?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The primary objective of the present study was to evaluate predictive parameters of the acute pain score during induction of an inflammatory heat injury.
Patients and methods
Healthy volunteers (50 females/50 males) were included in the study. The predictive potential of gender, anthropometric (body surface area, body mass index), psychological (anxiety, depression, vulnerability), and psychophysical (quantitative sensory testing, conditioned pain modulation) variables in estimating the pain response to a validated heat injury (47°C, 7 minutes, area 12.5 cm2) were investigated. All assessments were made in duplicate sessions separated by 21 days (median).
Results
There were three main findings in this study. First, a predictive model of pain sensitivity during the heat injury, including both genders and using multiple regression technique, could account for 28% of the variance (P < 0.0001), but gender-related differences in the final model could not be demonstrated. Second, the results confirmed significant gender-related differences in perception of electrical, pressure, and cold pressor stimuli (P < 0.002). Third, positive correlations between anthropometric data and pain perception during electrical and pressure stimuli were demonstrated (P < 0.001 and P < 0.005, respectively).
Conclusion
The study demonstrated predictability of acute pain sensitivity, and although gender-related differences in pain perception were demonstrated, no gender-related differences in pain sensitivity could be shown. Interestingly, positive correlations between anthropometric data and pain perception were shown for the first time.
Introduction
Pain sensitivity, defined as the proneness to react to standardized experimental or pathological stimuli, varies widely between subjects. Pain ratings of seemingly identical noxious stimuli may range from “no pain” to “excruciating” pain.Citation1 Pain sensitivity is determined by complex interactions between ethnic,Citation2,Citation3 psychophysical,Citation4,Citation5 psychological,Citation6–Citation8 genetic,Citation5 and social factors.Citation9 Prediction of pain sensitivity has been used preoperatively as a measure to prevent development of severe acute pain,Citation4,Citation10 and to attenuate its transition to persistent postsurgical pain. In addition, prediction of experimental pain sensitivity prior to testing of analgesic drug efficacy may theoretically reduce the high attrition rate for new drug candidates by allowing initial testing in groups of high-pain and low-pain responders.Citation11,Citation12
Investigating the predictive parameters of pain sensitivity could therefore be of potential medical interest and have significant implications both for the health care systemCitation13 and for pharmaceutical research.Citation11 Moreover, investigations in both females and males is a necessity as 10 years of laboratory research has still not been successful in producing a clear and consistent pattern of possible sex differences in human pain sensitivity.Citation14
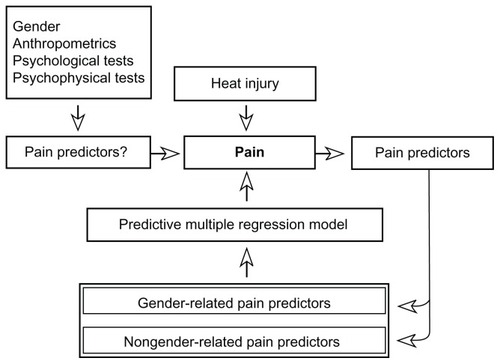
The primary objective of the present study was to evaluate the predictability of the pain intensity score during an inflammatory heat injury (in the present study defined as pain sensitivity), using standardized quantitative sensory testing (QST) and conditioned pain modulation (CPM [formerly called diffuse noxious inhibitory control, belongs to the endogenous pain inhibiting systems]) variables (). The heat-induced injury has been validatedCitation15,Citation16 and clinically used in prediction of postoperative pain.Citation12,Citation17 Potential variables predicting the heat-induced pain were anthropometric (body mass index [BMI], body surface area [BSA]), psychological (Hospital Anxiety and Depression Scale [HADS], Psychological Vulnerability Scale [PVS]), and psychophysical (QST, CPM test).Citation18–Citation21 All prediction interactions were examined for gender-related differences. The secondary objective was to analyze and characterize gender-related differences in psychophysical variables by anthropometric and psychological data.
Figure 1 The study algorithm: gender, anthropometric, psychological, and psychophysical variables are used as potential predictors of heat injury-induced pain.
Subjects and methods
Subjects
The protocol was approved by the Regional Ethics Committee (H-1-2009-132) and the Danish Data Protection Agency, and reported to ClinicalTrials.gov (NCT01345877). Healthy subjects were recruited from a website and bulletin boards at the University of Copenhagen. Inclusion criteria were healthy subjects between 20–40 years of age. Exclusion criteria were insufficient proficiency in Danish, participation in other clinical trials in the last 4 weeks prior to this study, skin lesions on the lower leg, intake of any medication 48 hours prior to the investigation, intake of analgesics 7 days prior to the investigation (except paracetamol as needed), current or former drug abuse, smoking, and BMI > 28 kg/m2. Following verbal and written information, all subjects provided written informed consent before inclusion. All subjects had a routine medical examination by a physician prior to inclusion. Subjects received compensation (€280) for the two study days.
Psychological tests
On day one, the subjects completed HADS and PVS.Citation22 HADS is a 14-item questionnaire used to evaluate the subject’s level of anxiety and depression; the subjects can rate between 0–21 with a score of eleven as the cutoff point for anxiety or depression.Citation23 PVS is a six-item questionnaire used to determine the subjects psychological vulnerability; the subjects can rate between 0–12 with a score of three as the cutoff point.Citation24,Citation25
Psychophysical tests
Environment and testing paradigm
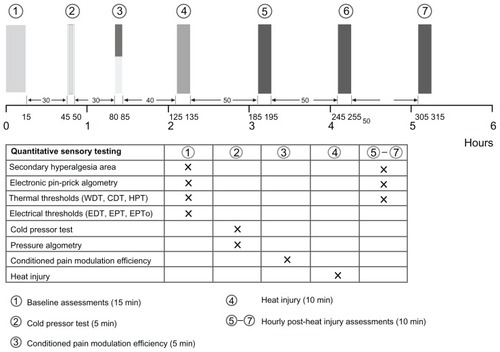
All tests were performed in a quiet, well-lit room (21°C–24°C, 24%–45% relative humidity) with the subjects comfortably relaxed in the supine position. Each test was explained prior to the test and standardized instructions were given during the tests (Appendix A). The subjects were blinded to the test results during the study. Subjects were allowed to ambulate between the testing sequences. In order to get familiarized with the QST procedures, the subjects underwent a training session prior to day one. The subject’s suitability in relation to ethical, psychomotor, and cognitive aspects were evaluated at this training session. Identical assessments were made on the two study days, each of 6 hours duration and separated by a minimum of 2 weeks. The testing paradigm is illustrated in .
