phantom pain after the injection of local anesthetic into the painful stump.Citation13,Citation35 Axonal nerve damage during an amputation initiates inflammation and regenerative sprouting which results in a neuroma. Afferent fibers in the neuroma develop ectopic activity, mechanical sensitivity, and chemosensitivity to catecholamines. Altered expression of transduction molecules, upregulation of voltage-sensitive sodium channels, downregulation of potassium channels, and the development of new nonfunctional connections between axons (ephapses) all serve to increase spontaneous afferent input to the spinal cord.Citation36 These changes may lead to spontaneous pain, and help explain the amplification in pain caused by emotional distress and/or exposure to cold that leads to increased sympathetic discharge and circulating catecholamines.Citation20
The spinal mechanisms for PAP are thought to center on functional changes in the dorsal horn of the spinal cord after deafferentation from a peripheral nerve injury. The loss of afferent input to the dorsal horn leads to decreased impulses from brainstem reticular areas, which normally exert inhibitory effects on sensory transmission.Citation37 Therefore the absence of inhibitory effects for sensory input arising from the missing peripheral body part cause increased autonomous activity of dorsal horn neurons, in effect becoming “sensory epileptic discharges.”Citation11,Citation12 The contribution of spinal cord mechanisms is illustrated by the fact that anticonvulsants, and lesions placed in the substantia gelatinosa, are effective in treating phantom pain.Citation38 Similar to cortical reorganization, a “spinal reorganization” process has also been described in which adjacent afferent fibers “invade” regions of the spinal cord that are functionally inactivated by injured afferent fibers.Citation38 Clinically, the evidence supporting spinal mechanisms is bolstered by: the development of phantom pain in lower-extremity amputees following new lumbar disc herniations, and new-onset phantom pain in an amputated upper extremity associated with herpes zoster infection, both of which have been successfully treated with epidural steroid injections;Citation39 the evocation of phantom pain with spinal analgesia;Citation40 and an unusual case in which longstanding PLP disappeared with the development of cauda equina compression by a tumor and recurred following decompression.Citation41
Multiple cellular, neurochemical and molecular changes underlie the peripheral and central reorganization phenomena that occur in the postamputation period. Studies of nonhuman primates have demonstrated that chronic deafferentation can cause distal axon sprouting and the formation of neuromas,Citation42 chromatolysis (dissolution of Nissl bodies in the neuronal cell body) and loss and fibrosis of dorsal root ganglion cells,Citation43–Citation46 atrophy or degeneration of central terminations of sensory neurons in the brainstemCitation46 and spinal dorsal horn,Citation45,Citation47 sprouting of sensory neuron terminations in the dorsal horn,Citation48,Citation49 decreased myelination, and changes in neuropeptide levels in dorsal root ganglia and the dorsal horn.Citation50 In the first month after amputation, nerve injury can cause transsynaptic atrophy of central neurons. Neurochemically, lower-limb amputations lead to decreased lectin binding and substance P levels and upregulated neuropeptide Y.Citation51,Citation52 These peripheral neuronal changes have been observed from about 2 months to as many as 38 years after amputation, and appear to be concurrent with changes in the brainstem. Immunohistochemical studies evaluating changes in the dorsal column nuclei of chronic upper- and lower-limb amputees have revealed some atrophy of cuneate and gracile nuclei ipsilateral to the amputation, as well as proliferation of astrocytes, reactive gliosis, and inflamed axons (spheroids).Citation53 These findings suggest that amputation triggers neurochemical/molecular changes that cause degenerative and regenerative changes in primary sensory axons in the dorsal column nuclei.
Ultimately, pain after amputation is likely caused by a combination of the above mechanisms as total spinal anesthesia, cordotomy, cordectomy, spinal cord stimulation, and regional anesthesia of the plexus or stump have at best yielded only modest relief of phantom pain. As noted above, in some cases spinal anesthesia can even rekindle phantom pain that previously subsided.Citation54,Citation55 Hence, the interactions between peripheral, spinal, and supraspinal phenomena are all thought to contribute to postamputation phenomena, and should all be considered when planning treatment.
Treatment
Injection therapy
There are few controlled trials available to guide pain practitioners in the optimal management of PAP, with most therapies extrapolated based on effectiveness in other neuropathic pain states. Presently, the state-of-the-art treatment of PAP involves a multimodal approach that includes injections, pharmacotherapy, complementary and alternative therapies, surgery, and prevention.
Based on available evidence, local injection therapy appears to be more efficacious in the treatment of RLP compared with PLP. This appears to be due to the greater contribution of peripheral mechanisms in RLP compared with PLP. Although regional nerve blocks using lidocaine and/or corticosteroid often results in immediate relief of RLP, the duration of pain relief is highly variable and temporary.Citation56,Citation57 An active area of research aims to prolong this effect. One small randomized controlled pilot study examined the Efficacy of focal chemo-denervation using perineural injection of botulinum toxin type A compared with combination lidocaine and depomedrol. The study found that while both therapies showed a trend toward Efficacy, botulinum toxin resulted in a statistically significant improvement in RLP at 1 month, which was sustained over the 6-month study period. Neither modality improved PLP.Citation58 Another small series found a similar short-term benefit for RLP with the local injection of botulinum toxin B.Citation59 A separate small case series found that for patients who experienced relief from a diagnostic lidocaine injection, pulsed radiofrequency was effective in relieving RLP, although the effects were mixed for PLP.Citation60 Another small case series found perineural injection of the tumor necrosis factor inhibitor to be effective in patients with PLP and RLP of less than one year duration and tender points on physical examination.Citation61 A small observational study demonstrated that sympathetic dysfunction may play a role in the pathogenesis of PAP, and that sympathetic nerve blocks may provide some short-term relief of PLP, and to a lesser extent RLP.Citation62 It is important to note that local injection therapy may have effects beyond providing local peripheral blockade of pain input. One small observational study found that contralateral myofascial injection with local anesthetic in unilateral amputees attenuated PLP and PSs in the affected limb, though follow-up was limited to 1 hour.Citation63 Whereas the precise mechanism for this effect is unclear, animal studies have demonstrated that blocking afferent inputs on the contralateral side can decrease spontaneous hyperactivity of wide dynamic response neurons on the injured side, suggesting that a spinal mechanism may be at work.Citation64
Pharmacotherapy
When choosing pharmacotherapy for patients with established PAP, the practitioner must consider chronicity, route of administration, and adverse effects. There are six groups of medications for which there is evidence in the treatment of PAP: N-methyl-D-aspartate (NMDA) receptor antagonists, opioids, anticonvulsants, antidepressants, local anesthetics, and calcitonin (see ).
