Abstract
Purpose
Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) present with a broad spectrum of symptoms, including headache. A simple, yet powerful tool – the pain drawing identifies essential aspects such as pain distribution. The aim with this study was to 1) evaluate the significance of pain drawing as a screening tool for cervicogenic headache using a predefined C2 pain pattern, 2) assess whether there was an association between dizziness/imbalance and a C2 pain pattern, and 3) compare subgroups according to the pain drawing with respect to pain characteristics and quality of life.
Patients and Methods
Pain drawings and clinical data from 275 patients investigated for ME/CFS were stratified into: 1) cervicogenic headache as determined by a C2 pain pattern, 2) headache with no C2 pain pattern, and 3) no headache. For inference logistic regression presented with odds ratios (OR) and 95% confidence intervals (95% CI) and Kruskal–Wallis test were applied.
Results
One hundred sixteen participants (42%) were stratified to the group for which the pain drawing corresponded to the C2 pain pattern, thus indicating putative cervicogenic origin of the headache. Dizziness/imbalance was strongly associated with a C2 pain pattern; OR 6.50 ([95% CI 2.42–17.40] p ˂ 0.00), whereas this association was non-significant for patients with headache and no C2 pain pattern. Those demonstrating a C2 pain pattern reported significantly higher pain intensity (p = 0.00) and greater pain extent (p = 0.00) than the other groups, and lower health-related quality of life (p = 0.00) than the group with no headache.
Conclusion
For patients with chronic fatigue who present with a C2 pain pattern (interpreted as cervicogenic headache) the pain drawing seems applicable as a screening tool for signs associated with neuropathic and more severe pain, dizziness and reduced quality of life as detection of these symptoms is essential for targeted treatment.
Introduction
The chronic fatigue syndrome (CFS), also referred to as myalgic encephalomyelitis (ME),Citation1 is characterized by post-exertional malaise, severe fatigue and sleep disturbance, problems with thinking and concentration, as well as pain, headache and dizziness.Citation2,Citation3 These patients have reported their quality of life to be very low.Citation4,Citation5 Headache that is centered at the craniocervical junction can arise from diseases or disorders that involve any of the tissues in the neck such as muscles, ligaments, joints, nerves and bones. Cervicogenic headache is thought to be referred pain arising from irritation caused by cervical structures innervated by spinal nerves C1, C2, and C3; thus, any structure innervated by the C1–C3 spinal nerves could be the origin of cervicogenic headache.Citation6,Citation7 Such secondary cervicogenic headache radiates from the neck to the back of the head and may spread along the scalp to the forehead, temple and area around the ear and/or eye.Citation8 Cervical findings have been linked to neurological symptoms among patients with ME/CFSCitation9,Citation10 and may give rise to persistent painCitation11 and headacheCitation12 along with impaired balance.Citation13–15
Pain drawing is an important part of the clinical assessment to help determine whether the pain is neuropathic in nature, as this is assessed in a step modelCitation16 where a pain drawing is mandatory in step 1 (verifying whether the pain has a neuroanatomically plausible distribution). In a previous study on the use of pain drawing in headache diagnostics,Citation17 the pain locality in patients with migraines was predominantly in the forehead and temples, while in cervicogenic headache usually suboccipitally, though sometimes overlapping.Citation17 Pain drawing as a diagnostic measuring instrument is under development.Citation18 A few studies have explored clinical applications with regard to the cervical spine, starting 2007, where the interrater reliability and between-session reliability was deemed adequate.Citation19–24 Using pain pattern recognition to guide spinal level specific diagnostics has very limited evidence.Citation22,Citation25 Involvement of the second cervical nerve root, hereafter referred to as C2 involvement, however, may be identified by the corresponding characteristic pain pattern,Citation17,Citation26–31 and the sometimes-accompanying symptoms such as dizziness. Critical for the function of the balance system is input from the vast number of mechanoreceptors of the upper neck joints and surrounding soft tissuesCitation32–35 (informing of head movement versus trunk) mediated via C2.Citation36,Citation37 As the vestibular nuclei compose central vestibular outputs on the grounds of information from mechanoreceptors, vestibular organs of the inner ear, and visual system, abnormal C2 input can give rise to distorted experience of movement or balance sense.Citation38–41
Dizziness sometimes accompanies neck pain or related disordersCitation42 but pain in the upper neck can nonetheless occur without comorbidity of dizziness. Therefore, the assessment of patients with this type of symptoms would most likely benefit from screening with pain drawing along with balance measures. Determining the most plausible pain mechanisms is essential as this can serve as a guide in selecting the most appropriate treatment.
