Abstract
Purpose
This study compared the pharmacokinetic profile, and systemic and local absorption of diclofenac, following dermal patch application and oral administration in Yorkshire-Landrace pigs.
Patients and methods
Twelve anesthetized, female, Yorkshire-Landrace pigs were randomized to receive either the dermal patch (FLECTOR® patch, 10 × 14 cm; Alpharma Pharmaceuticals, a subsidiary of Pfizer Inc, New York, NY) or 50 mg oral diclofenac (Voltaren®; Novartis, East Hanover, NJ). Tissue (skin area of 2 × 2 cm and underlying muscles approximately 2–3 cm in depth) and blood (10 mL) samples were collected at timed intervals up to 11.5 hours after initial patch application or oral administration. The concentrations of diclofenac in plasma, skin, and muscle samples were analyzed using validated ultra performance liquid chromatography tandem mass spectrometric methods.
Results
Peak systemic exposure of diclofenac was very low by dermal application compared with oral administration (maximum concentration [Cmax] values of 3.5 vs 9640 ng/mL, respectively). Absorption of diclofenac into underlying muscles beneath the dermal patch was sustained, and followed apparently zero-order kinetics, with the skin serving as a depot with elevated concentrations of diclofenac. Concentrations of diclofenac in muscles beneath the patch application site were similar to corresponding tissues after oral administration (Cmax values of 879 and 1160 ng/mL, respectively). In contrast to the wide tissue distribution of diclofenac after oral administration, dermal patch application resulted in high concentrations of diclofenac only on the treated skin and immediate tissue underneath the patch. Low concentrations of diclofenac were observed in the skin and muscles collected from untreated areas contralateral to the site of dermal patch application.
Conclusion
Dermal patch application resulted in low systemic absorption and high tissue penetration of diclofenac compared with oral administration.
Introduction
Interest has been growing in topical nonsteroidal anti-inflammatory drug (NSAID) preparations, which involve direct application of the NSAID to the site of injury to mediate localized pain relief. Treatment with topical NSAIDs has been shown to provide clinically effective analgesia at the site of application while minimizing systemic absorption.Citation1,Citation2 The benefits associated with topical NSAIDs include: (1) avoidance of first-pass metabolism and other variables associated with the absorption of drugs through the gastrointestinal tract; (2) reduced systemic side effects; (3) ease of dose termination in the event of adverse events; (4) sustained and controlled drug delivery over an extended period of time; (5) direct access to target site; (6) convenient administration; (7) improved patient acceptance and adherence; and (8) a viable solution for treatment when oral dosing is not possible.Citation3
The diclofenac epolamine topical patch (FLECTOR® patch, 10 × 14 cm; Alpharma Pharmaceuticals, a subsidiary of Pfizer Inc, New York, NY) is a topical patch containing 1.3% diclofenac epolamine, indicated for the treatment of acute pain due to minor strains, sprains, and contusions.Citation1,Citation4–Citation6 The diclofenac epolamine topical patch was found to be more effective than placebo in treating strains, sprains, and contusions,Citation4,Citation7,Citation8 and symptoms of primary osteoarthritis of the knee.Citation2 Diclofenac epolamine topical patch was also shown to be either superior or equivalent to oral diclofenac formulations or placebo for use in osteoarthritis of the knee and soft-tissue injuries.Citation9 In clinical studies, the plasma concentrations (in the order of 1 to 3 ng/mL) and systemic bioavailability (in the order of ≈1%) of the diclofenac epolamine patch were very low compared with oral intake.Citation5 As a result, the diclofenac epolamine topical patch was shown to be well tolerated in clinical studies, with minimal drug-related systemic adverse events.Citation1,Citation4–Citation6
While the efficacy and tolerability of the diclofenac epolamine patch compared with placebo or oral formulations has been previously investigated, there is a lack of data available that directly compares the pharmacokinetic (PK) profile, systemic absorption, and local tissue penetration of the topical preparation with that of oral formulations in the same model system. In the past, pigs have been used as a model for topical drug delivery due to similar fluxes of drugs between human and pig skin.Citation10 Therefore, the primary objective of the current study was to compare the PK profile, and systemic and local absorption of diclofenac epolamine, from a dermal patch and from oral diclofenac sodium, in Yorkshire-Landrace pigs.
