Abstract
Objective
To analyze the influencing factors of motor dysfunction in patients with limb herpes zoster and to construct a nomogram to predict the risk of this complication.
Methods
A total of 213 patients with limb herpes zoster in the First Hospital of Jiaxing from January 2015 to June 2020 were enrolled. The muscle strength of the affected limb was evaluated by the Medical Research Council (MRC) score. The muscle strength of the affected limb was less than grade 5, and lower than that of the contralateral limb (normal side) was defined as motor dysfunction. Univariate and multivariate logistic regression analyses were used to determine the influencing factors of motor dysfunction in patients with limb herpes zoster. The nomogram of the risk of motor dysfunction was drawn by R software. Discrimination and calibration of the prediction model were assessed by using the C-index and calibration plot. Internal validation was assessed by using bootstrapping validation.
Results
There were 58 cases in the motor dysfunction group and 155 cases in the nonmotor dysfunction group, and univariate analysis showed that the location of involvement (OR = 4.095, 95% CI: 2.097–8.466, P = 0.017), whether or not diseases were combined (OR = 0.520, 95% CI: 0.281–0.959, P = 0.036) and the number of affected centrums (OR = 0.336, 95% CI: 0.177–0.632, P = 0.001) were associated with the occurrence of motor dysfunction in patients with limb herpes zoster. Multivariate logistic regression analysis showed that upper limb involvement (OR = 3.811, 95% CI: 1.829–8.387, P = 0.001), noncombined diseases (OR = 0.493, 95% CI: 0.249–0.969, P = 0.041) and the number of affected centrums ≤3 (OR = 0.439, 95% CI: 0.218–0.881, P = 0.020) were independent influencing factors of this complication. The prediction model displayed good discrimination with a C-index of 0.72 and good calibration. A high C-index value of 0.71 could still be reached in the interval validation.
Conclusion
This study used the clinical data available to establish a nomogram model for motor dysfunction of limb herpes zoster and found that motor dysfunction is more likely to occur in patient who meets upper limb involvement, combined diseases and the number of affected centrums >3. This kind of high-risk group should be intervened as soon as possible.
Introduction
Herpes zoster is a skin infectious disease caused by varicella-zoster virus (VZV), which is common in the elderly.Citation1–Citation3 It is characterized by erythema and clustered blisters distributed in bands along one side of the peripheral nerve, accompanied by severe Pain.Citation4,Citation5 Its incidence is approximately 4–4.5/1000 person-year. With population aging, the global incidence of herpes zoster continues to rise. A total of 20–30% of people suffer from herpes zoster in their lifetime.Citation6
Motor dysfunction is a rare neurological complication of herpes zoster, accounting for only 0.3%-5.0%.Citation7 However, pain can cover up the symptoms of limb weakness, so its incidence is underestimated clinically. This complication seriously reduces the quality of life and work efficiency of patients and may lead to permanent disability.Citation8 Therefore, the early prevention of motor dysfunction is of great significance to reduce the permanent disability of patients with herpes zoster. At present, there are few studies on the related factors and is no study on the prediction model of motor dysfunction in patients with limb herpes zoster at home and abroad. Therefore, this study retrospectively analyzed the available clinical data of patients with limb herpes zoster and discussed the independent influencing factors of motor dysfunction. To construct a nomogram to predict the risk of motor dysfunction in patients with limb herpes zoster to provide some clinical guidance for early prevention and reduction of the occurrence of this complication.