Table 1 Randomized controlled trials of pharmacologic interventions for the treatment of postamputation pain
NMDA receptor antagonists including ketamine, dextromethorphan, and memantine are thought to block a cascade of events leading to sensitization of dorsal horn wide dynamic range neurons. In patients with PLP or RLP, Nikolajsen and colleagues found a significant decrease in pain intensity, wind-up-like-pain, and pressure-pain thresholds following a 45-minute low-dose ketamine infusion compared with a normal saline control.Citation65 Although most of the participants in this study had PAP following amputations for cancer, a more recent study by Eichenberger found 1 hour ketamine (0.4 mg/kg) and ketamine plus calcitonin infusions to be effective for up to 48 hours compared with placebo for participants with various etiologies of PLP. No benefit was noted for calcitonin either alone or combined with ketamine.Citation66 Likewise, oral dextromethorphan has been shown to be effective in reducing pain intensity in a small placebo-controlled crossover study comprised of patients with PLP,Citation67 but oral memantine has not been shown to have analgesic properties when treating patients with established PLP.Citation68–Citation70 Interestingly, memantine has been efficacious in combination with brachial plexus blockade to prevent PLP acutely, suggesting that the timing of administration may be important.Citation71 Whereas the evidence for ketamine is strongest, the short follow-up periods and high incidence of adverse effects, including alterations in consciousness, visual hallucinations, hearing impairment and mood changes, limit its long-term usefulness.
Opioids may be beneficial in the treatment of PAP due to its mechanism of action at both the spinal level, where it inhibits pain signaling pathways, and supraspinal level, where it may diminish the degree of cortical reorganization associated with pain intensity.Citation72 Both oral and intravenous opioids administered for up to 6 weeks have been shown to be effective for PAP.Citation72–Citation74 In fact, Huse and colleagues showed that cortical reorganization was reduced in two of three participants with PLP undergoing treatment with oral morphine at both 6 and 12 months follow-up for one patient, and during the treatment phase for the other.Citation72 Similarly, tramadol has been shown to be effective in treatment of long-standing PAP compared with placebo over a 1-month treatment period.Citation75 Among the two opioid class medications, morphine has the more severe adverse-event profile, which includes constipation, sedation, tiredness, dizziness, sweating, voiding difficulty, vertigo, itching, and respiratory problems.
Anticonvulsants have long been a mainstay in the treatment of neuropathic pain. However, studies examining gabapentin as a treatment for established PAP have been conflicting with both positive and negative trial results.Citation76,Citation77 Gabapentin was associated with significant side effects in both studies, including somnolence, dizziness, headache, and nausea.
Calcitonin has a direct central action which causes inhibition of neuronal firing in response to peripheral stimulation.Citation78 This mechanism of action has encouraged interest in calcitonin as an adjunctive medication in the treatment of PAP. However, studies to date have been variable. One early controlled crossover study by Jaeger and Maier demonstrated a reduction in pain intensity compared with placebo that was sustained through 1-year follow-up in 8 of 13 patients with postoperative PLP who received a single calcitonin infusion.Citation79 However, a more recent study by Eichenberger et al evaluating calcitonin as a treatment for established PLP found calcitonin to be ineffective compared with placebo, attributing its ineffectiveness to a possible lack of effect on central sensitization processes.Citation66 Adverse effects of calcitonin included headache, vertigo, drowsiness, nausea, emesis, and hot/cold flashes.
Sodium channel blockers have been shown to be effective for both neuropathic and inflammatory pain. One study assessing amitriptyline in the treatment of PLP or RLP did not show a significant difference compared with placebo after 6 weeks of treatment, although patients in the treatment group did experience adverse effects such as dry mouth and dizziness severe enough to cause dropouts.Citation80 Whereas tricyclic antidepressants act in part via the blockade of sodium channels, their main mechanisms of action involve enhancement of descending inhibitory pathways via the inhibition of serotonin and norepinephrine reuptake. Similarly, evidence for using primary sodium channel blocking medications has been mixed at best. One double-blind study by Wu et al found that whereas intravenous morphine provided significant immediate-term relief of both PLP and RLP compared with placebo, intravenous lidocaine alleviated only RLP.Citation73 In a placebo-controlled follow-up study by the same group in 60 patients with PAP, morphine but not the oral lidocaine analogue mexiletine provided significant pain relief at 6-week follow-up.Citation74
Complementary and alternative therapies
The refractory nature of PAP to traditional medical and interventional therapies underscores the importance of developing complementary and alternative therapies. Psychological interventions for PAP aim to facilitate adaptation to pain, body image, and negative emotions associated with amputation. In one randomized controlled trial of 20 patients with 6 months of RLP or PLP, hypnosis administered in three individual sessions reduced overall pain scores when compared with pre-intervention scores.Citation81 Evidence for other psychological techniques such as guided imagery (creating mental images that help promote relaxation and healing) and biofeedback (learned control over autonomic physiologic processes) is mostly anecdotal.Citation82,Citation83 In one case series by Beaumont et al in which six amputees with chronic PLP underwent visual-kinesthetic feedback therapy over 8 weeks, four participants demonstrated greater than 30% reduction of pain after the intervention but only one maintained this over 6 months of follow-up. The authors pointed out that psychological health, social support, and the degree of control prior to the intervention may be significant factors in determining those who benefit compared with those who do not.Citation82
Cognitive behavioral therapy (CBT) has been used successfully in patients with chronic neuropathic pain conditions.Citation84–Citation86 In particular, a case series by Tichelaar and colleagues involving three patients with complex regional pain syndrome type I suggested that CBT may address pain mediated by central cortical reorganization.Citation86 Although there are no controlled trials showing Efficacy for CBT in PAP, there is an ongoing randomized controlled trial by McQuaid and colleagues designed to test whether CBT plus mirror therapy is superior to supportive care in amputees.Citation87 Whereas psychological interventions such as hypnosis, biofeedback, guided imagery, and CBT are safe and minimally invasive, large-scale trials are lacking and there is little evidence for long-term benefit.