The aim with this study was to (1) evaluate the significance of pain drawing as a screening tool for cervicogenic headache using a predefined C2 pain pattern, (2) assess whether there was an association between dizziness/imbalance and a C2 pain pattern, and (3) compare subgroups according to the pain drawing with respect to pain characteristics and quality of life.
Materials and Methods
This single-center non-experimental cross-sectional correlational study was conducted at a private, publicly funded specialist clinic for ME/CFS where patients with persistent fatigue from all of Sweden are investigated. All study participants were referred from primary care physicians. Recommendations for referral were based on: If specialist medical care was necessary, if rehabilitation with a team-based approach was considered necessary, or in cases of unresolved diagnostics after initial management in primary healthcare.
Participants
Participants were recruited to a cross-sectional research project aiming to improve the investigation of patients with persistent pain and fatigue. All adults (≥18 years) with suspected ME/CFS who gave their written informed consent to participate in the project were consecutively enrolled from February 2019 to March 2020. Inclusion criteria were I) severe fatigue affecting physical and/or mental functioning for a minimum of 3 months; and II) being admitted for investigation at the specialist clinic. Exclusion criteria were I) any clinical condition judged not to be compatible with following the investigation, eg, known alcohol or drug abuse, severe psychiatric symptoms (comorbidity, previous surgical treatment allowed) and; II) barriers to communicating in English or Swedish. The entire project population (n = 278) was engaged in the present study, inferring some redundancy of data as the statistical requirements for this analysis were approximately 100 participants, based on a prevalence of C2 involvement of about 15% among study participants (prevalence of cervicogenic headache in the general population 2–10% (Denmark, Norway, USA)Citation43,Citation44 and approximately 3 times more common among patients at the recruiting clinic, ie, 6–30%); and a confidence interval of 95%.Citation45 This study was conducted in accordance with the Helsinki Declaration.Citation46 All inclusion of patients’ data was approved by the Swedish Ethical Review Authority (2018/1754).
Data Collection
Data was collected prior to (mailed surveys) and during the first visits to the clinic. The pain drawing was included in a set of questionnaires used to report to the National Quality Registry for Pain Rehabilitation (NRS).
Outcome Variables
Headache
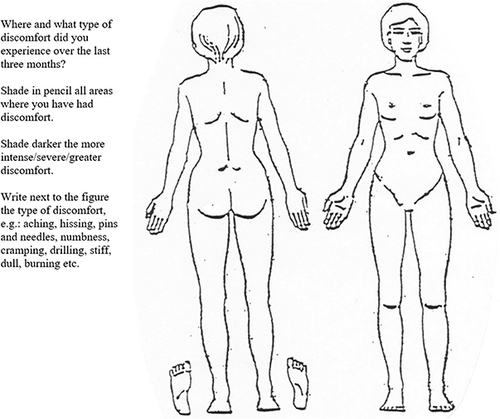
Was assessed with the paper pain drawing (the patient’s same drawing used for body pain extent, below) that has two silhouettes depicting the human body (frontal and dorsal view) ().Citation23 Markings in any part of the head, including the scalp and face was interpreted as headache. The drawing had an instruction that indicated the past three months and read to, “shade in pencil all areas where you have had discomfort”. Patients were also asked if appropriate to indicate next to the figure pain descriptors common in neuropathic pain,Citation47 for example, “pins and needles” or “dull pain”. Pain descriptors, marks outside the silhouette or marks made to direct attention to pain characteristics were not noted in the assessment.
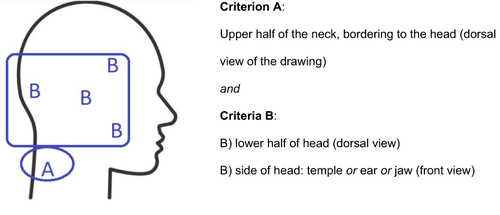
To address the research aim, a standardized screening key was defined to identify a C2 pain patternCitation26,Citation48–50 (). An identified C2 pain pattern meant that both criteria A and B were met. Criterion B was met if one locality had been marked, or two or more in combination. Criteria A and B must be on the same side (right/left). In cases where the entire silhouette was filled out, side of the face must have a marking. If in dorsal view the entire head had been filled out, the lower half of the head must be in a darker shade (indicating increased pain intensity). The drawings were screened by a medical nurse independent of the project and one of the researchers (GB). In the event of disagreement in interpretation (yes/no), a joint reassessment was made to reach consensus. Patients were stratified according to the pain drawing into three subgroups: 1) cervicogenic headache as determined by markings on the pain drawing corresponding to a C2 pain pattern, 2) headache with no C2 pain pattern, and 3) no headache.