Figure 2 The testing sequence: (1) baseline assessments (secondary hyperalgesia, electronic pinprick algometry, thermal and electrical thresholds), (2) cold pressor test and pressure algometry, (3) conditioned pain modulation efficiency, (4) heat injury, (5–7) postburn 1–3 hour assessments (comparable to baseline assessments, without electrical stimulation).
Testing areas
The testing area for thermal thresholds and secondary hyperalgesia was the upper, medial part of the nondominant lower leg.Citation12 The subjects were instructed to use a hair trimmer in the area, 2 days prior to the study, in order to avoid interference with the hair and sensory assessments. The three other testing areas were: the nondominant thumb and index finger for electrical thresholds, the nondominant index finger for pressure thresholds, and the dominant hand for CPM and CPT.
Electrical stimulation
Transcutaneous electrical stimuli were applied by a computerized, constant current stimulator (Pain Matcher®, Cefar Medical AB, Lund, Sweden).Citation26 The stimulator delivers square wave impulses with a frequency of 10 Hz and an amplitude of 15 mA. The stimulation intensity is automatically modulated by increasing the pulse width, in 4 μs increments, from 4 μs to a maximum of 396 μs. The subject pinches the two rubber electrodes between the nondominant thumb and index finger. By holding a steady grip on the electrodes, an incremental increase in the electrical energy is delivered. When releasing the pinch grip, a value between 1–100 is registered, which reflects the energy delivered. The electrical detection threshold (EDT), electrical pain threshold (EPT), and electrical pain tolerance (EPTo) were assessed.
Mechanical stimulation
Electronic pinprick algometry
The pinprick pain thresholds (PPT) were measured by an electronic pinprick algometer (Electronic von Frey, Somedic AB, Horby, Sweden)Citation27 and were assessed in and just outside the burn injury area on the nondominant lower leg (nonglabrous skin). The contact diameter with the skin was 0.2 mm (area 0.031 mm2). At each start-up, the algometer was calibrated against gravity (0 g) and a calibrated weight (20 g). In order not to exceed a range of skin pressures that could inflict tissue damage, the electronic pinprick algometer has a cutoff force value of 4.41 N. The subjects were told to indicate the pain threshold on an electronic visual analog scale (VAS) without indicator markings (electronic VAS, horizontal 10 cm line anchored by zero [no pain] and ten [maximum perceivable pain]). The PPT corresponded to a minimum detectable movement of the ruler (electronic VAS = 0.02).
Monofilaments
The area of secondary hyperalgesia in normal skin surrounding the area of the heat injury was determined with a nylon filament (nominal value 18 [mean ± standard deviation: 0.89 ± 0.05 N; Stoelting Co, Wood Dale, IL).Citation28 The border was determined by stimulating eight symmetric lines each separated by an angle of 45 degrees converging towards the center of the heat injury. The subjects, who had their eyes closed during the assessments, reported the occurrence of a definite uncomfortable change in sensation to a burning or stinging sensation. The corners of the octagon were marked on the skin and transferred to a transparent sheath. The secondary hyperalgesia areas were calculated (total area – area of the thermode) using a computer-based vector algorithm (Canvas 12.0; ACD Systems International, Victoria, Canada).
Thermal stimulation
Warmth detection threshold (WDT), cool detection threshold (CDT), heat pain threshold (HPT)
Thermal stimuli were delivered through a computerized system (MSA Thermotest®; Somedic AB) using a Peltier thermode with a contact area of 5.0 × 2.5 cm2.Citation12 Thermal neutrality was defined as 32°C and cutoff values for heat and cold stimuli were 50°C and 10°C, respectively. The ramp rates were ±2°C/second. The subjects were told to activate a button immediately at sensation of a change in temperature (WDT, CDT) and when the heat was perceived as pain (HPT). After activation of the button, the thermode returned to 32°C. On both study days, each test was performed in triplicate with random intervals of 2–6 seconds between the three runs, and the median value was used.
Heat injury
A first-degree heat injury was induced by the thermode (47°C, 420 seconds) applied to the lower nondominant leg. The injury is associated with development of erythema and tenderness. The subject used a VAS to rate the pain (zero = no pain, ten = maximum perceivable pain) immediately when 47°C had been reached, after 30 seconds, and thereafter every 60 seconds. The cumulated pain intensity during the heat injury (PHI) was calculated as:
CPM tests
Cold pressor test (CPT) and pressure algometry
A recirculating 0.9% saline-based chiller (model 11371P; VWR International, Radnor, PA) with a bath volume of 13 L was used for the CPT. The temperature was maintained at 0.3°C–0.5°C. First, an electronic algometer, with a neoprene-coated tip and an area of 1 cm2 (Somedic AB; rate: 30–40 mN/second), was applied on the dorsum of the distal phalanx of the dominant index finger (deep sensitivity pain). The subject was told to activate a button terminating the stimulus when the maximum tolerable pressure level was reached (pain pressure tolerance prior to CPT [PTo1]). Second, the subject then submerged the nondominant hand in the bath, maintaining the water level 1–2 cm above the wrist, spreading the fingers, and allowing water freely to circulate around the hand. The time to pain registration (cold pressor test pain threshold [CPP]) and time to pain tolerance (cold pressor test pain tolerance [CPTo]) were registered. Third, immediately after withdrawal of the hand from the bath, a second pressure algometry test at the dominant index finger was made (pain pressure tolerance immediately after CPT [PTo2]). The difference in pressure pain thresholds was calculated as ΔPTo = PTo2 − PTo1.
