Abstract
Objective
An immediate-release formulation of gabapentin is approved for treatment of postherpetic neuralgia (PHN). This formulation, however, requires multiple daily dosing, usually three times per day, and is associated with a high incidence of somnolence and dizziness. We assessed the tolerability and safety of a once-daily gastroretentive formulation of gabapentin (G-GR) in phase 3 clinical trials in patients with PHN.
Research design and methods
Data were pooled from two placebo-controlled studies involving 723 patients (G-GR 1800 mg, n = 359; placebo, n = 364). Patients (43% male, mean age 66 years) with PHN pain >4 (0–10 scale) for ≥3 months were enrolled. Summary statistics for the incidence of treatment-emergent adverse events (AEs) were performed. Laboratory parameters and vital signs were assessed.
Results
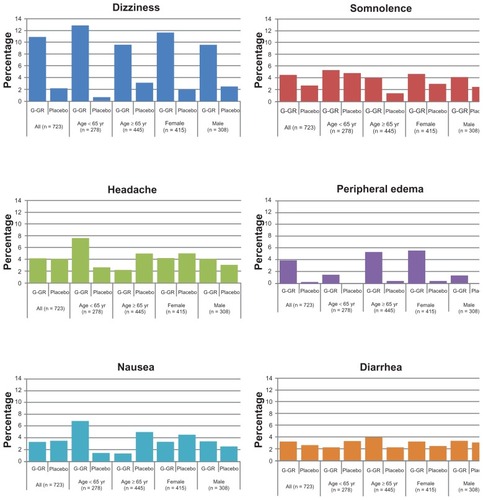
Treatment-emergent AEs were reported in 48% of patients (G-GR, 54%; placebo, 42%) and led to discontinuation in 8% of patients (G-GR, 10%; placebo, 7%). The most frequent (≥3% in any group) AEs were dizziness (G-GR, 11%; placebo, 2%), somnolence (G-GR, 5%; placebo, 3%), headache (G-GR, 4%; placebo, 4%), peripheral edema (G-GR, 4%; placebo, <1%), and diarrhea (G-GR, 3%; placebo, 3%). Serious AEs were reported in seven patients in the G-GR group (2%) and ten patients in the placebo group (3%). There were two deaths, both in the placebo group. No serious AEs were considered related to treatment. Mean values for laboratory parameters and vital signs at the end of each study were similar between groups.
Conclusion
G-GR was safe and well tolerated for the treatment of PHN.
Introduction
Postherpetic neuralgia (PHN) is a neuropathic pain syndrome characterized by persistence of pain after healing of acute herpes zoster rash (shingles). It is often defined by the persistence of pain for at least 3 months,Citation1 although the pain may resolve spontaneously in a considerable proportion of patients between 3 and 6 months.Citation2 PHN pain usually presents as constant burning, aching, throbbing pain or intermittent sharp, shooting/stabbing pain, and allodynia is reported by most patients.Citation3 PHN has been estimated to occur in up to 20% of patients with herpes zoster, and the incidence of both herpes zoster and PHN as well as the severity of PHN pain increases with patient age.Citation3
Immediate-release gabapentin (gabapentin TID) is approved by the US Food and Drug Administration for the treatment of PHN. However, that formulation has several limitations, including high incidences of dizziness (28% vs 8% for placebo) and somnolence (21% vs 5% for placebo).Citation4 Furthermore, because absorption of gabapentin occurs via a saturable transport mechanism, the amount of gabapentin delivered by gabapentin TID may overwhelm the transporters located in the proximal small intestine. Thus, as the dosage is increased, the proportion of drug absorbed is decreased,Citation5 and to maintain therapeutic levels, multiple daily doses are required.
A once-daily gastroretentive formulation of gabapentin (Gralise, referred to herein as G-GR) was recently approved for the treatment of PHN. The gastroretentive technology used in G-GR maintains the tablet in the fed stomach for up to 8 hours. While in the stomach, G-GR gradually releases gabapentin to its uptake transporters located in the proximal small intestine.Citation6 This gradual release helps optimize absorption of gabapentin by the saturable transporters.
The first placebo-controlled, phase 3 study of gastroretentive gabapentin (1800 mg/day) compared G-GR and a divided-dose regimen with placebo in 400 patients who had a duration of PHN ≥ 3 months. The study ran for 10 weeks (including 2 weeks of dose titration), followed by 1 week of dose tapering.Citation7 Although the study did not meet the primary efficacy end point (baseline observation carried forward [BOCF] least-squares mean change in average daily pain [ADP] score from baseline to week 10) due to an unexpectedly high placebo response, post hoc analysis demonstrated that the placebo response was especially evident in patients whose rash had healed <6 months prior to study entry. Furthermore, most secondary efficacy end points showed statistically significant improvement in the G-GR arm compared with placebo.