Pain Intensity
Patients reported their current pain intensity with a paper version of the 100 mm visual analogue scale (VAS): endpoints 0 = “no pain” and 100 = “worst possible pain”.Citation51 The instruction read, “How much pain are you experiencing at this moment? (Please mark with an X on the line)”.
Body Pain Extent
It was assessed with the paper pain drawing (the patient’s same drawing used for headache, above), using the body region method by Margolis,Citation52 where the drawing is divided into 45 anatomical regions. Each region is assigned a percent surface area, aggregated into a sum score 0–100%.
Dizziness/Imbalance
Verbally reported dizziness or imbalanceCitation53 by the patient at the first visit to the physician was assessed from the patients’ medical record. Dizziness or imbalance was logged as present regardless of phrasing by the attending physician. Examples of record entries (four different physicians): “All along he has had […] and balance issues”, “In addition, dizziness.”, “Bouts of dizziness.”, and “Sometimes when she experiences […] her gait tends to become wide.”.
Health-Related Quality of Life
Self-reported current health status was assessed with a paper version of the Euroqol 100 mm vertical VAS (EQ-VAS) with the endpoints 0 = “worst imaginable health” and 100 = “best imaginable health”.Citation54
Data Analyses
Descriptive data were analyzed using non-parametric statistics due to the non-normal distribution of data. Interval data was handled as inherently ordinal variables.Citation55 To avoid possible bias from excluding all cases where values were missing, we used MICECitation56 with 10 imputations using predictive mean matching for creating the VAS replacement data. For EQ-VAS, stochastic regression imputation was used.Citation56 Binomial logistic regressionCitation57 was used to analyze association between the dependent variable dizziness/imbalance (yes/no) and the independent variables pain pattern (categorized), pain intensity (continuous) and body pain extent (continuous) and covariates age (19–59 years/≥60 years) and gender, presented with odds ratio (OR) and 95% confidence intervals (95% CI). The significance of the differences between subgroups as evaluated with pain drawings (above) was determined by Kruskal–Wallis tests.Citation58
Level of significance was set to p < 0.05. All data analysis was performed in R version 4.1.1 (2021–08-10) Copyright ©2021 The R Foundation for Statistical Computing.Citation59
Results
Over a period of 13 months (February 2019–March 2020), 278 subjects agreed to participate in the study. Three cases where study participants had refrained from symptom-reporting with the pain drawing were excluded from the analysis. For the remaining 275 participants, missing data consisted of 30 reports (11%) of self-rated quality of life, and 4 reports of pain intensity (1,5%). An overview of participants’ characteristics is presented in .
Table 1 Characteristics of the Study Participants (n = 275)
Each of the 275 drawings had a unique marking distributed over the figure. Drawings with the largest pain extent included all body quadrants and the axial area, and some drawings were blank. The assessors were in agreement for 96% of drawings in their interpretation by means of the standardized C2 pattern pain drawing key (yes/no). For 12 drawings, there was a renewed joint assessment by discussion until consensus was reached. Of all participants, 116 (42%) had markings in the pain drawing that corresponded to the specific C2 pain pattern ().
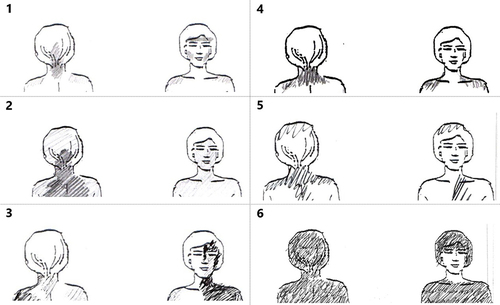
Figure 3 Drawings from study participants. To the left (1–3) are examples where C2 involvement was assessed to be present. To the right (5–6) are drawings with examples of a negative outcome. (1) Criterion A is met (upper neck) and 3 of criteria B (pain involving back of head, both jaws and left temple). (2) Criterion A is met and 1 of criteria B: back of head. (3) Criterion A is met and 2 of criteria B: ear and jaw. (4) Criterion A is met, not B. (5) Criterion A is met, not B. (6) Neither A or B is met (homogenous shading, ie, no pain punctum maximum in C2 supplied areas).
The proportion of patients in the total population who reported having dizziness or imbalance was 25%. Consequently, the remaining 75% did not report dizziness/imbalance in the initial consultation. There was a strong association between dizziness/imbalance and a C2 pattern, as compared to the group with no headache: OR 6.50 (95% CI 2.42–17.40, p-value: 0.00), and to the group with headache without the C2 pain pattern: OR 3.15 (95% CI 1.54–6.41, p-value: 0.00) (). There was no difference between men and women (p > 0.05), and no effect of any of the parameters age, pain intensity and body pain extent (p > 0.05). In the case of headaches without C2 pain patterns, the odds for dizziness were shown to be higher than for patients whose drawings indicated no headache, although a difference within the margin of error (not statistically significant).