Clinical Materials and Methods
Study Patients
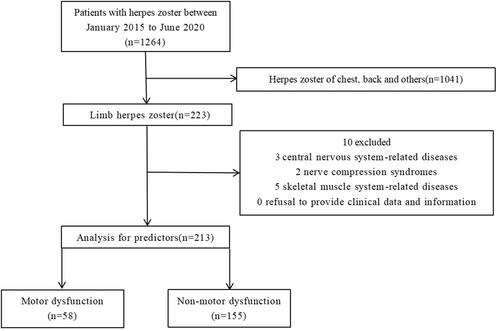
We retrospectively collected all patients with limb herpes zoster hospitalized in the First Hospital of Jiaxing from January 2015 to June 2020, and finally, after inclusion and exclusion, 213 patients were included in the final study. The process for patient selection is presented in . The study protocol was approved by the First Hospital of Jiaxing. We confirm that our study complies with the Declaration of Helsinki. This project was conducted as a retrospective observational study, and the study protocol involved minimal risk and did not threaten the health of the subjects. All participating patients signed informed consent forms.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) fit the diagnostic criteria of herpes zoster; (2) herpes zoster located in the extremities; (3) aged from 18 to 85 years old; (4) moderate and severe pain patients with numerical rating scale scores (NRSs) > 3; and (5) complete clinical data.
The exclusion criteria were as follows: (1) central nervous system-related diseases: such as cerebral infarction, cerebral hemorrhage; (2) nerve compression syndromes: such as carpal tunnel syndrome; (3) skeletal muscle system-related diseases: such as cervical spondylosis, lumbar spondylosis, shoulder periarthritis; and (4) refusal to provide clinical data and information.
Diagnostic Criteria of Motor Dysfunction
MRC score was adopted: 0: no muscle contraction; 1: muscle contraction but no joint activity; 2: full range movement of joint under no gravity; 3: full range movement of joint under anti-gravity state; 4: joint can resist partial resistance activity, but worse than normal; 5: normal muscle strength, that is, joint can achieve full range movement under maximum resistance. If the muscle strength was less than grade 5 and lower than that of the contralateral limb (normal side), it was defined as motor dysfunction, and if the muscle strength was equal to grade 5 or less than grade 5, but the same as the contralateral limb, it was defined as nonmotor dysfunction.
Data Collection
The data of sex, age, course of disease, location of involvement (upper limb/lower limb), number of affected centrums, presence of combined diseases (such as diabetes, immune system disease, cancer), NRS score and treatment (epidural nerve block or pulse radiofrequency of posterior root of spinal nerve) were collected from the hospital database.
Statistical Analysis
Statistical analyses were performed using SPSS software version 26.0 (Chicago, Illinois, USA) and R software version 4.1.0 (The R Foundation for Statistical Computing). All data were tested for normality using the Shapiro–Wilk test and histograms. Normally distributed continuous data are presented as the mean±SD, nonnormally distributed continuous data are presented as medians and interquartile ranges (IQRs), and categorical data are presented as numbers and percentages (%). Independent t-tests were used to compare normally distributed continuous data, while Mann–Whitney U-tests were used for nonnormally distributed continuous data and chi-square tests were used for categorical data. Univariate analysis and multivariate logistic regression analysis were used to analyze the risk factors for motor dysfunction in patients with limb herpes zoster, and the partial regression coefficients with statistical significance were screened to determine the risk factors for motor dysfunction and construct the nomogram. Discrimination and calibration of the prediction model were assessed using the C-index and calibration plot. Internal validation was assessed using bootstrapping validation.
Results
Patient Characteristics
The flow of patients through this retrospective study is summarized in . There were 1264 inpatients with herpes zoster admitted to the pain Department of the First Hospital of Jiaxing from January 2015 to June 2020. Among these patients, 1041 patients with herpes zoster were excluded because herpes zoster was located in chest, abdomen, head and face. After inclusion and exclusion criteria, a total of 213 patients with limb herpes zoster were included, Among them, motor dysfunction occurred in 58 cases, and nonmotor dysfunction occurred in 155 cases. The overall incidence of impaired motor function was 4.59% (58/1264). The details are shown in .