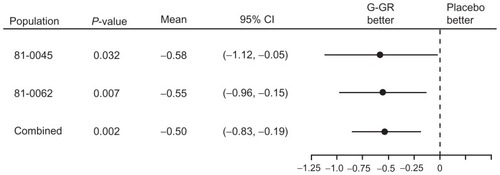
Given those promising results, a second 10-week, placebo-controlled, phase 3 study was conducted using 1800 mg G-GR in patients whose duration of PHN was ≥ 6 months (n = 452).Citation8 In that study, the change in ADP intensity score (the primary end point) was −2.1 for once-daily gabapentin vs −1.6 for placebo (P = 0.013, BOCF analysis). The between-group difference reached statistical significance at week 1 and remained significant throughout the study. shows the change in ADP intensity score over placebo for each of the two phase 3 studies with the pooled change in ADP intensity score data from the two studies.
Figure 1 Forest plot of change in average daily pain score from baseline, placebo subtracted, last-observation-carried-forward methodology.
In this pooled analysis, we examined the tolerability and safety of G-GR in patients with PHN from the two 11-week phase 3 clinical trials.
Patients and methods
This was an analysis of data pooled from two randomized, placebo-controlled studies of patients with PHN who had a pain intensity score of at least 4 on the Likert numerical rating scale (0–10) and were treated with either G-GR (1800 mg/day) or placebo. The studies included in this analysis have been described in detail elsewhere (clinicaltrials.gov identifiers NCT00335933 and NCT00636636).Citation7,Citation8 Briefly, both studies recruited patients at least 18 years of age with neuropathic pain of either ≥3 monthsCitation7 or ≥6 monthsCitation8 after the healing of a herpes zoster skin rash. One study was conducted entirely within the United States,Citation7 whereas the other enrolled 259 patients from the United States, 161 patients from Russia, and 32 patients from Argentina.Citation8 Patients being treated with drugs that could affect pain scores were required to undergo an initial washout period.
Exclusion criteria included: prior lack of response to treatment with gabapentin at doses of ≥1200 mg/day or pregabalin at doses ≥300 mg/day; dose-limiting adverse effects with gabapentin or hypersensitivity to gabapentin; neurolytic/neurosurgical treatment for PHN; severe pain unrelated to PHN; use of injected anesthetics or steroids within 30 days; immunocompromised state; creatinine clearance (CrCl) <50 mL/minute; gastric reduction surgery; history of substance abuse within the previous year; or any skin condition that could modify sensation in the affected area. Patients underwent a 1-week pretreatment period to establish baseline measurements, and those who continued to meet the pain score eligibility requirements were randomized to 10 weeks of treatment with G-GR 1800 mg dosed once daily (titrated up over 2 weeks) or placebo followed by 1-week of gabapentin dose tapering. One of the studies also included patients randomized to receive G-GR 1800 mg as an asymmetric divided dose,Citation7 but those data were not included in the current analysis.
Efficacy and safety data for each study have been reported previously.Citation7,Citation8 In this analysis, tolerability and safety data from both studies were pooled. The data included study medication compliance, the incidence of treatment-emergent adverse events (AEs), serious AEs (SAEs), study discontinuations, hematology, blood chemistry, and vital signs. All safety analyses were performed on the safety population consisting of all patients who received at least one dose of the study drug. AEs were coded using the Medical Dictionary for Regulatory Activities version 9.0. The number and percentage of patients reporting one or more AEs were categorized by AE preferred term and AE system organ class and summarized by treatment group. Summary statistics for the analyses of pooled study data were performed using SAS (Cary, NC) software (version 9.1.3 or higher).
Results
A total of 722 patients received at least one dose of study medication and were included in the safety population (G-GR, n = 359; placebo, n = 364). Baseline characteristics of the patients are shown in . Over half the patients were female, both groups were mostly Caucasian (88.5%), and the mean age of patients was approximately 66 years. Overall compliance with study medication was slightly higher for patients in the G-GR group compared with placebo (mean compliance, 99.2% vs 98.5%). Reasons for study discontinuation are listed in . Although there were more AEs in the G-GR group (194/359, 54.0%) than in the placebo group (154/364, 42.3%), discontinuations were slightly lower overall for patients in the G-GR group compared with the placebo group (16.4% vs 19.5.%). AEs were the most common reason for discontinuation and were more common in the G-GR group than in the placebo group (9.7% vs 6.0%). Lack of efficacy was a more common reason for discontinuation in the placebo group than in the G-GR group (6.0% vs 2.5%). The most frequently occurring AE that led to discontinuation was dizziness (G-GR, 8/359, 2.2%; placebo, 2/364, 0.6%); no other AE led to discontinuation in more than 1% of patients in the G-GR group. All incidences of these AEs except for two (0.6%) in the G-GR group and one (0.3%) in the placebo group were mild or moderate in severity. Almost all patients (95.0% of G-GR patients and 93.4% of placebo patients) were able to achieve an 1800-mg dose within 2 weeks. Only 3.6% of G-GR patients and 3.0% of placebo patients discontinued during the titration period due to an AE. SAEs were reported more frequently in the placebo group (G-GR, 1.9%; placebo, 2.7%). All except one SAE were considered unrelated to the study drug. An SAE of chronic pancreatitis in one patient (G-GR group) was deemed probably related to treatment, although the relationship to the study drug was challenged when the patient was seen by a local gastroenterologist. There were two deaths in the placebo group, both from myocardial infarction. Both patients were in their 70s and had a history of dyslipidemia. One patient had a history of hypertension, coronary bypass graft, and stenting. For the second patient, hypertension and hyperlipidemia were listed as secondary causes of death.