Mirror therapy exploits the brain’s predilection to prioritize visual information over somatosensory feedback and is believed to treat PAP by influencing cortical reorganization. Flor et al showed that the degree of phantom pain correlates with the degree of maladaptive reorganization of somatosensory pathways using functional MRI, and that reorganization can be reversed by mirror therapy with a corresponding reduction in pain.Citation88,Citation89 Also known as visual mirror feedback, mirror therapy involves placing a mirror adjacent to the intact limb to give the illusion that the amputated limb is present and can be purposefully moved. Since it was introduced in 1992 by Ramachandran and Altschuler,Citation90 multiple studies have demonstrated short-term pain reduction using visual feedback using both mirrors and virtual reality or video modalities.Citation91,Citation92 Chan et al randomly assigned six patients each to one of three groups, mirror therapy, covered mirror therapy, and trained visual imagery, and showed that after 4 weeks, pain decreased in the mirror therapy group, stayed the same in the covered mirror therapy group, and increased in the visual imagery group. Nine of the patients in the covered mirror and visual imagery groups crossed over to the mirror therapy group, with a mean decrease in pain of 75% over the next 4 weeks compared with their baselines.Citation93 Because sensory experiences can be evoked by visual stimuli, mirror therapy increases spinal motor and cortical excitability.Citation94 The simplicity and noninvasiveness of this treatment modality has led to its application not only following limb loss, but also for the prevention of PAP.Citation95
Surgical therapies
Although surgical interventions have not demonstrated significant benefit in well designed trials, patients with chronic intractable PAP who fail the aforementioned treatment modalities may sometimes be considered for surgical management.Citation12 Surgical modalities fall into two general categories: neuromodulatory techniques and reconstructive. Neuromodulatory therapies are by definition mechanism driven, targeting maladaptive neuroplastic changes at the peripheral, spinal, and supraspinal levels. Although there are no randomized controlled trials to demonstrate Efficacy and safety for peripheral nerve stimulation (PNS), PNS has the potential to be especially effective in patients for whom the majority of pain is confined to the distribution of one or two peripheral nerves. Historically, peripherally placed electrodes required surgical dissection of the nerve in order to place the electrodes along the nerve trunk.Citation96 Recently, Rauck and colleagues demonstrated that a PNS lead inserted percutaneously and remotely from the target nerve in a single patient with RLP could lead to both pain relief and improvement in quality of life outcomes at 2 weeks follow-up.Citation97 The emergence of percutaneous techniques may change the risk/ benefit for patients with refractory RLP, and represents a promising area of future study. Whereas targeting peripheral mechanisms may be sufficient in patients with RLP, those with central sensitization or deafferentation, as occur with PLP, require neuromodulation at spinal or supraspinal levels. Spinal cord stimulation has been shown to be effective in a number of neuropathic pain states, but the evidence for its Efficacy in PAP is less robust, with several studies demonstrating inferior outcomes compared with peripheral neuropathic pain.Citation98–Citation102 The evidence that does exist is mainly limited to small case series that report “successful outcome,” with the criteria for a successful outcome varying drastically between studiesCitation98–Citation104 (see ). Nonetheless, spinal cord stimulation is reimbursed by Medicare specifically “to treat intractable pain caused by phantom limb syndrome that has not responded to medical management.”Citation105 Although still considered investigational by the Food and Drug Administration, motor cortex stimulation in PAP is very promising. One meta-analysis of 155 patients from nine studies of motor cortex stimulation in various chronic pain states show that 53% of patients with PLP were treated successfully, with follow-up periods that ranged from 6 months to 10 years.Citation106 Motor cortex stimulation directly targets the site of cortical reorganization and pain by using precise positioning techniques such as preoperative functional MRI and awake intraoperative stimulation. Deep brain stimulation has been shown to be more effective in nociceptive pain conditions such as failed back surgery syndrome rather than in deafferentation central pain states, and is associated with mixed results in patients with PA P.Citation107
Table 2 Studies evaluating neuromodulation for the treatment of postamputation pain
The contribution of peripheral mechanisms is greater in RLP compared with PLP; therefore, surgical reconstruction can be successful in treating RLP associated with distinct pathologic lesions. For example, heterotopic ossification is highly prevalent in patients with traumatic amputation, with a prevalence rate of up to 63%.Citation115 In the same study by Potter and colleagues, 20 of 25 patients with traumatic amputations whose heterotopic ossifications were excised were able to discontinue or reduce their opioid and/or neuropathic pain medication consumption at an average of 12 months follow-up.Citation115 Neuromas are an inevitable sequelae of major nerve injury or transaction, and clinically significant neuromas may occur in up to 80% of cases, presenting as a discrete area of pain and abnormal sensation in the distribution of a single peripheral nerve.Citation116 Frequently, a Tinel’s sign can be elicited. Whereas this typically manifests as pain in the residual limb, referred pain from the stump into the phantom limb can also occur. Unlike with heterotopic ossifications, the long-term outcomes of peripheral nerve surgery are mixed. Several older studies have reported only a short duration of pain relief, with recurrence of the neuromas and RLP redeveloping months after initial resection.Citation117–Citation119 Nonetheless, more recent retrospective and prospective studies have shown that peripheral nerve reconstructive techniques could lead to improvement in pain and quality of life for both RLPCitation120 and PLP.Citation121 Currently, peripheral nerve reconstruction remains a viable option for RLP refractory to interventional and pharmacologic treatment modalities.