CPM efficiency
The CPM efficiency test has been used in clinical studies to predict the development of chronic postoperative pain.Citation20,Citation29
In the CPM efficiency paradigm, repeated short heat stimuli (47°C, 4 seconds; ) were applied to the nondominant lower leg (nonglabrous skin) in relation to submersion of the nondominant hand in the CPT water bath (0.3°C–0.5°C, 30 seconds).Citation29 During the heat stimuli, the subjects rated their maximal pain (CPM1–4; Appendix A) on a VAS. The subjects were instructed only to evaluate the pain from the heat stimulation. The CPM efficiency was calculated as
Figure 3 The conditioned pain modulation efficiency with repeated phasic heat stimuli (47°C, 4 seconds [1, 3–5]) in relation to submersion of the nondominant hand (2) in the cold pressor test (0.3°C–0.5°C, 30 seconds).
![Figure 3 The conditioned pain modulation efficiency with repeated phasic heat stimuli (47°C, 4 seconds [1, 3–5]) in relation to submersion of the nondominant hand (2) in the cold pressor test (0.3°C–0.5°C, 30 seconds).](/cms/asset/71d768a7-16c9-4e2d-a0c1-c363fd93fe24/djpr_a_33925_f0003_b.jpg)
Statistical analyses
The power analysis was based on the number of variables that could be included in the multiple regression analyses. The authors a priori considered ten variables for the analyses would make a sufficient statistical basis for conclusions, corresponding to the inclusion of at least 100 subjects.Citation30
All data were tested for normal distribution using the Shapiro–Wilk test of normality, analyses of skewness and kurtosis, and residual plots. In cases of nonnormal distribution, simple transformations were tried and, if unsuccessful, nonparametric statistics were applied. The tests for gender differences were with unpaired t-tests or Mann–Whitney U tests, depending on the underlying distribution.
Mean values for day one and day two assessments were used. All variables were tested for correlation with PHI using linear regression, and interactions with gender were examined. All variables, with P ≤ 0.10 in the linear regression model, were tested in a multiple regression with backward elimination. Variables were included and analyzed with and without interaction with gender in the multiple regression model if interaction with gender was found. In all regression analyses, the double-sided significance level was set at P < 0.05. In all nonregression analyses, the double-sided level of significance was set at P < 0.01 in order to reduce the likelihood of type I error due to multiple comparisons. Continuous data are presented as mean ± standard error of the mean unless otherwise stated.
Results
Subjects
The intention-to-treat and per-protocol groups were 57/58 (males/females) and 50/50, respectively (). A significant gender difference was seen in height, weight, BMI, and BSA (; unpaired t-test P < 0.0001), with lower values for female subjects compared to male subjects. A trend in gender- related difference in age distribution was seen (unpaired t-test P = 0.026). The median interval between day one and day two was 21 (15–30 [25%–75% interquartile range]) days.
Figure 4 Consolidated Standards of Reporting Trials diagram of the study illustrating patient enrollment, inclusion, the two study days, and analysis.
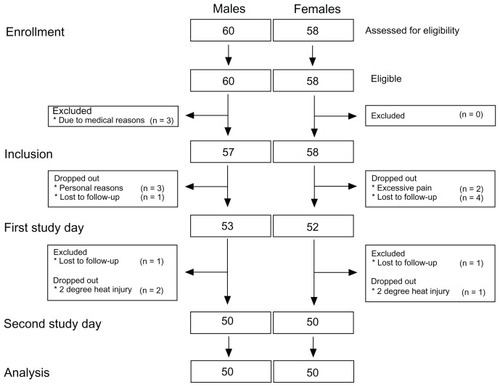
Table 1 Anthropometric data for the per-protocol group
The assessments were performed by three investigators: PR (female [52% of the test]), RF (male [29%]), and APS (male [19%]). All subjects were instructed by the same investigator (PR) on the training day, and 79% of the subjects were examined by the same investigator on both study days. No significant gender difference was seen in the number of subjects examined by a male or female investigator (P = 0.439).
Due to a malfunctioning alarm system, the contact thermode overheated by 1°C–2°C. This caused the development of blisters in the application area, observed 12–24 hours after induction of the injury, in 18 subjects. Data from this period of equipment failure were analyzed in detail and compared to earlier data, but no temporal changes attributable to overheating were observed. Data from these subjects were therefore included in the perprotocol analyses.
Psychological variables
Two subjects (one male, one female) had HADS subscores demonstrating anxiety (score of twelve and eleven, respectively); in addition, the male subject had a HADS subscore indicating depression (score of eleven). Eleven subjects (five males, six females) had PVS scores demonstrating psychological vulnerability (average scores of 3.6 and 4.7, respectively). No significant correlations between psychological variables and PHI (χ2 > 0.153) or gender (χ2 > 0.315) were observed.
Psychophysical tests
A comprehensive analysis of predictive variables from the psychophysical tests prior to the heat injury is presented in .
Table 2 Analyses of predictive variables
Electrical stimulation
Gender-related differences
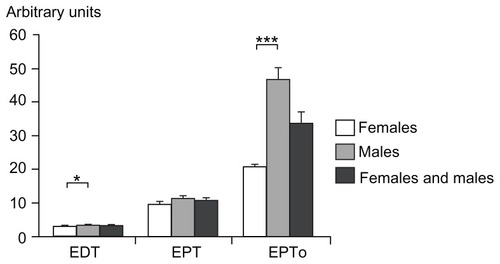
Significant gender differences were seen for EDT and EPTo (P < 0.002; and ), but not for EPT (P = 0.045). Associations between PHI and EDT, EPT, and EPTo in females (P < 0.012) and EPTo in males (P = 0.044) were observed, while no associations were found for EDT and EPT in males (P > 0.109). Combining both genders, an association was found between PHI and EDT and EPTo (P < 0.002), but not with EPT (P = 0.080). An interaction between EDT and EPTo and gender was observed (P < 0.006).
Figure 5 Electrical stimulation: electrical detection threshold, electrical pain threshold, and electrical pain tolerance for females, males, and both genders combined (mean ± standard error of the mean).
Abbreviations: EDT, electrical detection threshold; EPT, electrical pain threshold; EPTo, electrical pain tolerance.
Anthropometric-related differences
Separate linear regression analyses, using BSA and BMI as the independent variables, and the electric stimulation tests as the dependent variables, showed a relationship between BSA and EDT and EPTo (P < 0.0001, R2 = 0.138–0.196). A significant positive correlation between BMI and EPTo (P = 0.0001, R2 = 0.184), but not between BMI and EDT (P = 0.078, R2 = 0.031), was seen.