Methods
Animal model and experimental design
This study was conducted in twelve adult, female, Yorkshire-Landrace pigs (Mark Lavallée’s Farm, St Charles Borromée, Canada) weighing 16.1 to 19.2 kg at the onset of dosing. All animals were subject to thorough health assessments and clinical evaluations at arrival, prior to randomization, and within 24 hours of dosing. Animals were housed individually in stainless steel cages equipped with an automatic watering system, in a temperature- and humidity-controlled room (21°C ± 3°C and relative humidity of 50% ± 20%), and under a 12-hour light/12-hour dark cycle. A standard, certified, commercial chow (Teklad Certified Miniswine Diet #7037C; Harlan Laboratories Inc, Indianapolis, IN) was provided to the animals twice daily, except during the study procedures. Water (municipal tap water, which had been exposed to ultraviolet light and purified by reverse osmosis) was available to the study animals ad libitum, except during the study procedures. Animals underwent a 6- to 9-day acclimatization period prior to the start of any experimental procedures. The care of the laboratory animals was in accordance with national and international guidelines.
Study design and experimental procedures
Following an overnight fast, animals were randomized to receive either a single application of a dermal patch containing 1.3% diclofenac epolamine (FLECTOR® Patch 10 × 14 cm) (n = 6; group 1) or 50-mg oral diclofenac sodium (Voltaren®; Novartis, East Hanover, NJ) (n = 6; group 2). Animals fasted overnight prior to the day of dosing.
On the day of dosing, the animals were anesthetized with isoflurane gas, and remained under isoflurane anesthesia for the entire dosing and sample collection period. Animals were intubated, and placed on a ventilator supplemented with oxygen (rate: 10–15 breaths/min, pressure: 15–30 cm H2O), or manually ventilated to maintain oxygenation. Local anesthetic (lidocaine spray, 10% w/w) was applied to the glottis before intubation. Intravenous fluid therapy was given throughout the anesthesia (sterile Lactated Ringer’s solution at a rate of 100 mL/h), and body temperature was maintained at approximately 37°C by placing the animals on a heating pad, and monitored during the experimental procedure using a rectal thermometer. Prior to the application of the patch, the animals were sedated using a mixture of ketamine (21.8 mg/kg) and acepromazine (0.22 mg/kg), administered by intramuscular injection.
Treatment
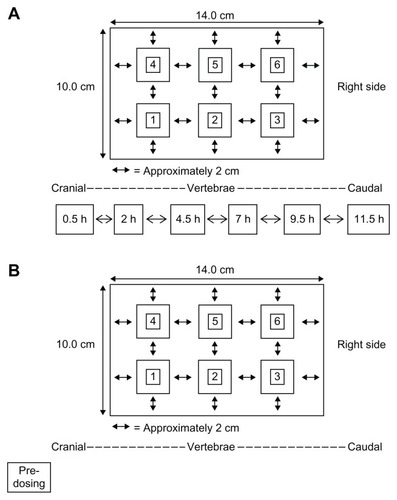
In the animals randomized to group 1, the right- and left-dorsal back of each animal was shaved and cleaned using sterile water prior to dermal patch application. Each patch was divided into six equal pieces (approximately 5.0 × 4.7 cm) and applied to the lower right-dorsal region of the animal in a longitudinal direction, parallel to the spine such that the final area covered by the six pieces was the same size as the original patch (10.0 × 14.0 cm). A schematic for the placement of the dermal patch and the sampling locations for animals in group 1 (dermal treatment) is depicted in .
Figure 1 Schematic for the placement of the dermal patch (divided into six pieces) and sampling for animals in group 1 (dermal treatment group) (A) and group 2 (oral treatment group) (B).