Table 2 Logistic Regression of Associations Between Having Dizziness/Imbalance and Headaches Among Patients in Investigation for Possible ME/CFS
presents the differences between the three pain pattern subgroups. These results showed that the outcomes with respect to pain and quality of life differed significantly depending on the subgroup (p < 0.05). The group that presented with the pre-defined C2 pattern had higher pain intensity (VAS median 66.5 mm, range 49.0–79.0), and more vast body pain extent (61.4%, range 44.6–76.3), than the other groups. Consistently, the group that presented with a C2 pattern in pain drawing had significantly lower self-rated quality of life (EQ-VAS median 26.5 mm, range 20.0–38.3) than those who presented with no headache according to the pain drawing (p = 0.00).
Table 3 Comparison Between Scores of Pain and Health-Related Quality of Life of the Three Subgroups Based on the Pain Drawing
Discussion
The findings demonstrated that a C2 pattern (putative cervicogenic headache) as determined by pain drawing was common among patients with chronic fatigue. Complaints of dizziness/imbalance were more prevalent in this group as compared to patients without a C2 pain pattern. Besides, self-reported pain was worse in patients who did present with the C2 pain pattern. The results thus imply that there might be a cervical spinal nerve involvement in patients with chronic fatigue who present with cervicogenic headache. Similar findings have been reported previously.Citation10,Citation60 Lindal et alCitation60 studied common pain localities in a group of women with CFS after many years of illness, and found neck pain to be the most common site. Rowe et alCitation10 studied surgical treatment for three patients diagnosed with severe ME/CFS and concluded that cervical stenosis could be part of the pathogenesis of ME/CFS. Studies have also shown a link between central sensitization and persistent symptoms after a neck injury.Citation61,Citation62 Central sensitization includes increased pain sensitivity and generalized pain.Citation63 This was paralleled in the present study, in that measures that were rated high among patients (pain intensity and body pain extent), were associated with self-reported cervical symptoms, suggestive of the C2 pain pattern reflecting or indicating a condition in the upper neck.
The link between dizziness/imbalance and cervicogenic headache, the latter in this study measured as a C2 pattern with a pain drawing, is in agreement with earlier research,Citation64 including whiplash-associated disordersCitation65 as well as ME/CFS:Citation9,Citation66 Serrador et alCitation66 found impaired balance in standing to be present in CFS (27 patients compared with 22 matched controls). They also found that a decreased vestibular function occurred with a comorbidity of fibromyalgia.Citation66 A hallmark of fibromyalgia is central sensitization.Citation67 Thus, in theory, the covariation in the study by Serrador et alCitation66 between imbalance and a worse degree of pain seems to have been in harmony with our results. No firm conclusions can however be drawn from such extrapolation. Matsui et alCitation9 evaluated treatment of cervical musculature in ME/CFS through low-frequency electrical stimulation and far-infrared irradiation. The greatest effect was achieved in relation to vertigo or dizziness, nausea or stomachache, and depression (recovery rate more than 70%). Their results have clear points of contact with the present study in that the neck had significance for the health status and that there was a covariation between neck and dizziness. Gustavsen et alCitation42 studied associations between musculoskeletal pain, dizziness and health-related quality of life, with pain as the dependent variable. They found that musculoskeletal pain was associated with increasing dizziness, and the combination of neck pain and dizziness was proposed as a possible trigger of persistent postural-perceptual dizziness (PPPD).Citation68 PPPD is classified as a chronic vestibular disorder,Citation68 but because no structural abnormality has yet been identified, it is so far called functional, ie, the vestibular system operates irregularly although we do not know why. A painful abnormality of the joint capsules and deep muscles at the craniocervical junction might indeed disrupt the normal functions of the central vestibular system, as it combines not only vestibular and visual but also somatosensory signals from all muscles and joints and foremost of all the upper neck.Citation69
Quality of life was shown to be worse for the group with headaches and a C2 pattern than for the group without headache. This is in line with previous research of negative effects on perceived health from headacheCitation70 as well as from dizziness, and where patients found the impaired function to be an important factor, rather than emotional aspects.Citation71,Citation72
Using pain drawing to examine patients with chronic fatigue has been investigated in some previous studies. Lindal et alCitation60 studied common pain localities in CFS with the same method used in the present study for the assessment of body pain extent, to divide the pain drawing into 45 body regions.Citation52 No pain in the suboccipital area was however found in the study by Lindal et al.Citation60 Though not mentioned by the authors, about 30% had reported headache, although in the two regions, each representing the entire half side of the head in frontal view, thus providing an unspecified description of headaches (unknown whether a marking referred to the forehead, temple or jaw, etc.).Citation60 Another small case study (12 patients with fibromyalgia)Citation73 studied pain treatment by means of pain drawing (counting painful body regions and measuring total painful area with the help of a grid). However, specifics about the outcomes were not reported. There are challenges in studying the usefulness of new interpretations of pain drawing with properties that aim to aid in the screening of patients. The drawings used in research often have different designs, why results can be limited to the study context, rather than contributing to a refinement of standard drawing design. This limits the usefulness of historical comparisons. However, to evaluate modes of interpretation which can be applied successfully regardless of the drawing’s design is very valuable as was done in our study.