Electronic pinprick algometry
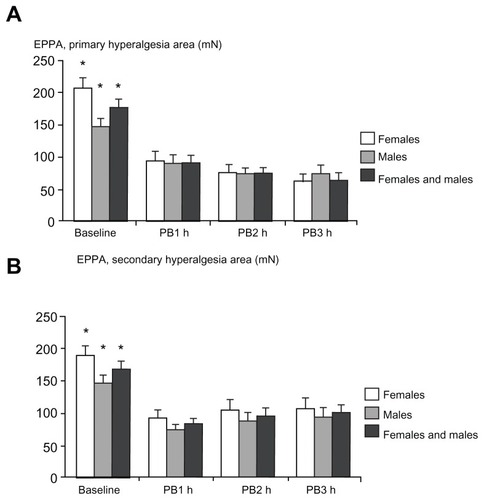
PPT assessments in the primary hyperalgesia area () and the secondary hyperalgesia area () during baseline and postburn measurements did not demonstrate a gender difference (P > 0.017; ). A negative correlation was found between the baseline measurements of PPT and PHI, in primary and secondary hyperalgesia areas, in females and both genders combined (P < 0.004). A negative correlation with PHI was also found for males in the primary hyperalgesia area (P = 0.007), but not in secondary hyperalgesia area (P = 0.062). Significant interactions between gender and all PPT assessments were observed (P < 0.001).
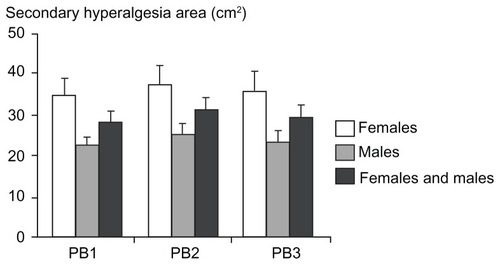
Figure 6 Assessment of pinprick pain thresholds with an electronic pinprick algometer in (A) the primary hyperalgesia area and (B) the secondary hyperalgesia area at baseline (preburn), 1 hour, 2 hours, and 3 hours after the burn injury.
Abbreviations: EPPA, electronic pinprick algometer; PB1, 1 hour postburn; PB2, 2 hours postburn; PB3, 3 hours postburn.
Monofilaments
Twelve subjects did not report secondary hyperalgesia in the postburn measurements. No significant gender differences in the secondary hyperalgesia areas were found in any of the postburn measurements (P > 0.072; ).
Thermal stimulation
Thermal thresholds (WDT, CDT, HPT)
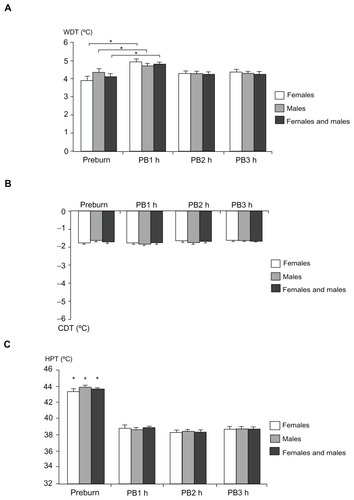
No significant gender-related differences in WDT, CDT, or HPT were observed (; P > 0.084 [preburn and postburn]). The linear regression analyses did not demonstrate a correlation between PHI and WDT for females, males, and both genders combined or between PHI for CDT and HPT in males (P > 0.064). A negative correlation was found between PHI and CDT and HPT in females and both genders combined (P < 0.016). An interaction was found between HPT and gender (P = 0.007).
Figure 8 Thermal stimulation: (A) warmth detection threshold, (B) cool detection threshold, and (C) heat pain threshold at baseline (preburn) and 1, 2, and 3 hours after the burn injury for females, males, and both genders combined (mean ± standard error of the mean).
Abbreviations: CDT, cool detection threshold; HPT, heat pain threshold; PB1, 1 hour postburn; PB2, 2 hours postburn; PB3, 3 hours postburn; WDT, warmth detection threshold.
Heat injury
No gender difference was seen in PHI (P = 0.477; ).
Table 3 Cumulated pain intensity during heat injury
CPM tests
CPT and pressure stimulation
Gender-related differences
A gender difference was seen in CPTo (P = 0.001; and ), but not in CPP (P = 0.468). A negative correlation was observed between CPP and PHI in females and in the genders combined (P = 0.025), but not in males (P = 0.629).
Figure 9 Pressure pain tolerance (left Y-axis) before and immediately after the cold pressor test, and the difference between the two assessments, for females, males, and both genders combined (mean ± standard error of the mean).
Abbreviations: CPTpain, cold pressor pain threshold; CPTo, cold pressor pain tolerance; PPTo1, pressure pain tolerance before the cold pressor test; PPTo2, pressure pain tolerance immediately after the cold pressor test; ΔPTo, difference in pressure tolerance before and after the cold pressor test.
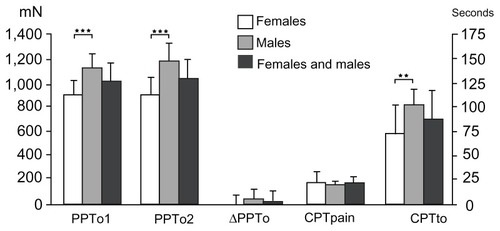
Significant gender differences were observed in PTo1 and PTo2 (P < 0.0001; ), with the lowest tolerance values for females. An interaction between gender and PTo1 and PTo2 was observed (P < 0.010) No significant gender differences were seen in ΔPTo (P = 0.103). No correlation was found between ΔPTo and PHI (P = 0.304), and no interaction between gender and ΔPTo was observed (P = 0.388).
Anthropometric-related differences
Separate linear regressions with BSA and BMI as the independent variables and CPTo as the dependent variable did not show any correlation with BSA (P = 0.547, R2 = 0.004) or BMI (P = 0.301, R2 = 0.011).
Separate linear regressions with BSA and BMI as the independent variables and PTo1 and PTo2 as the dependent variables showed a correlation with BSA (P < 0.001, R2 = 0.118–0.154) and BMI (P < 0.005, R2 = 0.080–0.125).