Table 1 Patient disposition and baseline characteristics of the safety population
AEs were further stratified by age (<65 years vs ≥65 years) and sex (male vs female) to examine potential influences of these factors (). Although the incidences of AEs within each treatment group, including dizziness and somnolence, were generally consistent regardless of patient age or sex, there were a few notable differences. For example, compared with the patients ≥65 years, the younger patients (<65 years) reported nausea more frequently (<65 years: G-GR, 6.8%; placebo, 1.4% vs ≥65 years: G-GR, 1.3%; placebo, 5.0%). And patients ≥65 years of age reported more incidences of peripheral edema (≥65 years: G-GR, 5.3%; placebo, 0.5%; <65 years: G-GR, 1.5%; placebo, 0%). Peripheral edema was also more frequently reported in females who received G-GR (females: G-GR, 5.6%; placebo, 0.5% vs males: G-GR, 1.4%; placebo, 0%).
Figure 3 Subgroup analyses, adverse events (with >3% incidence in patients in G-GR cohort).
Adverse events related to vital sign abnormalities were reported more frequently for patients in the G-GR group compared with the placebo group (G-GR, 15.0% vs placebo, 6.6%). Of these, dizziness and weight gain were the most common related AEs. There were no meaningful clinical differences between groups for the mean baseline values and mean changes from baseline for vital signs, weight, or measured laboratory parameters (blood panel, serum lipid profile, liver function tests, renal panel).
Discussion
This analysis of the pooled safety data from two phase 3 studies provides further support for the safety and tolerability of the once-daily G-GR formulation of gabapentin. The most common treatment-emergent AEs were the same as those previously identified in studies of gabapentin TID. The current analysis, as well as data from each of the component studies,Citation7,Citation8 demonstrate that the incidences of dizziness and somnolence in patients treated with G-GR (1800 mg/day) were lower than reported in previous studies with gabapentin TID.Citation9,Citation10 Placebo-controlled trials of gabapentin TID in patients with PHN that used 1800 or 2400 mg/dayCitation9 or up to 3600 mg/dayCitation10 reported rates of treatmentassociated dizziness from 24%–33%, and rates of treatmentassociated somnolence of 17%–27%.Citation9,Citation10 However, because the G-GR studies used in this integrated analysis did not directly compare G-GR with gabapentin TID, we cannot exclude the possibility that other differences between the studies contributed to differences in the AE profiles. Almost all patients were able to achieve the 1800 mg dose in 2 weeks. This is in contrast to data obtained from a retrospective database analysis that showed that only 14% of PHN patients prescribed gabapentin TID were able to achieve an 1800 mg dose and that it took on average 10 weeks to achieve this dose.Citation11
It is notable that the incidences of dizziness and somnolence reported in a study with an extended-release formulation of a gabapentin prodrug (gabapentin encarbil) appeared to be unrelated to either the peak concentration or the absolute level of gabapentin.Citation12 It is possible that treatment-associated AEs with G-GR are mitigated in part by the more gradual elevation in serum levels of gabapentin than the immediate-release formulation. In clinical trials with gabapentin TID, dizziness and somnolence are most frequently observed during the initial titration period,Citation13 and plasma concentrations of that formulation also demonstrate more marked fluctuations early in the course of treatment.Citation14 Alternatively, the apparent reduction in AEs with G-GR could be in part a result of gabapentin released from G-GR reaching peak concentrations during the period when most patients are asleepCitation15 and therefore less likely to notice them. The tolerability of G-GR did not appear to be affected by patient age. Given that PHN is a disease for which the risk and duration of PHN increases with age,Citation1 medications such as gabapentin, with good tolerability and a low propensity for drug–drug interactions,Citation16 are particularly well suited. In addition, in the one study from which data were available, the rate of AEs in G-GR-treated patients with CrCl from 50 mL/minute to <80 mL/minute was only slightly higher compared with those having CrCl ≥ 80 mL/minute, suggesting that mild or moderate renal impairment (CrCl ≥ 50 mL/minute) did not markedly affect tolerability of G-GR.