A strength with the present study is that the variable for dizziness was free from attention-induced bias. We chose not to include data from the patient pre-admission questionnaire for the Canadian Consensus Criteria, section for autonomous manifestations (“Dizziness and/or lightheadedness”)Citation74 likely to have low content validity. If checklists are used to gather information from patients, bias can come from some psychosocial events, shown for example for symptom reporting after concussions.Citation75 Mechanisms are believed to be that expectations become etiology, a tendency to overestimate your good health earlier in life, and to exaggerate symptoms.Citation75 Some studies support spontaneously reported symptoms as a reliable source of information, representing a more sensitive index of patients’ actual problems than can be obtained from structured surveys.Citation76,Citation77 It is unknown whether attention-induced bias appeared regarding the VAS, pain drawing and EQ-VAS instruments. It is conceivable that the pain drawing could be favored (comparatively lower degree of attention-induced bias) due to the relatively open-ended instruction of how to fill out the drawing. Moreover, option to compare the epidemiology of ME/CFS across studies is limited since the diagnostic criteria used vary,Citation78 but the study population was representative in terms of age range (ME/CFS most common between 40 and 60 years) and gender distribution (about 80% women) according to the Centers for Disease Control and Prevention.Citation2
The study’s limitations were that the data derived from medical records were logged by several physicians where quality and accuracy were likely to vary, and it cannot be ruled out that dizziness/imbalance existed in more patients but did not emerge. The study group had been investigated within the primary health-care system prior to being referred to the specialist clinic, and thus asked to join the study, which implies that other causes of dizziness/imbalance had been excluded (other than a cervicogenic cause), but no action was taken in the study to verify the reported symptoms using a more objective measurement method, which would be needed to assess any under- or overreporting. Generalization of results must therefore be made with caution. The confidence interval showed a wide range for the actual odds ratio of the entire population of interest to fall within, an effect of the relative occurrence of events within the sample. A more extensive sample size with a nearer to normal distribution of the sample odds ratios would improve precision. Despite the result being imprecise, it has clinical value, and suggests that a pain drawing in a straightforward and manageable test can be used for mapping parts of the nature and the severity of conditions. It can be one way to increase the effectiveness in the investigation of people who have persistent non-malignant pain.
The association shown here between dizziness/imbalance and the C2 pain pattern is supportive to a limited degree of the C2 pain pattern being condition specific and thereby potentially valuable as a complementary tool for understanding the pathophysiology of headaches and associated disorders.
Conclusion
For patients with chronic fatigue who present with a C2 pain pattern (interpreted as cervicogenic headache) the pain drawing seems applicable as a screening tool for signs associated with neuropathic and more severe pain, dizziness and reduced quality of life as detection of these symptoms is essential for targeted treatment.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors would like to thank all the study participants for making this work possible and senior statistical consultant Måns Thulin for his helpful comments and review of this paper.
References
- Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100. doi:10.1186/s12967-020-02269-0
- Myalgic encephalomyelitis/Chronic fatigue syndrome. Centers for disease control and prevention. Atlanta, GA: Myalgic encephalomyelitis/Chronic fatigue syndrome; 2022. Available from: https://www.cdc.gov/me-cfs/index.html. Accessed August 24, 2022.