Table 1 Demographic or Characteristic Date of Motor Dysfunction Group and Nonmotor Dysfunction Group
Analysis of the Risk Factors for Motor Dysfunction in Patients with Limb Herpes Zoster
The results of univariate analysis showed that the location of involvement, whether or not diseases were combined and the number of affected centrums were factors related to motor dysfunction in patients with limb herpes zoster (P<0. 05). Sex, age, course of disease, NRS score and treatment were not related to motor dysfunction of limb herpes zoster (P>0. 05). The details are shown in .
Table 2 Univariate Logistic Regression of Motor Dysfunction in Patients with Limb Herpes Zoster
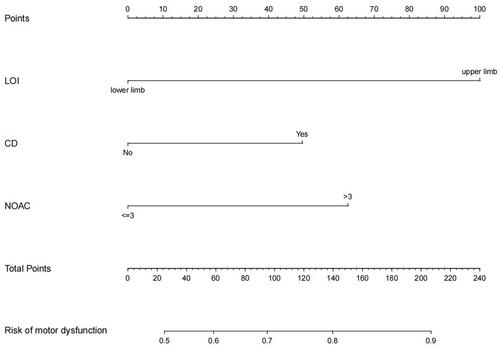
Multivariate logistic regression analysis took the occurrence of motor dysfunction as the dependent variable and the factors of P<0. 05 in as the independent variable. Multivariate logistic regression was performed by the step-by-step method. The results showed that upper limb involvement (OR=3.811, 95% CI=1.829–8.387, P=0.001), noncombined diseases (OR=0.493, 95% CI=0.249–0.969, P=0.041) and the number of affected centrums ≤3 (OR=0.439, 95% CI=0.218–0.881, P=0.020) were independent influencing factors of this complication. The details are shown in .
Table 3 Multivariable Logistic Regression of Motor Dysfunction in Patients with Limb Herpes Zoster
Construction and Application of a Nomogram of Motor Dysfunction in Patients with Limb Herpes Zoster
According to the results of multivariate logistic regression analysis, taking the location of involvement, whether diseases were combined with the number of affected centrums as predictive factors, and the occurrence of motor dysfunction as the clinical outcome, a nomogram was established to predict the risk of motor dysfunction in patients. The details are shown in . Through the values of different variables in the vertical line, the corresponding values are obtained on the score line at the top of the nomogram, and then all variables are added to obtain the total score. Finally, the corresponding predicted risk value is obtained through the total score line on the prediction line at the bottom of the nomogram.
Figure 2 Nomogram for prediction of motor dysfunction in patients with limb herpes zoster.
Nomogram Verification of Motor Dysfunction in Patients with Limb Herpes Zoster
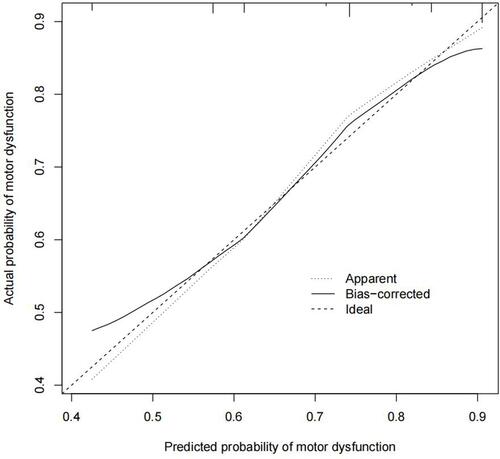
The calibration plot of the motor dysfunction nomogram for the prediction of this complication in limb herpes zoster patients demonstrated good agreement in this cohort, and the calibration plot is shown in . The C-index for the prediction nomogram was 0.72 for the cohort and was confirmed to be 0.71 through bootstrapping validation, which suggested the model’s good discrimination. In the motor dysfunction nomogram, apparent performance addressed a good prediction capability.
Figure 3 Calibration plot.