Prevention
In view of the inherent challenges and limited success observed in treating PAP, many investigators have attempted to find ways to preemptively treat acute PAP, thereby preventing acute PAP from becoming chronic. For the purpose of this review, preemptive interventions shall refer to interventions which take place preoperatively, intraoperatively, or in the early postoperative period (<2 weeks) after an amputation, with the goal being to avert long-term spinal sensitization by blocking nociceptive input after peripheral nerve injury.Citation122 In general, the evidence supporting preemptive interventions to reduce or prevent chronic pain have been limited to small trials of varying quality. A systematic review by Halbert et al identified eight controlled trials in which a preemptive intervention, including epidural treatments (three trials), regional nerve blocks (three trials), intravenous calcitonin infusion (one trial), and transcutaneous electrical nerve stimulation (TENS; one trial) was used to prevent or treat acute PLP.Citation123 The results were mixed for epidural treatments, with one small trial showing decreased PLP at 1 week, 6 months, and 1 year follow-up, another small trial showing decreased PLP which only reached statistical significance at 6 months follow-up, and the largest, most methodologically sound trial showing no difference between the two groups.Citation124–Citation126 The trials for peripheral nerve blocks, calcitonin, and TENS all showed no difference in pain control between intervention and control groups in long-term follow-up (6 months or greater).Citation127–Citation130 The authors also used a quality assessment instrument to determine the likelihood of bias in three areas: randomization, double-blinding, and withdrawals or dropouts.Citation131 In their discussion, they note that the reviewed trials were of poor overall quality due to the use of a variety of PLP outcome measures, which prevented examination of the treatment effect on pain. Overall, they concluded that the evidence does not support treatment of acute PLP in the acute postoperative period.Citation123
Treatment strategies for preventing the development of chronic postsurgical PAP primarily focus on blocking nociception at the spinal and peripheral nerve levels. The area that has been best studied is the use of epidural anesthesia for prevention of PAP. As summarized in , the evidence overall has been conflicting, with several promising early studies suggesting that preoperative epidural anesthesia could decrease incidence of PLP.Citation124,Citation125,Citation132 However, more recent studies have been mixed at best, with several showing no effect.Citation126,Citation133,Citation134 One recent study by Karanikolas et al showed that optimized epidural or systemic analgesia started 48 hours preoperatively was similarly effective in reducing the incidence of PLP at 6 months.Citation135 Overall, these studies suggest that timing may be critical; compared with studies that demonstrate no benefit for preemptive epidural analgesia, those that demonstrate effectiveness were more likely to implement treatment 24 hours or more preoperativelyCitation124,Citation125,Citation135 (see ). Another important factor affecting study results seems to be the effectiveness of the “control” treatment, as those studies in which the nonepidural or control treatment group was carefully managed have been less likely to demonstrate a benefit than those in which postoperative pain care was suboptimal.Citation134–Citation136
Table 3 Prospective studies evaluating epidural effect on preventing phantom limb pain
For perineural anesthesia, the available evidence is limited to several studies whose results are conflicting. An uncontrolled study by Borghi et al (n = 71)Citation137 showed that continuous peripheral nerve blockade that starts immediately preoperatively or intraoperatively and continues postoperatively can be effective in reducing the incidence of severe PLP, with the benefit persisting for up to 12 months. A randomized, controlled study that compared continuous postoperative brachial plexus block with the oral NMDA receptor antagonist memantine to brachial plexus blockade alone found that that the addition of memantine reduces the incidence of PLP at 4 weeks and 6 months, but not at 12 months.Citation71 However, a small (n = 21) randomized, controlled study by Pinzur et al found that sciatic nerve blockade begun immediately postoperatively fails to prevent phantom pain compared with a control group that received saline, though it did decrease postoperative opioid consumption.Citation130 Collectively, these studies do not support the routine use of postoperative perineural anesthesia to prevent PLP, though the effect of preoperative nerve blockade warrants further investigation.
Systemic therapies have demonstrated mixed results in preventing PAP. A small placebo-controlled crossover study done in the early 1990s (n = 21) evaluating intravenous calcitonin early in the postoperative period found a reduction in lower extremity PLP for up to 24 hours that persisted in an open-label phase for most patients throughout their 1-year follow-up.Citation79 Yet, a larger and more methodologically sound study comparing gabapentin – a first-line medication for neuropathic painCitation138 – with placebo starting on postoperative day 1 and continued over a 30-day period failed to show any benefit during the 6-month follow-up period.Citation139
Several studies have examined the effects of complementary and alternative treatments in preventing PAP. A randomized, controlled study conducted in 51 patients with acute lower extremity amputations compared TENS with sham TENS plus chlorpromazine, and sham TENS alone, for the prevention of PLP, re-operation rates, and postoperative wound healing.Citation140 Although a lower incidence of PLP occurred at 4 months in the active TENS group, no differences were observed at 4 weeks or 1-year post- amputation. Of note, the active TENS group experienced more rapid stump healing and a lower re-amputation rate than the control groups. Despite the recognition of the importance of supraspinal mechanisms in the treatment of existing chronic PAP, few studies have been performed evaluating the utilization of supraspinal modalities for the prevention of PAP. In a four-patient case series by Hanling and colleagues evaluating 2-weeks of preemptive mirror therapy prior to elective limb amputation, one patient did not experience PLP, two patients reported rare episodes of mild PLP, and one patient had moderate PLP, for up to 1-month post-surgery.Citation95 This suggests that preventative strategies targeting supraspinal mechanisms such as mirror therapy, may be a promising area for future research.
Conclusion and future directions
PAP remains a highly prevalent but difficult-to-treat condition for patients undergoing amputations. Treatment must be multimodal and mechanism-based in nature, taking into consideration supraspinal, spinal, and peripheral mechanisms. Further investigation into the mechanisms responsible for and the factors associated with the development of PAP is needed to provide an evidence-based foundation to guide current and future treatment approaches. The authors believe that the following developments have the potential to provide additional tools to the pain practitioner.