- Ravindran MK, Zheng Y, Timbol C, Merck SJ, Baraniuk JN. Migraine headaches in chronic fatigue syndrome (CFS): comparison of two prospective cross-sectional studies. BMC Neurol. 2011;11:30. doi:10.1186/1471-2377-11-30
- Hvidberg MF, Schouborg Brinth L, Olesen AV, Petersen KD, Ehlers L. The health-related quality of life for patients with myalgic encephalomyelitis/ chronic fatigue syndrome (ME/CFS). PLoS One. 2015;10(7):e0132421. doi:10.1371/journal.pone.0132421
- Eaton-Fitch N, Johnston SC, Zalewski P, Staines D, Marshall-Gradisnik S. Health-related quality of life in patients with myalgic encephalomyelitis/chronic fatigue syndrome: an Australian cross-sectional study. Qual Life Res. 2020;29(6):1521–1531. doi:10.1007/s11136-019-02411-6
- Silverberg ND, Martin P, Panenka WJ. Headache trigger sensitivity and avoidance after mild traumatic brain injury. J Neurotrauma. 2019;36(10):1544–1550. doi:10.1089/neu.2018.6025
- Lane R, Davies P. Post traumatic headache (PTH) in a cohort of UK compensation claimants. Cephalalgia. 2019;39(5):641–647. doi:10.1177/0333102418812091
- Al Khalili Y, Ly N, Murphy PB. Cervicogenic Headache. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Matsui T, Hara K, Iwata M, et al. Possible involvement of the autonomic nervous system in cervical muscles of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). BMC Musculoskelet Disord. 2021;22(1):419. doi:10.1186/s12891-021-04293-7
- Rowe PC, Marden CL, Heinlein S, Edwards CC. Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis. J Transl Med. 2018;16(1):21. doi:10.1186/s12967-018-1397-7
- Glassford JA. The Neuroinflammatory etiopathology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Physiol. 2017;8:88. doi:10.3389/fphys.2017.00088
- He L, Yue J, Yang L, et al. Cervicogenic headache alleviation after cervical coblation nucleoplasty: a prospective cohort study. Medicine. 2016;95(39):e4786. doi:10.1097/MD.0000000000004786
- Olszewski J, Majak J, Pietkiewicz P, Luszcz C, Repetowski M. The association between positional vertebral and basilar artery flow lesion and prevalence of vertigo in patients with cervical spondylosis. Otolaryngol Head Neck Surg. 2006;134(4):680–684. doi:10.1016/j.otohns.2005.11.023
- Yacovino DA, Hain TC. Clinical characteristics of cervicogenic-related dizziness and vertigo. Semin Neurol. 2013;33(3):244–255. doi:10.1055/s-0033-1354592
- Henderson FC, Francomano CA, Koby M, Tuchman K, Adcock J, Patel S. Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg Rev. 2019;42(4):915–936. doi:10.1007/s10143-018-01070-4
- Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
- Uthaikhup S, Barbero M, Falla D, Sremakaew M, Tanrprawate S, Nudsasarn A. Profiling the extent and location of pain in migraine and cervicogenic headache: a cross-sectional single-site observational study. Pain Med. 2020;21(12):3512–3521. doi:10.1093/pm/pnaa282
- Shaballout N, Neubert TA, Boudreau S, Beissner F. From paper to digital applications of the pain drawing: systematic review of methodological milestones. JMIR Mhealth Uhealth. 2019;7(9):e14569. doi:10.2196/14569
- Bertilson B, Grunnesjö M, Johansson SE, Strender LE. Pain drawing in the assessment of neurogenic pain and dysfunction in the neck/shoulder region: inter-examiner reliability and concordance with clinical examination. Pain Med. 2007;8(2):134–146. doi:10.1111/j.1526-4637.2006.00145.x
- Southerst D, Stupar M, Côté P, Mior S, Stern P. The reliability of measuring pain distribution and location using body pain diagrams in patients with acute whiplash-associated disorders. J Manipulative Physiol Ther. 2013;36(7):395–402. doi:10.1016/j.jmpt.2013.05.023
- Barbero M, Moresi F, Leoni D, Gatti R, Egloff M, Falla D. Test-retest reliability of pain extent and pain location using a novel method for pain drawing analysis. Eur J Pain. 2015;19(8):1129–1138. doi:10.1002/ejp.636
- Bernhoff G, Landén Ludvigsson M, Peterson G, Bertilson BC, Elf M, Peolsson A. The pain drawing as an instrument for identifying cervical spine nerve involvement in chronic whiplash-associated disorders. J Pain Res. 2016;9:397–404. doi:10.2147/JPR.S104747
- MacDowall A, Robinson Y, Skeppholm M, Olerud C. Pain drawings predict outcome of surgical treatment for degenerative disc disease in the cervical spine. Ups J Med Sci. 2017;122(3):194–200. doi:10.1080/03009734.2017.1340372
- Pitance L, De Longhi B, Gerard E, et al. Digital pain drawings are a useful and reliable tool for assessing patients with temporomandibular disorders. J Oral Rehabil. 2021;48(7):798–808. doi:10.1111/joor.13168
- Albert HB, Hansen JK, Søgaard H, Kent P. Where do patients with MRI-confirmed single-level radiculopathy experience pain, and what is the clinical interpretability of these pain patterns? A cross-sectional diagnostic accuracy study. Chiropr Man Therap. 2019;27:50. doi:10.1186/s12998-019-0273-8