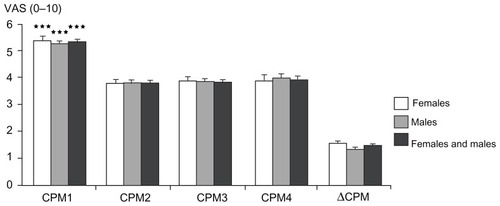
CPM efficiency
The ΔCPM was 28.8% for females and 25.7% for males, with no significant gender differences (P = 0.229; ). No correlation was seen between ΔCPM and PHI (P = 0.389; ).
Figure 10 Conditioned pain modulation efficiency: pain ratings with visual analog scale (0–10) during the four phasic heat stimuli during the test for conditioned pain modulation (compare to ) for females, males, and both genders combined (mean ± standard error of the mean).
Abbreviations: CPM1, conditioned pain modulation rating before the cold pressor test; CPM2, conditioned pain modulation rating during the cold pressor test; CPM3–4, conditioned pain modulation rating after the cold pressor test; VAS, visual analog scale; ΔCPM, conditioned pain modulation efficiency.
Prediction of pain sensitivity
The variables that reached statistical significance level (P ≤ 0.10) in the simple linear regression analyses with PHI as the dependent variable were used in a new multiple regression model. Ten variables were included: EDT, EPTo, PPT in the primary hyperalgesia area, PPT in the secondary hyperalgesia area, HPT, PTo1, PTo2, CPP, EPT, and CDT. The final model contained three variables: EPTo, PPT in the primary hyperalgesia area, and CDT (P = 0.0001, R2 = 0.282). Interaction with gender was included in the eight first parameters but was not significant in the final model.
Repeated assessments
Day one and day two preburn and postburn data, combined and divided between gender, were compared (paired t-tests). Out of 108 comparisons, seven differed significantly (P < 0.009): PHI in females, secondary hyperalgesia areas in males (1–3 hours postburn), and secondary hyperalgesia areas in both genders combined (1–3 hours postburn).
Discussion
There were three main findings in the present study. First, a predictive multiple regression model for experimental pain sensitivity accounted for 28.2% of the variance in pain induced from a heat injury. In this predictive regression model, no signs of a gender-related difference in experimental pain sensitivity were observed. Second, the study confirmed significant gender-related differences in the perception of electrical and cold pressor stimuli. Third, highly significant positive correlations between BSA, BMI, and pain perception during electrical, pressure, and cold pressor stimuli were demonstrated.
Prediction of pain sensitivity
Preoperative QST assessments have been used to predict the probability of developing severe acute or chronic postsurgical pain.Citation4,Citation10 A number of studies have used short phasic heat stimulation as a preoperative predictor variable.Citation31–Citation37 The heat injury model used in the present study has been validated and used in a number of physiologic and pharmacodynamic studies. The main advantage compared to phasic heat stimuli is that the tonic heat stimulus induces a short-lasting (<48 hours) tissue injury leading to inflammation with hyperalgesia, erythema, edema, and hyperthermia. The pain studied is thus of inflammatory origin, mimicking clinical tissue damage, and has successfully been used in preoperative prediction of postoperative pain.Citation12 Reviews indicate that 4%–54% of the variances in acute postoperative pain and opioid requirements are predictable by preoperative QST assessments.Citation10
Pain sensitivity is influenced by the efficacy of the descending inhibitory system.Citation19–Citation21,Citation38 In the present study, the endogenous analgesia system was tested by the CPM efficiency test but no gender-related difference was seen. A recent review on gender differences in pain modulation indicates that males have a more efficient CPM system than females,Citation39 although several conflicting studies exist.Citation40
Psychological variables, anxiety, depression and psychological vulnerability have been shown in a number of clinical studies to correlate with pain.Citation41–Citation43 However, in the present experimental study a correlation between psychological variables and pain was not observed, which most likely is due to a very low representation of psychological variables outside the normal range in this group of healthy individuals.
Gender-related differences in pain perception
Electrical stimuli
There is evidence for higher pain sensitivity to electrical stimulation in females compared to males from a number of volunteerCitation44,Citation45 and patient studies,Citation40,Citation46–Citation50 which is corroborated by the present study. Interestingly, studies with assessments of preoperative electrical thresholdsCitation10 have shown significant predictive potential of postoperative pain intensity in individuals undergoing caesarean section,Citation51,Citation52 but not in an all-male inguinal herniotomy study.Citation31
It is essentially not understood why females seem to have lower electrical thresholds than males. It has been hypothesized that psychological,Citation40,Citation47,Citation53 social,Citation40 genetic,Citation39,Citation40 and endocrinological factors,Citation40,Citation47,Citation53 nociceptive input integration in the central nervous system,Citation45,Citation47 and modulation of the afferent input by antinociceptive activity from supraspinal structuresCitation40,Citation47 may contribute. Another explanation is that while the electrical stimulation areas in most studies are the same for subjects, the relative area of motor stimulation (relative to total muscle area) is higher for females, leading to lower thresholds than in males.Citation54 However, a contribution of higher intraepithelial nerve fiber density in females is probably an important factor.Citation55–Citation57
The significant positive correlations between electrical thresholds and anthropometric data are also noticeable. This finding could be interpreted as gender-related; however, it does not imply a causal relationship since there are significant correlations with gender and BSA and BMI (higher in males compared to females). The present study does not have sufficient statistical power to decide if it is gender-related, anthropometric-related, or both.
A significant positive correlation with BSA was only seen with electrical stimuli applied at the fingers, and not with thermal or mechanical stimuli applied at the lower leg or hand. A possible explanation is that the fingertips have a higher myelinated nerve fiber density and possess a higher number of specialized receptors than the nonglabrous testing site, ie, the lower leg.Citation58–Citation60 Correspondingly, a higher density of Meissner’s corpuscles, intrapapillary myelinated nerve fibers, and epidermal nerve fibers have been demonstrated in females compared to males.Citation58,Citation60 Furthermore, the fingertip areas are generally smaller in females than in males,Citation58 indicating that less of the constant current stimulator’s finger pad area is covered by the female subject (the stimulator delivers fixed energy quanta). Thus, these two factors may theoretically contribute to the increased pain sensitivity in females: a higher nerve and specialized receptor density in glabrous skin and a smaller stimulation area, leading to a higher influx of electrical energy by the constant current simulator.
