Abstract
Neuropathic pain constitutes a significant portion of chronic pain. Patients with neuropathic pain are usually more heavily burdened than patients with nociceptive pain. They suffer more often from insomnia, anxiety, and depression. Moreover, analgesic medication often has an insufficient effect on neuropathic pain. Spinal cord stimulation constitutes a therapy alternative that, to date, remains underused. In the last 10 to 15 years, it has undergone constant technical advancement. This review gives an overview of the present practice of spinal cord stimulation for chronic neuropathic pain and current developments such as high-frequency stimulation and peripheral nerve field stimulation.
Introduction
Neuropathic pain constitutes a significant portion of chronic pain. The International Association for the Study of Pain defines neuropathic pain as pain caused by a lesion or disease of the somatosensory nervous system. Patients with neuropathic pain usually are more heavily burdened than patients with nociceptive pain and suffer more often from insomnia, anxiety, and depression.Citation1 Moreover, analgetic medication often has an insufficient effect on neuropathic pain.Citation2
Spinal cord stimulation (SCS) constitutes a therapy alternative that, to date, remains underused.Citation3,Citation4 It was described for the first time in 1967 and has become a standard therapy in many hospitals since the middle of the 1980s. Nonetheless, there is still a significant underuse of this treatment. In the last 10–15 years, SCS has undergone constant technical advancement. Stimulation patterns can now be adjusted according to the patient’s needs, thus increasing the efficacy of stimulation.
SCS for the treatment of pain must be seen in the general context of neuromodulative therapies. Neuromodulation has undergone rapid development in the last few years. Today, neurostimulation systems are used not only in the therapy of chronic pain but also in a multitude of different disorders, such as epilepsy, psychiatric diseases, and movement disorders, as well as gastrointestinal and urological diseases. This review gives an overview of the present practice of SCS for chronic neuropathic pain and current developments in the field.
Outline of the history of SCS and its uses
The development of SCS was one of the consequences of the gate control theory of Melzack and Wall.Citation5 They stated that external and internal pain stimuli are recorded by pain receptors in the skin, muscles, joints, and internal organs and switched to the second neuron of the pain pathway within the dorsal horn of the spinal cord. Here, many peripheral neurons converge to a single neuron termed the wide dynamic range neuron. According to their theory, activation of myelinated Aβ fibers inhibits pain transmission and is enhanced by activation of thinly myelinated Aδ fibers and unmyelinated C fibers.Citation5
Shealy et al employed SCS in 1967 for the first time in an animal model, using a technique that they termed dorsal column stimulation at that time. They showed that dorsal column stimulation, as well as stimulation of the anterior spinocerebellar tract, inhibited paw withdrawal after painful stimuli in cats.Citation6 In the same year, they treated a patient who suffered from intractable pain resulting from a progressive-state inoperable bronchial carcinoma. Electrode implantation was performed over a thoracic laminectomy at the level TH 2/3. After starting the stimulation, pain was drastically reduced and analgetic medication could be stopped. The patient died after 6 days from a previously undetected bacterial endocarditis.Citation7 In 1970, Shealy et al published a series of six patients with various diagnoses who had been treated with SCS; three of them had a very good outcome.Citation8
Nashold and Friedman published the first larger study on SCS in 1972. In this study, patients with neuropathic pain responded better to stimulation than patients with nociceptive pain such as bone pain, joint pain, and discal pain.Citation9 The same year, a two-step procedure with percutaneous testing of the electrode before final implantation of the impulse generator was proposed by Hosobuchi et al.Citation10 A study of 50 patients published in 1974 showed that SCS led to better results in phantom pain than in peripheral nerve lesions.Citation11 Before 1980, intra- or subdural electrodes were often employed,Citation12 sometimes leading to grave complications such as intraspinal bleedingCitation13 or spinal cord damage.Citation14 Therefore, great caution is required in comparing the treatment data from that time with those of our time.