Discussion
The neurological complications of herpes zoster include postherpetic neuralgia, meningoencephalitis, myelitis, Guillain–Barre syndrome, polycranial neuritis and motor dysfunction.Citation9–Citation13 When varicella-zoster virus invades the anterior nerve root, patients experience decreased muscle strength and motor dysfunction, which usually occurs within a few weeks after the appearance of the rash.Citation14 Since Broadbent first reported a case of motor dysfunction caused by cervical herpes zoster in 1866, a number of cases have been reported,Citation15–Citation18 including literature reporting that the incidence of motor dysfunction in patients with herpes zoster was only 0.3%-5.0%, but Mondelli et al found that more than half of the patients with herpes zoster had limb motor nerve involvement, indicating that a large number of patients were missed.Citation19 In our study, when MRC score rather than electromyography was used, the overall incidence of motor dysfunction reached 4.59% (58/1264), which may be underestimated. Because the lesions of these patients are located in the extremities and the location is special, there is no evidence-based treatment at present, and they cannot be treated with chemical or physical destruction of the corresponding nerve roots, which is more difficult than the treatment of lesions in the chest and abdomen.Citation20–Citation22 This complication seriously reduces the patient’s quality of life and work efficiency, which may lead to permanent disability. Therefore, it is necessary to further identify high-risk patients and take proactive personalized preventive measures according to the risk factors for impaired motor function.
In this study, 8 potential variables were analyzed in 213 patients with limb herpes zoster. The following three independent risk factors were identified: location of involvement, whether diseases were combined and the number of affected centrums. A simple and easy-to-use prediction nomogram for predicting motor dysfunction in patients with limb herpes zoster was developed by multivariate analysis for the first time. Stepwise regression was used to filter out six variables for the line map. The prediction nomogram has good diagnostic performance (C-index=0.72) and has been verified internally using a random sampling method. In addition, the calibration curve shows good consistency.
Thomas and Liu et al reviewed 61 and 8 patients, respectively, and concluded that the upper limb was more prone to motor dysfunction than the lower limb.Citation7,Citation23 Jones et al reviewed 49 patients and found that lower limb involvement was more common, accounting for 55%,Citation24 while Molloy and Cruz-Velarde reviewed 15 and 10 patients, respectively, and concluded that there was no difference in the incidence of motor dysfunction between the upper limb and the lower limb.Citation25 Therefore, in this paper, the location of the lesion (upper limb or lower limb) was included in the study to explore the relationship between the occurrence of upper limb or lower limb and motor dysfunction. There was no difference in the number of cases of motor dysfunction between the left limb or the right limb, so the left and right limbs were not included in the risk factors in this study. In this study, the results of multivariate analysis showed that upper limb involvement was an independent factor affecting the motor function of limb herpes zoster, and the probability of motor dysfunction caused by herpes zoster in the upper limb was 3. 811 times higher than that in the lower limb. The reasons may be related to the following two points. First, from the analysis of neurofunctional anatomy, the movement mode of the lower limb was simple, while that of the upper limb was complex. The increasingly complex labor of human beings makes the nerves innervating the upper limbs become increasingly developed, the division of nerve cells is increasingly detailed, and the virus is more likely to lurk and invade. Then, some of the muscles related to the lower limbs are innervated bilaterally, while the upper limbs are innervated unilaterally, which makes the upper limbs more vulnerable to neuropathy and myopathy.
Noncomplicated disease is a protective factor for motor dysfunction in patients with limb herpes zoster. In particular, basic diseases such as diabetes, immune system diseases and malignant tumors can reduce the immunity of patients, and the spread of the virus is difficult to limit, thus invading the anterior nerve root.Citation26–Citation29 Diabetes easily causes pathological changes in the peripheral blood vessels, thus losing nourishment to the nerves, and the virus is more likely to invade. Immune system diseaseCitation30 is a group of diseases that mainly invade joints, bones, muscles, blood vessels and related soft tissue or connective tissue; such patients are more vulnerable to virus invasion, resulting in decreased motor function. Thomas and Howard found that the incidence of malignant tumors in patients with motor dysfunction caused by herpes zoster is three times higher.Citation23 Malignant tumors and motor dysfunction caused by herpes zoster influence and promote each other,Citation31 which is consistent with the results of this study.