An obvious and important question is: are QST assessments correlated with the density of epidermal nerve fibers? The authors are not aware of any systematic sensory research in glabrous skin, but a recent study in nonglabrous skin suggests that thermal stimuli are only capable of detecting relatively large differences in epidermal innervation (12.2 epidermal nerve fibers/mm), while mechanical noxious stimuli have the ability to detect small changes in innervation (4.18 epidermal nerve fibers/mm).Citation60
Since there is a mathematical relationship between BMI and BSA,Citation61 it is not surprising that significant positive correlations between BMI and electrical (EPTo) and pressure algometry tolerance (PPTo1, PPTo2) were also observed.
Thermal thresholds
Quantitative perception of heat pain has generally been found to be more pronounced in female subjects,Citation40,Citation62,Citation63 particularly for tolerance assessments but also for pain thresholds; although this could not be reproduced in six of 23 studies.Citation64–Citation66 In the present study, the threshold assessments involving heat pain stimuli (HPT) indicated a trend towards higher pain sensitivity in females than males.
CPM tests
CPT and pressure stimulation
In the CPTo assessments, significantly lower thresholds were demonstrated in female subjects, but a significant correlation with BSA was not shown. Interestingly, although the study by Selim et al used nonglabrous skin, the epithelial nerve fiber density – after accounting for BMI – was still significantly higher in females compared to males.Citation60 If this finding can be extrapolated to the hand, a relatively higher sensory innervation density could probably account for the gender-related difference in spite of a smaller stimulation area in the female subject (the submerged hand).
Females demonstrated lowered tolerance compared to males during pressure algometry (PPTo1, PPTo2), a finding that corroborates the findings from a number of previous studies.Citation40,Citation67
CPM efficiency
In three experimental studies, CPT has been used as a conditioning stimulus and contact heat as a test stimulus. Citation19,Citation67,Citation68 Although the temperature of the conditioning stimuli varied between 5°C–18°C and the exposure time between 60–120 seconds (compared to 0.4°C and 30 seconds in the present study), no difference in CPM efficiency between genders was observed, which is in concordance with results from the present study. However, the weighted means of CPM efficiency in a recent review of ten studies were 15.6% for females and 28.6% for males, suggesting a higher CPM effect for males.Citation39 This finding was based on a plethora of different conditioning stimuli (cold, electrical, heat, ischemia, muscle pain) and test stimuli (chemical, electrical, heat, pressure), which seems to impede proper evaluation of data. At present, no reconciliatory view exists on the most reliable way to quantify the inhibitory effect of the CPM system.Citation68,Citation69
Methodology: limitations and advantages
A limitation of the study is the multiple comparisons made, leading to an increased probability of type I errors. Although a significance level of 0.01 was used, it cannot be ruled out that some results are a consequence of mass significance. In order to mitigate type II errors, a sufficient sample size was used to calculate the primary outcome by the multiple regression analyses. The validity of data was augmented by repeated assessments, the use of a limited number of investigators, and the use of standardized methods. Since repeatability data are influenced by variances in characteristics of QST equipment, testing paradigm, number of observers, test site, and baseline skin temperature,Citation70,Citation71 repeat data sampling – as in the present study – could be considered indicators of test homogeneity and, as such, a prerequisite for correct data interpretation.Citation72 It is important to consider that the repeated data assessments demonstrated that in 7% of the comparisons, statistical significant differences between test and retest data were observed. This could be attributed to a type I error, but a carryover effect between the test days, which have been demonstrated in the heat injury model, cannot be excluded.Citation73,Citation74 However, the risk of carryover effects within the same session was minimized by the use of relatively long intervals between the tests. Likewise, the risk of carryover effects between sessions was minimized by a minimum of 2 weeks between the sessions.
An important objection to the study is that the menstrual phase was not taken into consideration in female subjects. In a classic review of pain perception across the menstrual cycle, it was concluded that for pressure stimulation, cold pressor pain, thermal heat stimulation, and ischemic muscle pain, higher thresholds were apparent in the follicular phase compared to other phases.Citation75 In contrast, recent systematic reviews have presented evidence for much more inconsistent menstrual cycle effects.Citation40,Citation76 In an experimental study, assessments of responses to cold pressor, heat, and ischemic pain during early follicular, late follicular, and luteal phases in female subjects were evaluated.Citation77 The pain perception during each test was not influenced by the menstrual cycle nor did it affect the demonstrated gender differences in pain sensitivity. Furthermore, a recent study in females undergoing in vitro fertilization did not demonstrate a relationship between estrogen levels and thermal perception, pain threshold, and pain tolerance.Citation78
Conclusion
The present study demonstrates predictability of acute pain sensitivity in healthy volunteers that could account for nearly 30% of the variance. Significant correlations between anthropometric variables (BSA and BMI) and pain perception during electrical and pressure stimulation were observed. Future studies are needed to confirm the role of explanatory variables for gender-related differences in pain perception and, most importantly, to translate these findings into a clinical perspective.