Following confirmation of anesthesia, diclofenac was administered orally to the animals in group 2 as follows: an endoscope was advanced into the gastrointestinal tract of each animal until it was positioned immediately in front of the pylorus; once the pylorus was centered in the field of vision, the distal segment of the endoscope was inserted into the duodenum; a diclofenac tablet was then released into the duodenum and the duodenum flushed with approximately 20 mL of reverse osmosis water.
Skin and muscle sampling
In group 1 animals, full-thickness skin and muscle samples were collected from the dermal patch site (right-dorsal side) at 0.5, 2, 4.5, 7, 9.5, and 11.5 hours post-application. Skin and muscle biopsies were also taken from the left-dorsal side of each animal (as depicted in ) before application of the patch, and at 2, 7, and 11.5 hours post-application.
At each time point one piece of dermal patch was removed and the skin cleaned to remove any residual adhesive present, and then skin/subcutis and epaxial lumbar muscle samples (approximately 2 × 2 cm and 2- to 3-cm depth) were removed from the appropriate site using sterile surgical equipment. Special attention was paid to minimize any cross contamination between the skin/subcutis and the muscle samples by using separate equipment for each skin/subcutis sample and muscle sample. Following excision of the tissue sample, the removed piece of dermal patch was reapplied as closely as possible to the original site. Each skin/subcutis or muscle sample was trimmed and weighed, and stored frozen until later homogenized for analysis.
In animals from group 2, collection of skin and muscle samples was performed using a grid (14 × 10 cm) marked on the right-dorsal lumbar area prior to oral administration of diclofenac. A schematic for the sampling locations for animals in group 2 (oral treatment) is depicted in . As with the animals in group 1, skin/subcutis and epaxial lumbar muscle samples of approximately 2 × 2 cm and 2-to 3-cm depth were removed from the sampling locations using sterile surgical equipment. Each biopsy site was evenly spaced from one another by approximately 2 cm. Samples were taken predose (on the left-dorsal side), and at 0.5, 2, 4.5, 7, 9.5, and 11.5 hours postdose (on the right-dorsal side).
Tissue sample homogenization, extraction, and ultra performance liquid chromatography tandem mass spectrometric analyses
Skin samples were homogenized using a FastPrep 24 tissue homogenizer (MP Biomedicals Inc, Santa Ana, CA) after incubation with 0.1 M sodium hydroxide for 24 hours at 60°C. Muscle samples were homogenized using a FastPrep 24 tissue homogenizer, following the addition of aqueous 25% acetonitrile with 0.001 M sodium hydroxide. Diclofenac in the tissue homogenates was extracted by a liquid–liquid extraction method using ethyl acetate as the extraction solvent, after the addition of hydrochloric acid in water (0.1 M for skin samples or 0.01 M for muscle samples) and an internal standard solution (diclofenac-d4 in 50% aqueous dimethyl sulfoxide). The ethyl acetate extracts were transferred into clean tubes, evaporated to dryness, and reconstituted with 0.1 mL of 50% aqueous dimethyl sulfoxide, then transferred to autosampler vials and analyzed for diclofenac concentrations.
The concentrations of diclofenac in skin and muscle extracts were measured using an ultra performance liquid chromatographic system (Acquity UPLC®, Waters Corporation, Milford, MA) equipped with a triple quadrupole tandem mass spectrometer (API 5000™ AB Sciex LLC, Foster City, CA). Separation of diclofenac was accomplished using a Waters BEH C18 column (2.1 × 100 mm, 1.7 μm) operated at 50°C. The mobile phase consisted of 5 mM ammonium bicarbonate with 0.01% formic acid and 5% methanol in water (mobile phase A), and 100% methanol (mobile phase B). The analysis was performed under isocratic conditions (55% B) with a flow rate of 0.600 mL/min. Mass spectrometric detection of diclofenac (mass transition 296.0→213.9 amu) and its internal standard (diclofenac-d4: mass transition 300.1→213.9 amu) were achieved using multiple reaction monitoring under Positive Ion TurboIonSpray™ mode, using the Analyst® data acquisition software version 1.4.2 (AB Sciex LLC).