Mechanisms of action
The first explanation of the effects of SCS according to the gate control theory was that the nociceptive signal in the dorsal horn would be inhibited by antidromic activation of collateral fibers of the dorsal columns. This explanation later turned out to be only partially true. According to this explanation, acute nociceptive pain would be inhibited most effectively by SCS, which in fact is not the case. In addition, the pain-free interval after cessation of stimulation cannot be explained in that way. Apparently, there is also an orthodromic stimulation, which becomes manifest in the paresthesia felt by the patient under stimulation. Supraspinal centers are also involved in SCS effects.Citation15 At the time the gate control theory was first described, there was only a little knowledge about supraspinal control of pain transmission, and SCS was thought to act at the segmental level.Citation16
In animal models, a couple of possible mechanisms of action have been described. Overexcitability of wide dynamic range (WDR) neurons in the dorsal horn can at least partially be overcome by SCS.Citation17 This seems to be related to an increased basal release of glutamate and to a dysfunction of the γ-amino-butyric acid (GABA) system. SCS counteracts this mechanism by increased GABA release. However, GABA release was only observed in animals that also had pain relief effected by SCS.Citation18 Moreover, SCS led to a decreased extracellular glutamate concentration in the dorsal horn.Citation19 Here, activation of the GABAB receptor seems to play a crucial role.Citation19–Citation21 Cholinergic transmitter systems are also involved in SCS effects. Release of acetylcholine was observed under SCS and later attributed to activation of the M4 muscarine receptor.Citation22 Moreover, low doses of muscarine receptor agonists led to amplification of the SCS effects in rats,Citation23 and it was shown that serotonergic pain-modulating descending pathways were involved in this effect.Citation24
Moreover, supraspinal centers are also engaged in the effect of SCS. Here, in particular, a dorsal column– brainstem–spinal loop is of importance.Citation25–Citation27 Barchini et al recently addressed the question of the relative proportions of supraspinal and segmental mechanisms in SCS effects. They studied the effect of SCS on neuropathic pain after lesioning the dorsal columns and stimulating rostral and caudal to the lesion under the influence of different receptor antagonists. They found that segmental as well as supraspinal mechanisms contribute to the effects of SCS and that stimulation rostral and caudal to the lesion leads to activation of different synaptic circuits and transmitter systems.Citation16
The question of how stimulation of the spinal ganglia and SCS differ clinically and electrophysiologically was studied by Guan et alCitation28 by means of single-cell electrodes in WDR neurons. They found that SCS inhibited wind-up in the WDR neurons, whereas stimulation of the spinal ganglia did not. The effect of SCS continued, at least partially, for about 30–45 minutes after cessation of the stimulation.Citation28
Patient selection/screening, including psychological
SCS can have diverging clinical effects in different patients. It has been known for many years that some patients do not profit from SCS, but others do. Therefore, a two-step procedure with a test phase before implantation of the impulse generator (IPG) has become routine in most hospitals since the 1970s. It has become apparent that about 17%–20% of the patients have a negative trial resultCitation29 and do not proceed to IPG implantation, even when the test electrode has the optimal position and the paraesthesia coverage of the painful region is complete. Moreover, SCS effects diminish over time after IPG implantation in some patients. The extent of decrease of SCS effect over time is reported variously in the literature. Some studies state that the effect has decreased 25%–50% after 2 years,Citation30 but others only see a slight loss of efficacy over time.Citation31 Often technical factors such as electrode problems (dislocation, breakage) have been cited to explain the loss of efficiency. However, psychological factors also come into account with regard to both the loss of efficacy over time and a negative trial result.