Because there are different mechanisms involved in PAP, a systematic method for classifying patients is needed. Based on current understanding of the disorder, a phenotypic model may be helpful. For example, classifying patients by diagnostic category (ie, RLP versus PLP), referral patterns, descriptors, associated signs and symptoms, as well as chronicity may help determine which mechanisms predominate and guide therapy. These phenotype therapies would not necessarily be multimodal since they would be individualized to each patient’s predominant pain mechanism. Future studies could be designed to elucidate how phenotypic groups respond to different mechanism-specific therapies.
Controlled studies exploring multimodal treatments and preventative measures may also be on the horizon. Gilron and colleagues have described similar studies for the use of preemptive multimodal analgesics in the perioperative period for patients undergoing abdominal hysterectomy.Citation141
Monoclonal antibody-based therapeutics have revolutionized the treatment of oncologic and inflammatory disorders, and hold promise in the treatment of chronic pain.Citation142 For patients with PAP, antibody-based treatments could offer safer and more effective alternatives to currently available treatments. In addition to tumor necrosis factor alpha inhibitors, antibodies against several other pain-specific targets are currently under development. For example, anti–nerve growth factor antibodies have demonstrated Efficacy in the treatment of pain in patients with osteoarthritis and chronic low back pain.Citation143,Citation144 This may herald the advent of a new class of medications for the treatment of PAP.
Finally, though phenotypic classification of patients may be more applicable in the near term, preclinical studies have shown that a portion of individual variability in pain thresholds and susceptibility can be explained by differences (or mutations) in genotypes and gene expression.Citation145,Citation146 Costigan et al identified one single nucleotide polymorphism within KCNS1, the gene encoding a voltage-gated potassium channel, which is constitutively expressed in sensory neurons downregulated following nerve injury.Citation147 In this study, six different cohorts of chronic pain patients to include two separate groups of lumbosacral radiculopathy subjects, women with post-mastectomy pain, PAP, and PLP, along with a control group of healthy adults, were tested for experimental pain. Collectively, these patients showed a significant increase in self-reported pain that was associated with a specific single nucleotide polymorphism of KCNS1. Further genotypic studies of this nature may help to identify individuals at higher risk for developing neuropathic pain after amputation who need aggressive early pain management to prevent the development of PAP.
Disclosure
The authors did not receive any grants, consulting fees, honorariums, support for travel, or costs associated with writing or editing this manuscript.
References
- PadulPAFriedmannLWAcquired amputation and prostheses before the sixteenth centuryAngiology19873821331413548491
- MitchellSWMorehouseGRKeenWWGunshot Wounds and other Injuries of NervesPhiladelphiaJB Lippincott and Co1864
- HendersonWRSmytheGEPhantom limbsJ Neurol Neurosurg Psychiatry1948118811218861109
- EwaltJRRandallGCMorrisHThe phantom limbPyschosom Med19479118123
- WoolfCJPain: moving from symptom control toward mechanism-specific pharmacologic managementAnn Intern Med200414044145115023710
- WoolfCJMaxMBMechanism-based pain diagnosis: issues for analgesic drug developmentAnesthesiology20019524124911465563
- OwingsMFKozakLJAmbulatory and inpatient procedures in the United States, 1996Vital Health Stat1319981391119
- DillinghamTPezzinLMacKenzieELimb amputation and limb deficiencies: epidemiology and recent trends in the United StatesSouth Med J20029587588312190225
- EphraimPWegenerSMacKenzieEDillinghamTPezzinLPhantom pain, residual limb pain, and back pain in amputees: results of a national surveyArch Phys Med Rehabil2005861910191916213230
- ShermanRAShermanCJPrevalence and characteristics of chronic phantom limb pain among American veterans: results of a trial surveyAm J Phys Med19836252272386624883
- JensenTSKrebsBNielsenJRasmussenPImmediate and long-term phantom limb pain in amputees. incidence, clinical characteristics and relationship to pre-amputation limb painPain19852132672783991231
- DavisRWPhantom sensation, phantom pain, and stump painArch Phys Med Rehabil19937479918380543
- JensenTSKrebsBNielsenJRasmussenPPhantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputationPain19831732432566657285
- EhdeDMCzernieckiJMSmithDGChronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputationArch Phys Med Rehabil20008181039104410943752
- SchleyMTWilmsPToepfnerSPainful and nonpainful phantom and stump sensations in acute traumatic amputeesJ Trauma200865485886418849803
- MontoyaPLarbigWGrulkeNFlorHTaubEBirbaumerNThe relationship of phantom limb pain to other phantom limb phenomena in upper extremity amputeesPain1997721–287939272791
- BermanJSBirchRAnandPPain following human brachial plexus injury with spinal cord root avulsion and the effect of surgeryPain1998752–31992079583755
- HainsBCWaxmanSGActivated microglia contribute to the maintenance of chronic pain after spinal cord injuryJ Neuroscience2006261643084317
- HansenDMKehletHGartnerRPhantom breast sensations are frequent after mastectomyDan Med Bull2011584A425921466766
- BartelsKCohenSPRajaSNPostamputation painBenzonHRajaSNFishmanSMLiuSSCohenSPEssentials of Pain Medicine3rd edPhiladelphia, PAElsevier Health2011365369
- SmithDGEhdeDMLegroM WPhantom limb, residual limb, and back pain after lower extremity amputationsClin Orthop Relat Res1999361293810212593
- CohenSPRajaSNPathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint painAnesthesiology200710659161417325518
- DyckPJDaviesJLLitchyWJLongitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohortNeurology1997492292399222195