In anticipation of the different concentration ranges in the skin and muscle samples with the different treatments, two separate calibration ranges were established for the measurement of diclofenac in skin and muscle homogenates. The “low-level methods” had calibration ranges of 0.0075 to 7.50 ng/mL and 0.00075 to 0.75 ng/mL for skin and muscle homogenates, respectively. The “high-level methods” had calibration ranges of 2.00 to 2000 ng/mL and 1.00 to 1000 ng/mL for skin and muscle homogenates, respectively.
Plasma sampling and diclofenac analyses
Blood samples, collected into tubes containing tripotassium ethylenediaminetetraacetic acid as an anticoagulant, were obtained from all animals via a catheter surgically inserted into the right femoral vein or via venipuncture in the left jugular vein. In both groups 1 and 2, 10 mL blood samples were collected predose, and at 0.5, 2, 4.5, 7, 9.5, and 11.5 hours postdose, and plasma samples were obtained by centrifugation. A 0.5 mL aliquot of the plasma sample was extracted with ethyl acetate after the addition of hydrochloric acid (1 N) and internal standard solution (diclofenac-d4 in 50% dimethyl sulfoxide). The plasma ethyl acetate extracts were processed using similar methods to those used for tissue homogenate extracts.
Plasma concentrations of diclofenac were determined using a validated ultra performance liquid chromatography tandem mass spectrometric method with the same conditions as described for the tissue homogenate analyses. The plasma assay had a calibration curve range of 0.001 to 1.00 ng/mL. Samples above the quantitation limit were diluted into the calibration range with control pig plasma.
All the bioanalytical methods (skin and muscle homogenates, low- and high-level methods, and plasma) were validated according to established best practices for bioanalytical method validation.Citation11–Citation14 Bioanalytical analyses were carried out according to the acceptance criteria outlined in the conference reports and white papers published as a result of the American Association of Pharmaceutical Scientists/Food and Drug Administration Bioanalytical Workshops.Citation12
Pharmacokinetc and statistical analysis
The estimated PK parameters included maximum tissue/plasma drug concentration (Cmax), time at which Cmax was first observed (Tmax), and area under the tissue/plasma drug concentration-time curve from 0 to 11.5 hours (AUC0–11.5 h). Mean plasma or tissue concentration data for diclofenac over time are also presented. The PK parameters of diclofenac in plasma, skin, and muscle from individual pigs were calculated using a noncompartmental approach in WinNonlin (version 6.1, Pharsight Corporation, Mountainview, CA). Mean PK parameters were calculated using Excel 2007 (Microsoft Corporation, Redmond WA). The AUC values were estimated using the linear trapezoidal rule.Citation15 Data are presented as mean and standard deviation (SD) for each treatment group. No statistical analyses were conducted on data obtained in this study.
Results
Bioanalytical method performance
The analytical performance (precision and accuracy) of the bioanalytical methods for the determination of diclofenac in skin and muscle homogenates, and in plasma samples, was assessed by the overall bias and coefficient of variation values of the quality control samples analyzed with each batch of study samples. The analytical performance results are summarized in . The average percent bias and percent coefficient of variation for the measurement of diclofenac were within ±10% for all five methods used in analyzing tissue and plasma samples from this study; this indicated that diclofenac was measured with a high degree of precision and accuracy despite the very low concentrations (ie, low pg/mL) in some samples (eg, very low plasma concentrations in the dermal treatment group and low skin concentrations in the oral treatment group) and the wide range of concentrations observed between the two treatment groups.
Table 1 Analytical performance of the UPLC/MS-MS methods used for the analyses of diclofenac in tissue homogenates and plasma samples from Yorkshire-Landrace pigs
PK profile of diclofenac in plasma, skin, and muscle after oral or dermal administration
The mean PK parameters of diclofenac in plasma, skin, and muscle after oral or dermal administration are shown in . In animals treated with the dermal patch, the mean Tmax was observed at 5.3, 6.7, and 5.2 hours in plasma, treated muscle, and treated skin, respectively. In animals that received the oral dose, the mean Tmax was observed at 2 hours in the plasma and muscle, and at 3.3 hours in the skin.