Acknowledgments
This work was supported by an unrestricted educational grant from Norpharma A/S Denmark. The authors are grateful to Dorthe Tvinnemose for valuable discussions and Per Rotboll Nielsen for assistance with medical examination of the subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
- NielsenCSStubhaugAPriceDDVassendOCzajkowskiNHarrisJRIndividual differences in pain sensitivity: genetic and environmental contributionsPain20081361–2212917692462
- CampbellCMEdwardsRRFillingimRBEthnic differences in respon-ses to multiple experimental pain stimuliPain20051131–2202615621360
- Rahim-WilliamsFBRileyJL3rdHerreraDCampbellCMHastieBAFillingimRBEthnic identity predicts experimental pain sensitivity in African Americans and HispanicsPain20071291–217718417296267
- AbrishamiAChanJChungFWongJPreoperative pain sensitivity and its correlation with postoperative pain and analgesic consumption: a qualitative systematic reviewAnesthesiology2011114244545721245740
- AfariNMostoufiSNoonanCC-reactive protein and pain sensitivity: findings from female twinsAnn Behav Med201142227728321785898
- BaumCHuberCSchneiderRLautenbacherSPrediction of experimental pain sensitivity by attention to pain-related stimuli in healthy individualsPercept Mot Skills2011112392694621853779
- EdwardsRRCampbellCMFillingimRBCatastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responsesJ Pain20056533833915890636
- OostermanJMDijkermanHCKesselsRPScherderEJA unique association between cognitive inhibition and pain sensitivity in healthy participantsEur J Pain201014101046105020493746
- HastieBARileyJL3rdRobinsonMECluster analysis of multiple experimental pain modalitiesPain2005116322723715964682
- WernerMUMjoboHNNielsenPRRudinAPrediction of postoperative pain: a systematic review of predictive experimental pain studiesAnesthesiology201011261494150220460988
- ChizhBAPriestleyTRowbothamMSchafflerKPredicting therapeutic efficacy – experimental pain in human subjectsBrain Res Rev200960124325419168094
- WernerMUDuunPKehletHPrediction of postoperative pain by preoperative nociceptive responses to heat stimulationAnesthesiology2004100111511914695732
- SchulzEZherdinATiemannLPlantCPlonerMDecoding an individual’s sensitivity to pain from the multivariate analysis of EEG dataCereb Cortex20122251118112321765182
- RacineMTousignant-LaflammeYKlodaLADionDDupuisGChoiniereMA systematic literature review of 10 years of research on sex/gender and experimental pain perception – part 1: are there really differences between women and men?Pain2012153360261822192712
- NaertALKehletHKupersRCharacterization of a novel model of tonic heat pain stimulation in healthy volunteersPain2008138116317118207325
- PedersenJLKehletHSecondary hyperalgesia to heat stimuli after burn injury in manPain19987633773849718256
- WernerMUDuunPKraemerOLassenBKehletHArthroscopic knee surgery does not modify hyperalgesic responses to heat injuryAnesthesiology20039951152115714576553
- Arendt-NielsenLYarnitskyDExperimental and clinical applications of quantitative sensory testing applied to skin, muscles and visceraJ Pain200910655657219380256
- GranotMWeissman-FogelICrispelYDeterminants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter?Pain20081361–214214917720319
- YarnitskyDCrispelYEisenbergEPrediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at riskPain20081381222818079062
- YarnitskyDConditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain statesCurr Opin Anaesthesiol201023561161520543676
- BjellandIDahlAAHaugTTNeckelmannDThe validity of the hospital anxiety and depression scale. An updated literature reviewJ Psychosom Res2002522697711832252
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- BisgaardTKlarskovBRosenbergJKehletHCharacteristics and prediction of early pain after laparoscopic cholecystectomyPain200190326126911207398
- BorlyLAndersonIBBardramLPreoperative prediction model of outcome after cholecystectomy for symptomatic gallstonesScand J Gastroenterol199934111144115210582767
- Stener-VictorinEKowalskiJLundebergTA new highly reliable instrument for the assessment of pre- and postoperative gynecological painAnesth Analg200295115115712088960
- MollerKAJohanssonBBergeOGAssessing mechanical allodynia in the rat paw with a new electronic algometerJ Neurosci Methods1998841–241479821632
- WernerMURotboll-NielsenPEllehuus-HilmerssonCHumidity affects the performance of von Frey monofilamentsActa Anaesthesiol Scand201155557758221827443
- PudDGranovskyYYarnitskyDThe methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humansPain20091441–2161919359095
- AltmanDGPractical Statistics for Medical ResearchLondonChapman and Hall1996
- AasvangEKHansenJBKehletHCan preoperative electrical nociceptive stimulation predict acute pain after groin herniotomy?J Pain200891094094418650130
- GranotMLowensteinLYarnitskyDTamirAZimmerEZPostcesarean section pain prediction by preoperative experimental pain assessmentAnesthesiology20039861422142612766652
- GranotMWeissman-FogelIThe effect of post-surgical neuroplasticity on the stability of systemic pain perception: a psychophysical studyEur J Pain201216224725522323377
- PanPHCoghillRHouleTTMultifactorial preoperative predictors for postcesarean section pain and analgesic requirementAnesthesiology2006104341742516508387
- RudinAWolner-HanssenPHellbomMWernerMUPrediction of post-operative pain after a laparoscopic tubal ligation procedureActa Anaesthesiol Scand200852793894518477083
- RudinAErikssonLLiedholmRListTWernerMUPrediction of postoperative pain after mandibular third molar surgeryJ Orofac Pain201024218919620401357
- StrulovLZimmerEZGranotMTamirAJakobiPLowensteinLPain catastrophizing, response to experimental heat stimuli, and post-cesarean section painJ Pain20078327327917113350
- NirRRGranovskyYYarnitskyDSprecherEGranotMA psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulationEur J Pain201115549149721035364
- PopescuALeRescheLTrueloveELDrangsholtMTGender differences in pain modulation by diffuse noxious inhibitory controls: a systematic reviewPain2010150230931820557999
- FillingimRBKingCDRibeiro-DasilvaMCRahim-WilliamsBRileyJL3rdSex, gender, and pain: a review of recent clinical and experimental findingsJ Pain200910544748519411059
- TaenzerPMelzackRJeansMEInfluence of psychological factors on postoperative pain, mood and analgesic requirementsPain19862433313423960574
- DornLDCampoJCThatoSPsychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disordersJ Am Acad Child Adolesc Psychiatry2003421667512500078
- McWilliamsLAGoodwinRDCoxBJDepression and anxiety associated with three pain conditions: results from a nationally representative samplePain20041111–2778315327811
- LautenbacherSRollmanGBSex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation methodPain19935332552648351155