- ChengWYJiangYDChuangLMQuantitative sensory testing and risk factors of diabetic sensory neuropathyJ Neurol199924639439810399873
- AttalNBrasseurLParkerFEffects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot studyEur Neurol1998401912009813401
- EisenbergEAlonNIshayALamotrigine in the treatment of painful diabetic neuropathyEur J Neurol1998516717310210828
- ZaslanskyRYarnitskyDClinical applications of quantitative sensory testing (QST)J Neurol Sci19981532152389511880
- RamachandranVSRogers-RamachandranDStewartMPerceptual correlates of massive cortical reorganizationScience19922585085115911601439826
- KewJJRiddingMCRothwellJCReorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputationJ Neurophysiol1994725251725247884476
- GirauxPSiriguASchneiderFDubernardJMCortical reorganization in motor cortex after graft of both handsNat Neurosci20014769169211426223
- RorichtSMeyerBUNiehausLBrandtSALong-term reorganization of motor cortex outputs after arm amputationNeurology199953110611110408544
- MercierCReillyKTVargasCDAballeaASiriguAMapping phantom movement representations in the motor cortex of amputeesBrain200612982202221016844715
- KarlABirbaumerNLutzenbergerWCohenLGFlorHReorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb painJ Neurosci200121103609361811331390
- LotzeMFlorHGroddWLarbigWBirbaumerNPhantom movements and pain. An fMRI study in upper limb amputeesBrain2001124112268227711673327
- CarlenPLWallPDNadvornaHSteinbachTPhantom limbs and related phenomena in recent traumatic amputationsNeurology1978283211217564474
- FlorHNikolajsenLStaehelin JensenTPhantom limb pain. A case of maladaptive CNS plasticityNat Rev Neurosci200671187388117053811
- IaconoR PLinfordJSandykRPain management after lower extremity amputationNeurosurgery19872034965002883607
- KronerKKrebsBSkovJJorgensenHSImmediate and long-term phantom breast syndrome after mastectomy, incidence, clinical characteristics and relationship to pre-mastectomy breast painPain19893633273342785259
- CohenSPVillenaFMaoJTwo unusual cases of postamputation pain from Operation Iraqi FreedomJ Trauma200762375976117414361
- SchmidtA PTakahashiMEde Paula PossoIDPhantom limb pain induced by spinal anesthesiaClinics (Sao Paulo)200560326326415962090
- AydinMDCesurMAydinNAliciHDisappearance of phantom limb pain during caudaequina compression by spinal meningioma and gradual reactivation after decompressionAnesth Analg200510141123112616192532
- DellonALMackinnonSEPestronkAImplantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formationAnn Plast Surg19841230406703605
- CarmelPWSteinBMCell changes in sensory ganglia following cortex following proximal and distal nerve section in the monkeyJ Comp Neurol19691351451664976342
- LissAGaf EkenstamFWWibergMLoss of neurons in the dorsal root ganglia after transection of a peripheral sensory nerveScand J Plast Reconstr Surg Hand Surg199630168711436
- LissAGWibergMLoss of nerve endings in the spinal dorsal horn after a peripheral nerve injury. An anatomical study in Macacafascicularis monkeysEur J Neurosci19979218721929421178
- LissAGWibergMLoss of primary afferent nerve terminals in the brainstem after peripheral nerve transection: an anatomical study in monkeysAnat Embryol19971962792899363850
- CsillikBKnyiharERakicPTransganglionic degenerative atrophy and regenerative proliferation in the rolando substance of the sensorimotor cortex of the primate spinal cord: discoupling and restoration of synaptic connectivity in the central nervous system after peripheral nerve lesionsFolia Morphol198230189193
- CalfordMBTweedaleRImmediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervationSomatosens Motor Res19918249260
- FlorenceSLGarraghtyPECarlsonMKaasJHSprouting of peripheral nerve axons in the spinal cord of monkeysBrain Res19936013433488431785
- ZhangXJuGEldeRHokfeltTEffect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galaninJ Neurocytol1993223423817686215
- FischerJCsillikBLectin binding: a genuine marker for transganglionic regulation of human primary sensory neuronsNeurosci Lett1985542632673991066
- HuntSPRossorMNEmsonPCClement-JonesVSubstance P and enkephalins in spinal cord after limb amputationLancet19821827910236176819
- OharaSTakahashiHKatoMNakamuraTTsukadaMTransganglionic gracile response following limb amputation in manActa Neuropathol200010046947411045668
- DevorMWallPDReorganisation of spinal cord sensory map after peripheral nerve injuryNature197827656837576570248
- MurphyJPAnandacivaSPhantom limb pain and spinal anaesthesiaAnaesthesia1984391886703275
- RasmussenMRKitaokaHBPatzerGLNonoperative treatment of plantar interdigital neuroma with a single corticosteroid injectionClin Orthop19963261881938620640
- NicholsonBEvaluation and treatment of central pain syndromesNeurology2004622S30S3615007162
- WuHSultanaRTaylorKBSzaboAA prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus lidocaine/depomedrol injection on residual and phantom limb painClin J Pain20122810811221750460
- KernUMartinCScheicherSMullerHEffects of botulinum toxin type B on stump pain and involuntary movements of the stumpAm J Phys Med Rehabil200483539639915100632
- WestMWuHPulsed radiofrequency ablation for the management of residual limb pain and phantom limb painPain Practice201010548549120230449
- DahlECohenSPPerineural injection of etanercept as a treatment for postamputation painClin J Pain200824217217518209523
- CohenSPGambelJMRajaSNGalvagnoSThe contribution of sympathetic mechanisms to postamputation phantom and residual limb pain: a pilot studyJ Pain201112885986721481650
- CasaleRCeccherelliFLabeebAABiellaGEPhantom limb pain relief by contralateral myofascial injection with local anaesthetic in a placebo-controlled study: preliminary resultsJ Rehab Med2009416418422
- Bilevicuite-LjungaraIBiellabGBellomibPSotgiubMContralateral treatment with lidocaine reduces spinal neuronal activity in mononeu-ropathic ratNeurosci Lett2001311315716011578818
- NikolajsenLHansenCNielsenJKellerJArendt-NielsenLJensenTThe effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral inputPain199667169778895233
- EichenbergerUNeffFSveticicGChronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholdsAnesth Analg200810641265127318349204
- AbrahamRMarouaniNWeinbroumADextromethorphan mitigates phantom pain in cancer amputeesAnn Surg Oncol200310326827412679312
- MaierCDertwinkelRMansourianNEfficacy of the NMDA receptor antagonist memantine in patients with chronic phantom limb pain-results of a randomized double-blinded, placebo-controlled trialPain2003103327728312791434
- WiechKKieferRTTopfnerSA placebo-controlled randomized crossover trial of the N-methyl-D-aspartic acid receptor antagonist, memantine, in patients with chronic phantom limb painAnesth Analg200498240841314742379
- SchwenkreisPMaierCPlegerBNMDA-mediated mechanisms in cortical excitability changes after limb amputationActa Neurol Scand2003108317918412911461
- SchleyMTopfnerSWiechKContinuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputeesEur J Pain200711329930816716615
- HuseELarbigWFlorHBirbaumerNThe effect of opioids on phantom limb pain and cortical reorganizationPain2001901–2475511166969
- WuCTellaPStaatsPAnalgesic effects of intravenous lidocaine and morphine on postamputation painAnesthesiology200296284184811964590
- WuCLAgarwalSTellaPKMorphine versus mexiletine for treatment of postamputation pain: a randomized, placebo controlled, crossover trialAnesthesiology2008109228929618648238
- Wilder-SmithCHHillLTLaurentSPostamputation pain and sensory changes in treatment-naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placeboAnesthesiology2005103361962816129989
- BoneMCritchleyPBuggyDGabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over studyReg Anesth Pain Med200227548148612373695
- SmithDEhdeDHanleyMEfficacy of gabapentin in treating chronic phantom limb and residual limb painJ Rehabil Res Dev200542564565416586190
- AzriaMPossible mechanisms of the analgesic action of calcitoninBone200230580S83S12008164
- JaegerHMaierCCalcitonin in phantom limb pain: a doubleblind studyPain19924821271738570
- RobinsonLCzernieckiJEhdeDTrial of amitriptyline for relief of pain in amputees: results of a randomized controlled studyArch Phys Med Rehabil20048511614970960
- RickardJAEffects of Hypnosis in the Treatment of Residual Stump and Phantom Limb Pain [dissertation]Pullman, WAWashington State University2004
- BeaumontGMercierCMichonPEMalouinFJacksonPLDecreasing phantom limb pain through observation of action and imagery: a case seriesPain Med201112228929921276185
- HardenRNHouleTTGreenSBiofeedback in the treatment of phantom limb pain: a time-series analysisAppl Psychophysiol Biofeedback200530839315889588
- WeteringEJLemmensKMNieboerAPHuijsmanRCognitive and behavioral interventions for the management of chronic neuropathic pain in adults – a systematic reviewEur J Pain201014767068120096614
- PrakashSPGolwalaPPhantom headache: pain-memory-emotion hypothesis for chronic daily headache?J Headache Pain201112328128621479704
- TichelaarY VGeertzenJHKeizerDVan WilgenC PMirror box therapy added to cognitive behavioural therapy in three chronic complex regional pain syndrome type I patients: a pilot studyInt J Rehabil Res200730218118817473633
- Department of Veterans AffairsCognitive behavior therapy (CBT) and mirror training for phantom limb painClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00731614NLM identifier: NCT00731614. Accessed December 7, 2012
- FlorHDiersMChristmannCKoeppeCMirror illusions of phantom hand movements brain activity mapped by fMRINeuro Image200631S159
- FlorHElbertTKnechtSPhantom-limb pain as a perceptual correlate of cortical reorganization following arm amputationNature199537565314824847777055
- RamachandranVSAltschulerELThe use of visual feedback, in particular mirror visual feedback, in restoring brain functionBrain20091321693171019506071
- MercierCSiriguATraining with virtual visual feedback to alleviate phantom limb painNeurorehabil Neural Repair20092358759419171946
- ColeJCrowleSAustwickGExploratory findings with virtual reality for phantom limb pain; from stump motion to agency and analgesiaDisabil Rehabil2009311084685419191061
- ChanBLWittRCharrowAPMirror therapy for phantom limb painN Engl J Med20073572206220718032777
- GarryMILoftusASummersJJMirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitabilityExp Brain Res200516311812215754176
- HanlingSRWallaceSCHollenbeckKJPreamputation mirror therapy may prevent development of phantom limb pain: a case seriesAnesth Analg201011061161419917622
- MobbsRJNairSBlumPPeripheral nerve stimulation for the treatment of chronic painJ Clin Neurosci20071421622317258129
- RauckRLKapuralLCohenSPPeripheral nerve stimulation for the treatment of postamputation pain – a case reportPain Pract201212864965522548686
- NielsonKDAdamsJEHosobuchiYPhantom limb pain: treatment with dorsal column stimulationJ Neurosurg1975423013071117328
- KrainickJUThodenURiechertTPain reduction in amputees by long-term spinal cord stimulation: long term follow-up study over 5 yearsJ Neurosurg1980523463506153710
- HuntWEGoodmanJHBinghamWGStimulation of the dorsal spinal cord for treatment of intractable pain: a preliminary reportSurg Neurol197541531561080900
- MilesJLiptonSPhantom limb pain treated by electrical stimulationPain19785373382740403
- Sanchez-LedesmaMJGarcia-MarchGDiaz-CascajoPGomez-MoretaJBrosetaJSpinal cord stimulation in deafferentation painStereotact Funct Neurosurg19895340452472663
- KumarKTothCNathRLaingPEpidural spinal cord stimulation for treatment of chronic pain – some predictors of success. A 15-year experienceSurg Neurol1998501101219701116
- BroggiGServelloDDonesICarboneGItalian multicenter study on pain treatment with epidural spinal cord stimulationStereotact Funct Neurosurg1994622732787631081
- Indication and limitations of coverage and/or medical necessity. Centers for Medicare and Medicaid Website http://www.findacode.com/medicare/policies-guidelines/display-medicare-info.php?type=LCD&type_id=26741Accessed October 30, 2012