Table 2 PK parameters of diclofenac in female Yorkshire-Landrace pigs following oral administration or dermal patch application
Systemic and local absorption of diclofenac by dermal or oral administration
After a 50-mg oral dose of diclofenac was administered to Yorkshire-Landrace pigs, the mean (±SD) plasma Cmax was 9640 (±906) ng/mL (). Maximum concentrations were achieved at 2 hours (Tmax) after oral dosing. The mean (±SD) AUC0–11.5 h was 46,000 (±4817) ng · h/mL. The PK of oral diclofenac observed in this study are similar to those reported in Yucatan miniature pigs given a 50-mg oral dose of diclofenac.Citation16
Systemic absorption of diclofenac, as assessed by Cmax and AUC, was lower following administration by dermal patch compared with oral dosing (dermal/oral Cmax ratio: 0.0004; dermal/oral AUC ratio: 0.0005) (; ). Systemic availability following dermal patch administration was also subject to greater variability than oral administration.
Figure 2 Diclofenac concentrations in the plasma (A), muscle (B), and skin (C) of female Yorkshire-Landrace pigs following oral administration or dermal patch application.
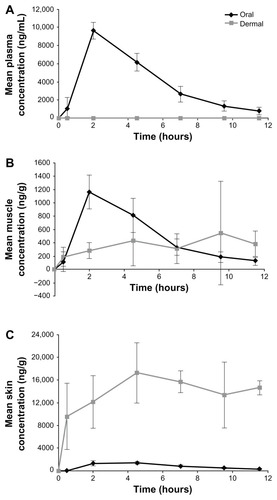
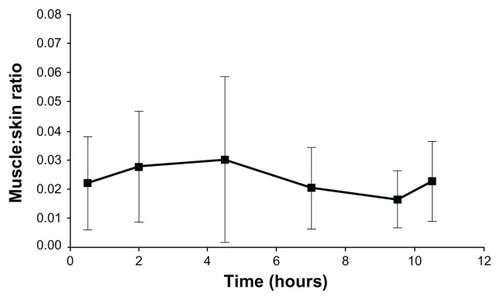
In the right-dorsal muscles, similar concentrations of diclofenac were achieved in animals by either the dermal or oral route of administration (dermal/oral Cmax ratio: 0.76; dermal/oral AUC ratio: 0.70) ( and ). The exposure to diclofenac was greater in skin biopsies from the right-dorsal patch application sites than in biopsies obtained following oral administration (dermal/oral Cmax ratio: 12.8; dermal/oral AUC ratio: 16.8) ( and ). In the dermal treatment group, the muscle to skin concentration ratios on the right side were more or less constant between 0.5 and 11.5 hours (), suggesting that the dermal absorption of diclofenac was sustained and followed apparent zero-order kinetics, with the diclofenac absorbed into the skin serving as a depot to deeper tissue layers such as the muscles.
Figure 3 Muscle to skin concentration ratios of diclofenac after the application of dermal patch in Yorkshire-Landrace pigs.
Following oral administration, skin diclofenac concentrations were similar to those recorded in muscle samples. However, unlike the wide tissue distribution of diclofenac after oral administration, high concentrations of diclofenac were only observed in skin and muscle samples on the right-dorsal (treated) side, whereas very low concentrations were observed in tissues from the left-dorsal (untreated) side. The differences in tissue concentrations between the treated and untreated sides demonstrate that diclofenac was absorbed locally from the dermal patch and penetrated to the underlying muscle layer, with minimal distribution to other tissues beyond the application site.