- MyliusVKunzMSchepelmannKLautenbacherSSex differences in nociceptive withdrawal reflex and pain perceptionSomatosens Mot Res200522320721116338828
- al’AbsiMFranceCHarjuAFranceJWittmersLAdrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and womenPsychosom Med200668229229816554396
- AshinaSBendtsenLAshinaMMagerlWJensenRGeneralized hyperalgesia in patients with chronic tension-type headacheCephalalgia200626894094816886930
- AyeshEEJensenTSSvenssonPSomatosensory function following painful repetitive electrical stimulation of the human temporomandibular joint and skinExp Brain Res2007179341542517146645
- LeongGWLauschkeJRutowskiSBWaitePMAge, gender, and side differences of cutaneous electrical perceptual threshold testing in an able-bodied populationJ Spinal Cord Med201033324925520737798
- NyklicekIVingerhoetsAJVan HeckGLHypertension and pain sensitivity: effects of gender and cardiovascular reactivityBiol Psychol199950212714210403201
- BuhagiarLCassarOABrincatMPPredictors of post-caesarean section pain and analgesic consumptionJ Anaesthesiol Clin Pharmacol201127218519121772677
- NielsenPRNorgaardLRasmussenLSKehletHPrediction of post-operative pain by an electrical pain stimulusActa Anaesthesiol Scand200751558258617430320
- RileyJL3rdRobinsonMEWiseEAMyersCDFillingimRBSex differences in the perception of noxious experimental stimuli: a metaanalysisPain1998742–31811879520232
- MaffiulettiNAHerreroAJJubeauMImpellizzeriFMBizziniMDifferences in electrical stimulation thresholds between men and womenAnn Neurol200863450751218300313
- BakkersMMerkiesISLauriaGIntraepidermal nerve fiber density and its application in sarcoidosisNeurology200973141142114819805731
- GoranssonLGMellgrenSILindalSOmdalRThe effect of age and gender on epidermal nerve fiber densityNeurology200462577477715007129
- MowlaviACooneyDFebusLKhosravianiAWilhelmiBJAkersGIncreased cutaneous nerve fibers in female specimensPlast Reconstr Surg200511651407141016217487
- NolanoMProviteraVCrisciCQuantification of myelinated endings and mechanoreceptors in human digital skinAnn Neurol200354219720512891672
- ProviteraVNolanoMPaganoACaporasoGStancanelliASantoroLMyelinated nerve endings in human skinMuscle Nerve200735676777517405136
- SelimMMWendelschafer-CrabbGHodgesJSVariation in quantitative sensory testing and epidermal nerve fiber density in repeated measurementsPain2010151357558120851518
- VerbraeckenJVan de HeyningPDe BackerWVan GaalLBody surface area in normal-weight, overweight, and obese adults. A comparison studyMetabolism200655451552416546483
- HashmiJADavisKDWomen experience greater heat pain adaptation and habituation than menPain2009145335035719632779
- NeziriAYScaramozzinoPAndersenOKDickensonAHArendt-NielsenLCuratoloMReference values of mechanical and thermal pain tests in a pain-free populationEur J Pain201115437638320932788
- FillingimRBMaixnerWKincaidSSilvaSSex differences in temporal summation but not sensory-discriminative processing of thermal painPain19987511211279539681
- JensenMTPetersenKLGender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteersJ Pain20067321121716516827
- JonesAZachariaeRArendt-NielsenLDispositional anxiety and the experience of pain: gender-specific effectsEur J Pain20037538739512935790
- EdwardsRRNessTJWeigentDAFillingimRBIndividual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variablesPain2003106342743714659526
- Tousignant-LaflammeYPageSGoffauxPMarchandSAn experimental model to measure excitatory and inhibitory pain mechanisms in humansBrain Res20081230737918652808
- YarnitskyDArendt-NielsenLBouhassiraDRecommendations on terminology and practice of psychophysical DNIC testingEur J Pain201014433920227310
- GeberCKleinTAzadSTest-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre studyPain2011152354855621237569
- SiaoPCrosDPQuantitative sensory testingPhys Med Rehabil Clin N Am200314226128612795516
- BlandJMAltmanDGMeasuring agreement in method comparison studiesStat Methods Med Res19998213516010501650
- PedersenJLKehletHHyperalgesia in a human model of acute inflammatory pain: a methodological studyPain1998742–31391519520228
- WernerMULassenBPedersenJLKehletHLocal cooling does not prevent hyperalgesia following burn injury in humansPain200298329730312127031
- RileyJL3rdRobinsonMEWiseEAPriceDDA meta-analytic review of pain perception across the menstrual cyclePain199981322523510431710
- ShermanJJLeRescheLDoes experimental pain response vary across the menstrual cycle? A methodological reviewAm J Physiol Regul Integr Comp Physiol20062912R245R25616484434
- KlatzkinRRMechlinBGirdlerSSMenstrual cycle phase does not influence gender differences in experimental pain sensitivityEur J Pain2010141778219217329
- SteningKDBergGHammarMInfluence of estrogen levels on thermal perception, pain thresholds, and pain tolerance: studies on women undergoing in vitro fertilizationJ Pain201213545946622480441
Appendix A
Each test was explained prior to the test and standardized instructions were given during the tests, as described below.
Electrical stimulation
Electrical detection threshold: “release the grip when you perceive the electrical stimulation;” electrical pain threshold: “release the grip when you perceive the electrical stimulation as painful;” and electrical pain tolerance: “release the grip when you perceive the electrical stimulation as intolerable.”
Mechanical stimulation
Secondary hyperalgesia: “please tell me when the sensation elicited by the pinprick stimulation changes from nonpainful to painful or uncomfortable.”
Thermal stimulation
Warmth detection threshold: “press the button as soon as you feel the thermode getting warmer;” cool detection threshold: “press the button as soon as you feel the thermode getting cooler;” heat pain threshold: “press the button as soon as you feel the stimulus changing from warm to painful;” mean pain intensity during the heat injury: “please rate the pain as soon as the thermode reaches 47°C, after 30 seconds, and then every minute for up to 7 minutes. I will tell you when to rate the pain;” cold pressor test pain threshold: “please tell me when you perceive the cold water stimulation as painful;” and cold pressor test pain tolerance: “please withdraw the hand when you perceive the cold water stimulations as intolerable.”
Conditioned pain modulation
Conditioned pain modulation efficiency: “you will receive four 4-second heat stimuli. After the first stimulus you will place your hand in the water bath and you will experience the second heat stimuli. I will tell you when to withdraw the hand and thereafter you will receive two additional heat stimuli. It is important that you only rate the pain from the heat stimuli and not from the water bath;” and pressure pain tolerance: “activate the button when you perceive the pressure stimulation as intolerable.”