For patients with herpes zoster, the area of rash is an independent factor affecting the occurrence of postherpetic neuralgia.Citation31–Citation33 The smaller the lesion area is, the less likely it is to develop postherpetic neuralgia. For patients with limb herpes zoster, the number of affected centrums ≤ 3 is also a protective factor for motor dysfunction in patients with limb herpes zoster. The number of affected centrums was lower, and the area of the lesion was also correspondingly reduced. The condition of patients with less affected centrums is milder than that of patients with more affected centrums, and complications of impaired motor function do not easily occur.
In addition, some scholars have found that the extent of muscle involvement is consistent with the skin distribution of sensory disorders, but there is no correlation between the severity of motor dysfunction and the severity of pain,Citation34 which is consistent with the results of this study. Akiyama found that herpes zoster patients with motor dysfunction were older than herpes zoster patients without motor dysfunction.Citation35 Jones et al found that the disease occurs more frequently in males than in females, and the elderly are the main disease groups,Citation24 indicating that advanced age and males may be the influencing factors of motor dysfunction in patients with limb herpes zoster. This is not consistent with the results of this study, which may be because limb herpes zoster often occurs in the elderly, and the age differentiation of patients with and without motor dysfunction is not large. In this study, the influencing factor of sex had no significant correlation with the occurrence of motor dysfunction, which may be caused by the small sample size.
In our research, there was no correlation between the course of disease and the occurrence of motor dysfunction, indicating that the process of varicella zoster virus invading the anterior root of the spinal nerve has nothing to do with the course of the disease. In addition, this study also found that there was no correlation between the treatment and the occurrence of motor dysfunction. The main treatment methods in this study are epidural nerve block or pulse radiofrequency of the posterior root of the spinal nerve, both of which have an analgesic effect on the posterior root of the spinal nerve.Citation21,Citation36 However, the effect on the anterior root of the spinal nerve may be weak or absent, so neither of these two treatments can prevent the occurrence of motor dysfunction. Therefore, new treatments and methods used to reduce or even prevent motor dysfunction urgently need to be proposed.
The weakness of motor function in patients with limb herpes zoster is a clinical problem that needs to be paid attention to. For patients with upper limb involvement, underlying diseases and a large number of involved segments (> 3), we should be highly vigilant against the possibility of motor dysfunction. Try to perfect the electromyography and other examinations to observe whether there is motor nerve involvement; patients with high-risk factors should be treated with nutritional nerve therapy as soon as possible to prevent the deterioration of the disease.
Finally, this study still has some limitations. First, the lack of external verification is one of the major limitations of our study. In this case, additional research is needed to replicate and externally verify the results of this study. Second, in this study, the MRC classification method was used for defined motor dysfunction rather than a more objective and accurate neuroelectrophysiological examination, so the diagnosis of motor dysfunction may lack strictness. Third, the sample size is limited. However, the prediction model in this study may still be universal. In the future, it is necessary to further carry out multicenter, prospective, large-sample clinical studies, include more patient data, and compare different algorithm models to establish a more accurate prediction model.
Conclusion
This study used the clinical data available to establish a nomogram model for motor dysfunction of limb herpes zoster and found that motor dysfunction is more likely to occur in patient who meets upper limb involvement, combined diseases and the number of affected centrums > 3. This kind of high-risk group should be intervened as soon as possible.
Acknowledgments
The authors thank the subjects of this study for participating in this study. This work was supported by funding from Zhejiang provincial and municipal construction plan for key disciplines (2019-ss-ttyx) and Jiaxing Key Laboratory of Neurology and Pain Medicine and Jiaxing Science and Technology Bureau (2019AD32150).