Discussion
This study was conducted to compare the PK of diclofenac by dermal versus oral administration in the same experimental model. The systemic and local absorption of diclofenac administered by a diclofenac epolamine 1.3% patch and oral diclofenac sodium (50-mg single oral dose) were evaluated in Yorkshire-Landrace pigs. Due to the morphological and physiological similarities between human and porcine skin, and the general comparative biology between humans and pigs, the Yorkshire-Landrace pig is a robust model for the evaluation of dermal absorption.Citation10,Citation17
The results showed very low systemic exposures of diclofenac when administered by dermal patch compared with oral administration. Plasma concentrations (mean Cmax ≈ 3.5 ng/mL by dermal patch) obtained in this study are comparable with those reported in healthy human subjectsCitation5 and, therefore, confirm the suitability of this animal model. Steady-state plasma diclofenac concentrations in studies of healthy human subjects were achieved before day 3 of patch administration (2 patches/day; t1/2 9–12 hours) and were approximately 1 to 3 ng/mL.Citation5 In healthy human volunteers, the Cmax values observed between 0 and 12 hours following twice-daily 180-mg diclofenac epolamine patch application for four consecutive days was 1.55 ng/mL.Citation18 Peak plasma concentrations of diclofenac following patch application have been reported to be 0.2% to 8.0% of those observed following oral dosing.Citation5,Citation19
Low systemic exposure to topical diclofenac is of clinical importance since it may result in reduced rates of dose-dependent NSAID-associated risks, such as gastrointestinal and cardiovascular events.Citation1 Topical NSAID preparations must effectively penetrate the stratum corneum of the skin to reach their site of action in the underlying musculoskeletal soft tissue and peripheral nerves.Citation1 More importantly, concentrations of diclofenac in underlying tissues following topical administration have not been measured successfully in clinical studies. Therefore, it is not clear whether the concentrations of diclofenac in deeper layers (eg, muscles) beneath the epidermis are due to direct penetration from the patch, or delivered via systemic circulation.Citation20 This study provides direct evidence of the absorption and tissue penetration of diclofenac from the dermal application site to the underlying tissues.
Data from the current study demonstrated the successful dermal absorption of diclofenac into the skin, penetration into underlying muscles (2–3 cm beneath the skin’s surface), and limited systemic bioavailability or distribution of diclofenac to other tissues beyond the immediate patch application site. Diclofenac concentrations on the right-dorsal side were comparable among animals treated with the dermal and oral routes of administration, showing that sufficient diclofenac exposure was achieved in muscles with the dermal patch relative to an efficacious oral dose of 50 mg. Low concentrations of diclofenac were observed in skin and muscle biopsies from the left (untreated) side of animals receiving the dermal patch. These results show that the muscle concentrations are due to direct drug absorption from the dermal patch and penetration to muscles rather than to delivery through systemic circulation. Physicochemical properties of the epolamine salt formulation of diclofenac used in the patch may act to enhance local tissue penetration; the epolamine salt is more soluble in both water and nonpolar solvents than other diclofenac salts, and also demonstrates surfactant behavior that may increase membrane permeability.Citation21
The PK data from the present study and previous evaluations in human subjects are largely supportive of the clinical data that have demonstrated the effectiveness of the diclofenac epolamine patch in the treatment of soft-tissue injuries, coupled with an acceptable safety and tolerability profile.Citation4,Citation7,Citation8,Citation22 Using a relevant animal model, this study directly demonstrated the low systemic absorption and high tissue penetration of diclofenac by dermal patch application compared with oral administration. Topical formulations such as the diclofenac epolamine patch may represent an effective and well-tolerated alternative for the treatment of soft-tissue injury.
Authorship contributions
Susanna Tse contributed to the analysis and interpretation of the data, and is the primary author responsible for the manuscript. Kendall D Powell contributed to the analysis and interpretation of the data, and critical revision of the manuscript. Stephen MacLennan, Allan R Moorman and Craig Paterson contributed to the design of the study and critical revision of the manuscript. Rosonald Bell contributed to the interpretation of the data and critical revision of the manuscript. All authors gave final approval of the version to be published.
Acknowledgments
The authors would like to thank Doug Rickert and CiToxLAB Group (formally LAB Research North American Inc) for their contribution to the study.
This study was sponsored by Pfizer Inc. Editorial support was provided by C Campbell, PhD, of PAREXEL, and was funded by Pfizer Inc.