One limitation of the current integrated analysis is that it only examined tolerability and safety data for a 10-week treatment period. But given the extensive clinical history of gabapentin TID for the treatment of neuropathic pain and the established safety of the gastroretentive technology,Citation17 it is reasonable to expect that the safety and tolerability of G-GR would be maintained for longer treatment periods. Another limitation of this analysis is that patients who had not previously responded to gabapentin at daily doses >1200 mg or who were intolerant to gabapentin were excluded. Although this exclusion criterion may have enriched the study population with patients likely to be tolerant of gabapentin, its intent was to treat patients ethically. It would be unfair to require a patient to enroll in a 11-week study investigating a once-daily formulation of a drug they had previously been unable to tolerate or from which they had obtained no pain relief. This same approach was used in previous studies of gabapentin TID and pregabalin in patients with PHNCitation9,Citation18,Citation19 and as such the patient populations in G-GR clinical trials and previous studies of gabapentin TID and pregabalin are comparable.
Conclusion
In conclusion, the results of this analysis, together with the previous demonstration of efficacy in this patient population,Citation8 suggest that G-GR has the potential to provide an efficacious, well-tolerated, and convenient treatment option in the treatment of PHN.
Acknowledgments
Editorial support was provided by Ed Parr, PhD, CMPP, of Envision Scientific Solutions, Southport, CT, and funded by Abbott. Medical writing support was provided by Stephanie Kareht, PhD, at Depomed. The authors thank Eric Sondag, PhD, formerly of Abbott, and Geertrui Vanhove, MD, PhD, from Depomed for their suggestions and advice during development of the manuscript, and Stephanie Kareht, PhD for assistance with the production of figures.
Disclosure
These analyses were sponsored by Depomed. Gordon Irving has previously received an honorarium from Depomed for participation on an advisory board. Michael Sweeney is an employee of Depomed.
References
- DworkinRHPortenoyRKPain and its persistence in herpes zosterPain1996672412518951917
- OxmanMNLevinMJJohnsonGRA vaccine to prevent herpes zoster and postherpetic neuralgia in older adultsNew Engl J Med20053522271228415930418
- JohnsonRWWasnerGSaddierPBaronRPostherpetic neuralgia: epidemiology, pathophysiology and managementExpert Rev Neurother200771581159517997705
- Pfizer Neurontin [package insert]New YorkPfizer2010
- StewartBHKuglerARThompsonPRBockbraderHNA saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasmaPharm Res1993102762818456077
- [No authors listed]Gabapentin extended-release – Depomed: gabapentin ER, gabapentin gastric retention, gabapentin GRDrugs R D2007831732017767396
- WallaceMSIrvingGCowlesVEGabapentin extended-release tablets for the treatment of patients with postherpetic neuralgia: a randomized, double-blind, placebo-controlled, multicentre studyClin Drug Investig201030765776
- SangCNSathyanarayanaRSweeneyMOnce-daily gabapentin (G-GR) reduces intensity of pain associated with post herpetic neuralgiaClin J Pain2012In press
- RiceASCMatonSGroupPNSGabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled studyPain20019421522411690735
- RowbothamMHardenNStaceyBBernsteinPMagnus-MillerLGabapentin for the treatment of postherpetic neuralgia: a randomized controlled trialJAMA1998280183718429846778
- JohnsonPBeckerLHalpernRSweeneyTDosing patterns, therapy outcomes and health care costs among patients with postherpetic neuralgia (PHN) treated with gabapentin or pregabalinPharmacoeconomics [Submitted.]
- CundyKCSastrySLuoWZouJMoorsTLCanafaxDMClinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentinJ Clin Pharmacol2008481378138818827074
- BackonjaMGlanzmanRLGabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trialsClin Ther2003258110412637113
- GordiTHouEKasichayanulaSBernerBPharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive-extended-release and immediate-release tablets: a randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjectsClin Ther20083090991618555937
- ChenCCowlesVEHouEPharmacokinetics of gabapentin in a novel gastric-retentive extended-release formulation: comparison with an immediate-release formulation and effect of dose escalation and foodJ Clin Pharmacol20115134635820484610
- JohnsonRWWasnerGSaddierPBaronRHerpes zoster and postherpetic neuralgia: optimizing management in the elderly patientDrugs Aging200825991100619021299
- BernerBCowlesVECase studies in swelling polymeric gastric retentive tabletsExpert Opin Drug Deliv2006354154816822228
- DworkinRHCorbinAEYoungJPJrPregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trialNeurology2003601274128312707429
- van SeventerRFeisterHAYoungJPJrStokerMVersavelMRigaudyLEfficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trialCurr Med Res Opin20062237538416466610