Several guidelines recommend that psychological testing should be performed before SCS implant. The first aim of this testing is to rule out major psychiatric diseases such as major depression, psychosis, or substance abuse. There is a vast consensus that patients with these diagnoses should not be subjected to SCS testing. However, it remains to be determined whether there are also psychological factors below the level of severe pathology, which can negatively influence SCS efficacy. Regarding this question, the literature remains somewhat inconclusive; regarding the long-term efficiency, there are studies that find a negative correlation between the level of depression and SCS efficacy,Citation32,Citation33 whereas others do not.Citation30,Citation33
In contrast, the level of depression remains one of the functional items that can show remarkable improvements after SCS implantation. Thus, even if SCS efficacy might be slightly lower in patients with mild depression, IPG implantation might be justified because apart from pain relief, depression can also improve under SCS.Citation31
Pre- and postimplant advice to patients
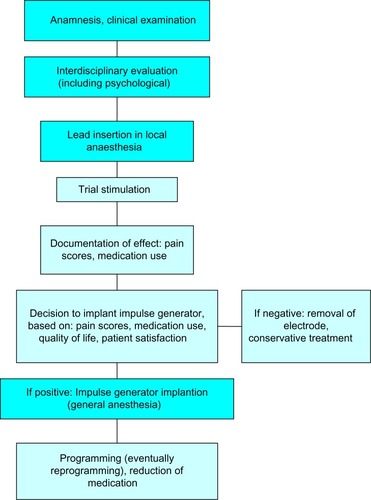
Preoperatively, the indication should be considered cautiously, taking the prior conservative medical physiotherapeutic and psychotherapeutic approaches into account. Differential diagnosis also requires determining the proportions of nociceptive and neuropathic pain; for instance, by means of diagnostic nerve root blocks. SCS should be considered when the radicular (neuropathic) part of the pain outweighs the nociceptive portion ().
Some guidelines, moreover, claim interdisciplinary decision-making before testing SCS. At least a psychological examination should be carried out before SCS implantation in any case. The psychological examination should not only contribute to decision-making about whether or not to implant the electrode, but should also prepare the patient for the postoperative course and render it easier for the patient to deal with having an implantable device.
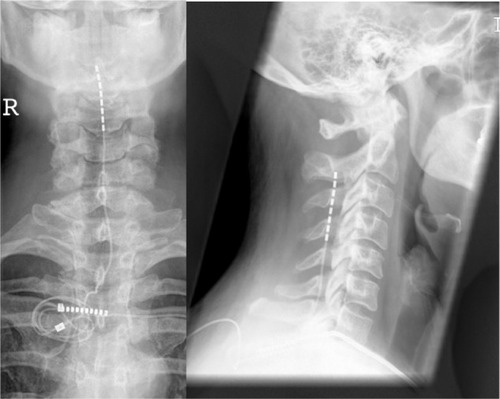
Electrode implantation usually is performed under local anaesthesia, using percutaneous-type electrodes, which can be inserted under fluoroscopic guidance. First, the spinal canal is punctured with a Touhy needle. Once the spinal canal is reached, intraspinal localization of the needle tip is ensured by means of a saline probe, a technique that can be strongly recommended to ensure the safety of the procedure and to prevent cerebrospinal fluid leaks. Then, the electrode is advanced to the desired level. In the case of leg pain, the usual electrode position is TH10–12 (). In thoracic pain (such as postherpetic neuralgia), the electrode position depends on the level of the pathology, and in brachial pain, final electrode position is at level C3–C6 (). Once the electrode is in the desired position, intraoperative test simulation is performed and the patient is asked to state where he feels the stimulation-induced paresthesia. At this stage, it is most important to obtain full paresthesia coverage of the painful area. Sometimes the electrode position must be changed before the optimal paraesthesia is achieved.
Figure 2 Anteroposterior and lateral view of a thoracolumbar spinal cord stimulation placement; the 8-pole lead (octrode) is positioned at level TH10–12, and the impulse generator is placed abdominally subcutaneously.
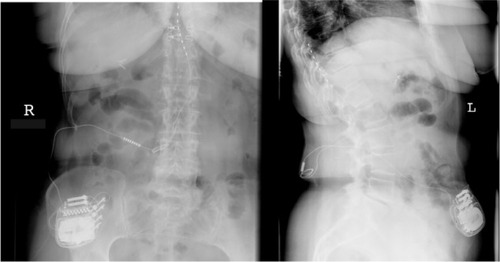
Figure 3 Anteroposterior and lateral view of a cervical spinal cord stimulation placement; the 8-pole lead (octrode) is positioned at level C3-5, and the spinal canal is entered at level TH2/3.
The patient then proceeds to a test phase, which usually lasts about 6–12 days. Some hospitals also prefer testing under domestic circumstances. Mostly, however, testing is performed in the hospital. The patient is asked to document his or her pain scores during the test phase. The decision whether or not to implant an IPG is dependent on whether or not more than 50% pain reduction is obtained, whether quality of life and moods are improved, whether analgetic medication can be reduced, and whether the patient wants the implant.