Disclosure
Susanna Tse is an employee of Pfizer Inc. Kendall D Powell is an employee of Tandem Labs, A LabCorp Company. Stephen MacLennan is an employee of BioCryst Pharmaceuticals Inc. Allan R Moorman was an employee of Pfizer Inc (legacy King Pharmaceuticals, Inc) at the time of the study; he is currently an employee of Alta Vetta Pharmaceutical Consulting, LLC. Craig Paterson is an employee of Salix Pharmaceutical, Inc. Rosonald Bell is an employee of Pfizer Inc.
The authors report no other conflicts of interest in this work.
References
- McCarbergBHArgoffCETopical diclofenac epolamine patch 1.3% for treatment of acute pain caused by soft tissue injuryInt J Clin Pract201064111546155320666849
- BookmanAAWilliamsKSShainhouseJZEffect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trialCMAJ2004171433333815313991
- StanosSPTopical agents for the management of musculoskeletal painJ Pain Symptom Manage200733334235517349504
- LionbergerDRBrennanMJTopical nonsteroidal anti-inflammatory drugs for the treatment of pain due to soft tissue injury: diclofenac epolamine topical patchJ Pain Res2010322323321197326
- PetersenBRovatiSDiclofenac epolamine (Flector) patch: evidence for topical activityClin Drug Investig200929119
- RainsfordKDKeanWFEhrlichGEReview of the pharmaceutical properties and clinical effects of the topical NSAID formulation, diclofenac epolamineCurr Med Res Opin200824102967299218814824
- GalerBSRowbothamMPeranderJDeversAFriedmanETopical diclofenac patch relieves minor sports injury pain: results of a multi-center controlled clinical trialJ Pain Symptom Manage200019428729410799795
- LionbergerDRJoussellinELanzarottiAYanchickJMagelliMDiclofenac epolamine topical patch relieves pain associated with ankle sprainJ Pain Res20114475321559350
- BanningMTopical diclofenac: clinical effectiveness and current uses in osteoarthritis of the knee and soft tissue injuriesExpert Opin Pharmacother20089162921292918937623
- QvistMHHoeckUKreilgaardBMadsenFFrokjaerSEvaluation of Göttingen minipig skin for transdermal in vitro permeation studiesEur J Pharm Sci2000111596810913754
- BansalSDeStefanoAKey elements of bioanalytical method validation for small moleculesAAPS J200791E109E11417408234
- ShahVPMidhaKKFindlayJWBioanalytical method validation – a revisit with a decade of progressPharm Res200017121551155711303967
- ViswanathanCTBansalSBoothBQuantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assaysPharm Res200724101962197317458684
- Food and Drug AdministrationGuidance for Industry: Bioanalytical Method ValidationUS Department of Health and Human Services, FDA, Center for Drug Evaluation and Research2001 Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdfAccessed June 5, 2012
- GibaldiMPerrierDPharmacokineticsNew YorkMarcel Dekker Inc1975
- OberleRLDasHWongSLChanKKSawchukRJPharmacokinetics and metabolism of diclofenac sodium in Yucatan miniature pigsPharm Res19941156987038058639
- BodeGClausingPGervaisFfor Steering Group of the RETHINK ProjectThe utility of the minipig as an animal model in regulatory toxicologyJ Pharmacol Toxicol Methods201062319622020685310
- GschwendMHMartinWArnoldPDetermination of the transdermal bioavailability of a newly developed diclofenac sodium patch in comparison with a reference preparationArzneimittelforschung200555740341316080280
- HeynemanCALawless-LidayCWallGCOral versus topical NSAIDs in rheumatic diseases: a comparisonDrugs200060355557411030467
- SinghPRobertsMSSkin permeability and local tissue concentrations of nonsteroidal anti-inflammatory drugs after topical applicationJ Pharmacol Exp Ther199426811441518301551
- FiniAFazioGGonzalez-RodriguezMCavallariCPasseriniNRodriguezLFormation of ion-pairs in aqueous solutions of diclofenac saltsInt J Pharm1999187216317310502622
- JenourePSegesserBLühtiUGremionGA trial with diclofenac HEP plaster as topical treatment in minor sport injuriesDrugs Exp Clin Res19931931251318112201