- Haldeman S, Dagenais S. Cervicogenic headaches: a critical review. Spine J. 2001;1(1):31–46. doi:10.1016/s1529-9430(01)00024-9
- Bogduk N, Govind J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8(10):959–968. doi:10.1016/S1474-4422(09)70209-1
- Johnston MM, Jordan SE, Charles AC. Pain referral patterns of the C1 to C3 nerves: implications for headache disorders. Ann Neurol. 2013;74(1):145–148. doi:10.1002/ana.23869
- Fujiwara Y, Izumi B, Fujiwara M, et al. C2 spondylotic radiculopathy: the nerve root impingement mechanism investigated by para-sagittal CT/MRI, dynamic rotational CT, intraoperative microscopic findings, and treated by microscopic posterior foraminotomy. Eur Spine J. 2017;26(4):1073–1081. doi:10.1007/s00586-016-4710-2
- Baek IC, Park K, Kim TL, Yang HM, Kim SH, Kim SH. Comparing the injectate spread and nerve involvement between different injectate volumes for ultrasound-guided greater occipital nerve block at the C2 level: a cadaveric evaluation. J Pain Res. 2018;11:2033–2038. doi:10.2147/JPR.S172692
- Xiao H, Peng B, Ma K, et al. The Chinese Association for the Study of Pain (CASP): expert consensus on the cervicogenic headache. Pain Res Manag. 2019;2019:9617280. doi:10.1155/2019/9617280
- Wyke B. Neurology of the cervical spinal joints. Physiotherapy. 1979;65(3):72–76.
- McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19(5):495–501. doi:10.1097/00007632-199403000-00001
- Karlberg M, Magnusson M, Malmström EM, Melander A, Moritz U. Postural and symptomatic improvement after physiotherapy in patients with dizziness of suspected cervical origin. Arch Phys Med Rehabil. 1996;77(9):874–882. doi:10.1016/s0003-9993(96)90273-7
- Boyd-Clark LC, Briggs CA, Galea MP. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine. 2002;27(7):694–701. doi:10.1097/00007632-200204010-00005
- Yin W, Willard F, Dixon T, Bogduk N. Ventral innervation of the lateral C1-C2 joint: an anatomical study. Pain Med. 2008;9(8):1022–1029. doi:10.1111/j.1526-4637.2008.00493.x
- Edwards IJ, Lall VK, Paton JF, et al. Neck muscle afferents influence oromotor and cardiorespiratory brainstem neural circuits. Brain Struct Funct. 2015;220(3):1421–1436. doi:10.1007/s00429-014-0734-8
- de Jong PT, de Jong JM, Cohen B, Jongkees LB. Ataxia and nystagmus induced by injection of local anesthetics in the Neck. Ann Neurol. 1977;1(3):240–246. doi:10.1002/ana.410010307
- Blouin J, Okada T, Wolsley C, Bronstein A. Encoding target-trunk relative position: cervical versus vestibular contribution. Exp Brain Res. 1998;122(1):101–107. doi:10.1007/s002210050496
- Lackner JR, DiZio P. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol. 2005;56:115–147. doi:10.1146/annurev.psych.55.090902.142023
- Armstrong B, McNair P, Taylor D. Head and neck position sense. Sports Med. 2008;38(2):101–117. doi:10.2165/00007256-200838020-00002
- Gustavsen I, Wilhelmsen K, Goode AP, et al. Dizziness and physical health are associated with pain in dizzy patients-A cross-sectional study. Physiother Res Int. 2021;26(4):e1923. doi:10.1002/pri.1923
- Page P. Cervicogenic headaches: an evidence-led approach to clinical management. Int J Sports Phys Ther. 2011;6(3):254–266.
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from Government Health Studies. Headache. 2018;58(4):496–505. doi:10.1111/head.13281
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi:10.1001/jama.2013.281053
- Heraughty M, Ridehalgh C. Sensory descriptors which identify neuropathic pain mechanisms in low back pain: a systematic review. Curr Med Res Opin. 2020;36(10):1695–1706. doi:10.1080/03007995.2020.1790349
- Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: a study in normal volunteers. Spine. 1990;15(6):453–457. doi:10.1097/00007632-199006000-00004
- Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. 2019;20(1):76. doi:10.1186/s10194-019-1023-y
- Lawson GE, Nolet PS, Little AR, et al. Medial branch blocks for diagnosis of facet joint pain etiology and use in chronic pain litigation. Int J Environ Res Public Health. 2020;17:21. doi:10.3390/ijerph17217932
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi:10.1016/0304-3959(83)90088-X
- Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65. doi:10.1016/0304-3959(86)90026-6
- Reiley AS, Vickory FM, Funderburg SE, Cesario RA, Clendaniel RA. How to diagnose cervicogenic dizziness. Arch Physiother. 2017;7:12. doi:10.1186/s40945-017-0040-x
- Brooks R. EuroQol: the current state of play. Health Policy (New York). 1996;37(1):53–72. doi:10.1016/0168-8510(96)00822-6
- Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand J Pain. 2016;13:67–75. doi:10.1016/j.sjpain.2016.06.012
- Van Buuren S. Flexible Imputation of Missing Data. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2018.