Disclosure
The authors declare no conflicts of interest in this work.
References
- Dayan RR, Peleg R. Herpes zoster-typical and atypical presentations. Postgrad Med. 2017;129:567–571. doi:10.1080/00325481.2017.1335574
- John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31:811–826. doi:10.1016/j.idc.2017.07.016
- Le P, Rothberg M. Herpes zoster infection. BMJ. 2019;364:k5095. doi:10.1136/bmj.k5095
- Johnson RW. Consequences and management of pain in herpes zoster. J Infect Dis. 2002;S83–S90. doi:10.1086/342970
- Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18:55–78. doi:10.1111/ddg.14013
- Rosamilia LL. Herpes zoster presentation, management, and prevention: a modern case-based review. Am J Clin Dermatol. 2020;21(1):97–107. doi:10.1007/s40257-019-00483-1
- Liu Y, Wu BY, Ma ZS, et al. A retrospective case series of segmental zoster paresis of limbs: clinical, electrophysiological and imaging characteristics. BMC Neurol. 2018;18(1):121. doi:10.1186/s12883-018-1130-4
- Gupta SK, Helal BH, Kiely P. The prognosis in zoster paralysis. J Bone Joint Surg. 1969;51(4):593–603. doi:10.1302/0301-620X.51B4.593
- Nagel MA, Mahalingam R, Cohrs RJ, et al. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets. 2010;10(2):105–111. doi:10.2174/187152610790963537
- Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system–Prognosis, diagnostics and treatment. J Infect. 2015;71(3):281–293. doi:10.1016/j.jinf.2015.06.004
- Islam B, Islam Z, Geurtsvan Kessel CH, et al. Guillain-Barré syndrome following varicella-zoster virus infection. Eur J Clin Microbiol Infect Dis. 2018;37(3):511–518. doi:10.1007/s10096-018-3199-5
- Niesvizky-Kogan I, Greca I, Gambhir HS. Varicella zoster presenting as cranial polyneuropathy. Am J Emerg Med. 2019;37(3):564.e5–6. doi:10.1016/j.ajem.2018.12.025
- Mercan A, Uzun ST, Keles S, et al. Immunological mechanism of postherpetic neuralgia and effect of pregabalin treatment on the mechanism: a prospective single-arm observational study. Korean J Pain. 2021;34:463–470. doi:10.3344/kjp.2021.34.4.463
- Kawajiri S, Tani M, Noda K, et al. Segmental zoster paresis of limbs: report of three cases and review of literature. Neurologist. 2007;13(5):313–317. doi:10.1097/NRL.0b013e31811e9d6d
- Chen GB, Tuan SH, Liou IH, et al. Segmental zoster paresis of unilateral upper extremity: a case report and literature review. Medicine. 2020;99:e20466. doi:10.1097/MD.0000000000020466
- Teo HK, Chawla M, Kaushik M, Rare A. Complication of herpes zoster: segmental zoster paresis. Case Rep Med. 2016;2016:7827140. doi:10.1155/2016/7827140
- Yoshioka M, Kurita Y, Hashimoto M, et al. A case of segmental zoster paresis with enhanced anterior and posterior spinal roots on MRI. J Neurol. 2012;259:574–575. doi:10.1007/s00415-011-6222-7
- Kim HS, Jung JW, Jung YJ, et al. Complete recovery of herpes zoster radiculopathy based on electrodiagnostic study: a case report. World J Clin Cases. 2021;9:4303–4309. doi:10.12998/wjcc.v9.i17.4303
- Mondelli M, Romano C, Rossi S, et al. Herpes zoster of the head and limbs: electroneumyographic and clinical findings in 158 consecutive cases. Arch Phys Med Rehabil. 2002;83(9):1215–1221. doi:10.1053/apmr.2002.33989