- NguyenJPLefaucheurJPRaoulSMotor cortex stimulation for the treatment of neuropathic painKramesESPeckhamPHRezaiARNeuromodulation1st edAmsterdamElsevier Science2009515526
- RascheDRinaldiPCYoungRFTronnierVMDeep brain stimulation for the treatment of various chronic pain syndromesNeurosurg Focus2006216E817341052
- KatayamaYYamamotoTKobayashiKKasaiMOshimaHFukayaCMotor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulationStereotact Funct Neurosurg2001771–415916212378068
- SolJCHCasauxJRouxFEChronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studiesStereotact Funct Neurosurg20017717217612378072
- BittarRGOteroSCarterHAzizTZDeep brain stimulation for phantom limb painJ Clin Neurosci200512439940415925769
- McAuleyJGroningenRVGreenCSpinal cord stimulation for intractable pain following limb amputationNeuromodulationEpub 9252012
- LongDMElectrical stimulation for relief of pain from chronic nerve injuryJ Neurosurg1973397187224357242
- NasholdBSJrGoldnerJLMullenJBBrightDSLong-term pain control by direct peripheral-nerve stimulationJ Bone Joint Surg Am1982641106976348
- CampbellJNLongDMPeripheral nerve stimulation in the treatment of intractable painJ Neurosurg1976456926991086348
- PotterBKBurnsTCLacapA PGranvilleRRGrajewskiDAHeterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excisionJ Bone Joint Surg20078947648517332095
- HertaFPhantom-limb pain: characteristics, causes, and treatmentLancet Neurol2002118218912849487
- SturmVKrogerMPenzholzHProblems of peripheral nerve surgery in amputation stump pain and phantom limbsChirurg1975463893911240797
- DellonALMackinnonSETreatment of the painful neuroma by neuroma resection and muscle implantationPlast Reconstr Surg1986774274382937074
- BurchielKJJohansTJOchoaJThe surgical treatment of painful traumatic neuromasJ Neurosurg1993787147198468601
- DucicIMesbahiANAttingerCEGrawKThe role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumpsPlast Reconstr Surg200812190891418317139
- PrantlLSchremlSHeineNEisenmann-KleinMAngelePSurgical treatment of chronic phantom limb sensation and limb pain after lower limb amputationPlast Reconstr Surg20061181562157217102729
- WoolfCJChongMSPreemptive analgesia: treating postoperative pain by preventing the establishment of central sensitizationAnesth Analg1993773623798346839
- HalbertJCrottyMCameronIDEvidence for the optimal management of acute and chronic phantom limb pain. A systematic reviewClin J Pain200218849211882771
- BachSNorengMFTjelldenNUPhantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockadePain1988332973013419837
- JahangiriMJayatungaA PBradleyJWDarkCHPrevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaineAnn R Coll Surg Engl19947653243267979074
- NikolajsenLIlkjaerSChristensenJHKronerKJensenTSRandomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputationLancet19973509088135313579365449
- ElizagaAMSmithDGShararSRContinuous regional analgesia by intraneural block: effect on postoperative opioid requirements and phantom limb pain following amputationJ Rehabil Res Dev1994311791877965876
- FinsenVPersenLLovlienMTranscutaneous electrical nerve stimulation after major amputationJ Bone Joint Surg Br1988701091123257494
- FisherAMellerYContinuous postoperative regional analgesia by nerve sheath block for amputation surgery: a pilot studyAnesth Analg1991723003031994757
- PinzurMSGarlaPGPluthTVrbosLContinuous postoperative infusion of a regional anesthetic after an amputation of the lower extremity. A randomized clinical trialJ Bone Joint Surg Am199678150115058876577
- JadadARMooreACarrollDAssessing the quality of reports of randomised clinical trials: is blinding necessary?Control Clin Trials1996171128721797
- SchugSABurrellRPayneJTesterPPre-emptive epidural analgesia may prevent phantom limb painReg Anesth1995202567547671
- LambertAWDashfeldAKCosgroveCWilkinsDCWalkerAJAshleySRandomized prospective study comparing preoperative epidural and intraoperative perineural analgesia for the prevention of postoperative stump and phantom limb pain following major amputationReg Anesth Pain Med200126431632111464349
- WilsonJANimmoAFFleetwood-WalkerSMColvinLAA randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputationPain200813510811817583431
- KaranikolasMArethaDTsolakisIOptimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trialAnesthesiology201111451144115421368651
- NikolajsenLIlkjaerSKronerKChristensenJHJensenTSThe influence of preamputation pain on postamputation stump and phantom painPain1997723934059313280
- BorghiBD’AddabboMWhiteP FThe use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndromeAnesth Analg201011151308131520881281
- DworkinRHO’ConnorABAudetteJRecommendations for the pharmacological management of neuropathic pain: an overview and literature updateMayo Clin Proc2010853S3S1420194146
- NikolajsenLFinnerupNBKrampSVimtrupASKellerJJensenTSA randomized study of the effects of gabapentin on postamputation painAnesthesiology200610551008101517065896
- FinsenVPersenLLøvlienMVeslegaardEKSimensenMGåsvannAKBenumPTranscutaneous electrical nerve stimulation after major amputationJ Bone Joint Surg Br19887011091123257494
- GilronIOrrETuDO’NeillJ PZamoraJEBellACA placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomyPain20051131–219120015621380
- ChessellIPDudleyABillintonABiologics: the next generation of analgesic drugs?Drug Discov Today2012171587587922464946
- LaneNESchnitzerTJBirbaraCATanezumab for the treatment of pain from osteoarthritis of the kneeN Engl J Med20103631521153120942668
- KatzNBorensteinDGBirbaraCEfficacy and safety of tanezumab in the treatment of chronic low back painPain2011152102248225821696889
- NielsenCSPriceDDVassendOStubhaugAHarrisJRCharacterizing individual differences in heat-pain sensitivityPain2005119657416298065
- FoulkesTWoodJNPain genesPLoS Genet200847e100008618654615
- CostiganMBelferIGriffinRSMultiple chronic pain states are associated with a common amino acid-changing allele in KCNS1Brain20101332519252720724292
- Katsuly-LiapisIGeorgakisPTierryCPreemptive extradural analgesia reduces the incidence of phantom pain in lower limb amputees [abstract]Br J Anaesth199676125