- Schober P, Vetter TR. Logistic regression in medical research. Anesth Analg. 2021;132(2):365–366. doi:10.1213/ANE.0000000000005247
- Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. doi:10.1080/01621459.1952.10483441
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.R-project.org/. Accessed August 24, 2022.
- Líndal E, Bergmann S, Thorlacius S, Stefánsson JG. The localization of pain in chronic fatigue syndrome on a pain drawing according to grid areas. Percept Mot Skills. 1996;83(2):508–510. doi:10.2466/pms.1996.83.2.508
- Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. 2013;17(3):299–312. doi:10.1002/j.1532-2149.2012.00193.x
- Malfliet A, Kregel J, Cagnie B, et al. Lack of evidence for central sensitization in idiopathic, non-traumatic neck pain: a systematic review. Pain Physician. 2015;18(3):223–236.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
- Devaraja K. Approach to cervicogenic dizziness: a comprehensive review of its aetiopathology and management. Eur Arch Otorhinolaryngol. 2018;275(10):2421–2433. doi:10.1007/s00405-018-5088-z
- Landén Ludvigsson M, Peterson G, Widh S, Peolsson A. Exercise, headache, and factors associated with headache in chronic whiplash: analysis of a randomized clinical trial. Medicine. 2019;98(48):e18130. doi:10.1097/MD.0000000000018130
- Serrador JM, Quigley KS, Zhao C, Findley T, Natelson BH. Balance deficits in chronic fatigue syndrome with and without fibromyalgia. NeuroRehabilitation. 2018;42(2):235–246. doi:10.3233/NRE-172245
- Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
- Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res. 2017;27(4):191–208. doi:10.3233/VES-170622
- Brandt T, Bronstein AM. Cervical vertigo. J Neurol Neurosurg Psychiatry. 2001;71(1):8–12. doi:10.1136/jnnp.71.1.8
- van Suijlekom HA, Lamé I, Stomp-van den Berg SGM, Kessels AGH, Weber WEJ. Quality of life of patients with cervicogenic headache: a comparison with control subjects and patients with migraine or tension-type headache. Headache. 2003;43(10):1034–1041. doi:10.1046/j.1526-4610.2003.03204.x
- Voorde MT, van der Zaag-Loonen HJ, Van Leeuwen RB. Dizziness impairs health-related quality of life. Qual Life Res. 2012;21(6):961–966. doi:10.1007/s11136-011-0001-x
- Hermansen A, Peolsson A, Hedlund R, Kammerlind A-S. Balance problems and dizziness after neck surgery - associations with pain and health-related quality of life. Physiother Theory Pract. 2020;36(10):1145–1152. doi:10.1080/09593985.2019.1571137
- Thimineur M, De Ridder D. C2 area neurostimulation: a surgical treatment for fibromyalgia. Pain Med. 2007;8(8):639–646. doi:10.1111/j.1526-4637.2007.00365.x
- Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international Consensus Criteria. J Intern Med. 2011;270(4):327–338. doi:10.1111/j.1365-2796.2011.02428.x
- Edmed SL, Sullivan KA. Base rates of postconcussion syndrome by method of symptom report. Appl Neuropsychol Adult. 2012;19(3):164–170. doi:10.1080/09084282.2011.643961
- Xu JQ, Choy JCP, Tang JYM, et al. Spontaneously reported symptoms by informants are associated with clinical severity in dementia help-seekers. J Am Geriatr Soc. 2017;65(9):1946–1952. doi:10.1111/jgs.14931
- Crochard A, El Hasnaoui A, Pouchain D, et al. Diagnostic indicators of restless legs syndrome in primary care consultations: the DESYR study. Mov Disord. 2007;22(6):791–797;quiz 907. doi:10.1002/mds.21325
- Estévez-López F, Mudie K, Wang-Steverding X, et al. Systematic review of the epidemiological burden of myalgic encephalomyelitis/chronic fatigue syndrome across Europe: current evidence and EUROMENE research recommendations for epidemiology. J Clin Med. 2020;9(5):1557. doi:10.3390/jcm9051557