- Dilip M, Paz-Soldan G, Carvajal M, et al. Successful ultrasound-guided erector spinae plane block for herpes zoster in the emergency department: a case report. J Emerg Med. 2021;60:e73–e76. doi:10.1016/j.jemermed.2020.11.006
- Tekin E, Ahiskalioglu A, Aydin ME, et al. High-thoracic ultrasound-guided erector spinae plane block for acute herpes zoster pain management in emergency department. Am J Emerg Med. 2019;37:375.e1–375.e3. doi:10.1016/j.ajem.2018.10.028
- Lee CH, Choi SS, Lee MK, et al. Comparison of the efficacy of continuous epidural block with epidural electrical stimulation and conventional continuous epidural block for management of zoster-associated pain beyond the acute phase: a retrospective study. Medicine. 2019;98:e17026. doi:10.1097/MD.0000000000017026
- Thomas JE, Howard FM. Segmental zoster paresis: a disease profile. Neurology. 1972;22(5):459–466. doi:10.1212/wnl.22.5.459
- Jones LK, Reda H, Waison JC, et al. Clinical, electrophysiologic, and imaging features of zoster-associated limb paresis. Muscle Nerve. 2014;50(2):177–185. doi:10.1002/mus.24141
- Molloy MG, Goodwill CJ. Herpes zoster and lower motor neurone paresis. Rheumatol Rehabil. 1979;18(3):170–173. doi:10.1093/rheumatology/18.3.170
- Saadatian-Elahi M, Bauduceau B, Del-Signore C, et al. Diabetes as a risk factor for herpes zoster in adults: a synthetic literature review. Diabetes Res Clin Pract. 2020;159:107983. doi:10.1016/j.diabres.2019.107983
- Ning L, Liu R, Li S, et al. Increased risk of herpes zoster infection in patients with inflammatory bowel disease: a meta-analysis of cohort studies. Eur J Clin Microbiol Infect Dis. 2020;39:219–227. doi:10.1007/s10096-019-03706-9
- Yasokawa N, Yasuda Y, Chin H, et al. Generalized herpes zoster and cutaneous metastasis during chemotherapy for non-small cell lung cancer: a case report. Thorac Cancer. 2021;12:117–121. doi:10.1111/1759-7714.13722
- Zhang ZY, Deng W, Wu QZ, et al. Tuberculosis, hepatitis B and herpes zoster in tofacitinib-treated patients with rheumatoid arthritis. Immunotherapy. 2019;11:321–333. doi:10.2217/imt-2018-0113
- Ryu HJ, Han JO, Lee SA, et al. Risk factors for herpes zoster in patients with rheumatic diseases: a nationwide cohort study in Korea. Rheumatology. 2021;60:2427–2433. doi:10.1093/rheumatology/keaa636
- Sim JH, Cho HS, Kim YD, et al. The association between herpes zoster and increased cancer risk: a nationwide population-based matched control study. Curr Oncol. 2021;28:2720–2730. doi:10.3390/curroncol28040237
- Wang XX, Zhang Y, Fan BF. Predicting postherpetic neuralgia in patients with herpes zoster by machine learning: a retrospective study. Pain Ther. 2020;9:627–635. doi:10.1007/s40122-020-00196-y
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–1821. doi:10.1016/j.mayocp.2017.10.009
- Papagianni M, Metallidis S, Tziomalos K. Herpes zoster and diabetes mellitus: a review. Diabetes Ther. 2018;9:545–550. doi:10.1007/s13300-018-0394-4
- Akiyama N. Herpes zoster infection complicated by motor paralysis. J Dermatol. 2000;27(4):252–257. doi:10.1111/j.1346-8138.2000.tb02160.x
- Liu DY, Chen JS, Fang ZZ, et al. Pulsed radiofrequency of the trigeminal ganglion for treating postherpetic neuralgia of the ophthalmic branch. Pain Res Manag. 2021;2021:6638392. doi:10.1155/2021/6638392