After implantation of the IPG, reprogramming of the device often is needed one or more times. Patients should be informed that slight changes in the SCS-induced paresthesia can occur and that these can be relieved by reprogramming the device. Patients often ask whether they should avoid physical activities to avoid electrode dislocation. In our experience, the risk for electrode dislocation is highest in the first few weeks after implantation, so we would not recommend heavy lifting or intense bending of the spine in this stage. After implantation of the IPG, the analgetic medication should also be reconsidered and possibly stopped. The patient can also better determine over time which simulation pattern, intermittent or continuous, is most suitable in his situation.Citation34
Current technological developments
Burst stimulation
One new development in SCS refers to the order and frequency of impulses. De Ridder et alCitation35 published a series of 12 patients who had been treated by means of a “burst stimulation”. This pattern of stimulation consists in so-called “bursts” of 5 impulses of 1 ms duration followed by a 1 ms interval, applied at a frequency of 500 Hz. These 5-impulse “bursts” are applied at 40 Hz. Under this stimulation pattern, pain was strongly relieved compared with using conventional stimulation. Moreover, no stimulation-induced paresthesia was necessary to obtain a pain-relieving effect. Furthermore, unlike “conventional” SCS, not only leg pain but also back pain was relieved.Citation35,Citation36
High-frequency stimulation
Recently, a prospective multicenter study on high-frequency SCS (continuous stimulation with 10 kHz) showed favorable results.Citation37 In this new technique, two octrodes are implanted in a staggered manner at level TH8 down to TH12. It has been observed that not only radicular leg pain but also back pain can be treated in this way. Eighty-two patients were treated in the study. An IPG was implanted in 72 patients. The mean intensity of back pain decreased from 8.2 to 2.7 on the visual analog scale and the mean intensity of the leg pain decreased from 5.4 to 1.4. The results also remained stable after 24 months.Citation38 One peculiarity of this stimulation technique is that no paresthesia is perceived by the patient. The effectivity of high-frequency stimulation was explained by desensitization of hyperactive WDR neurons and with control of the wind-up phenomenon of WDR neurons. However, no experimental evidence for this assumption was provided.Citation37 Moreover, a randomized study that compared a 5 kHz stimulation with a sham stimulation in a blinded manner did not find significant differences between the two stimulation techniques.Citation39
At the present stage, it is probably too early to draw final conclusions on the pain-relieving efficiency of high-frequency stimulation. However, as long as there is no clear neurophysiological hypothesis explaining the intense effect and the paresthesia-free pain relief, some reservation toward this new method might be indicated.
Position-adaptive SCS
The intensity of the SCS-induced paresthesia is dependent on body position.Citation40–Citation42 Under equal technical settings of the stimulator, the paresthesia is perceived more intensely in the supine position than in an upright position. Therefore, up to now, patients had to correct changes in stimulation intensity (ie, while standing up) by means of a handheld programming device. This position dependency of the stimulation is not caused by a dislocation of the electrode. In fact, it is based on the variable thickness of the cerebrospinal fluid layer around the spinal cord. Holsheimer et alCitation43 pointed out that the position of the spinal cord within the spinal canal exhibited considerable interindividual differences. By means of a computer model, they calculated the expected thresholds for stimulation on the basis of anatomical and radiological data. The calculated data corresponded to the thresholds measured in individual patients.Citation43 Likewise, Abejon and FelerCitation44 found that the impedance of the electrode is not dependent on the body position. Thus, changes in body position do not interfere with changes in the electrical conductivity of the electrode and the directly adjacent tissues.Citation44,Citation45
Recently, new position-adaptive stimulation devices have been introduced. These devices are able to detect whether the patient is lying down or standing. The stimulation intensity is then automatically fitted to previously set values. With this technique, there is no longer any need for the patient to adjust the stimulation intensity after changing his position.
A study with 15 patients showed that patients with automatic sensor-driven stimulation were significantly more satisfied than with manually adapted stimulation.Citation46 A recent multicenter study with 79 patients showed functional improvements; in particular, enhanced comfort during position changes, increased physical activity, and improved sleep.Citation47
Novel peripheral neurostimulation techniques
Two new developments of neurostimulation are addressed here that actually do not constitute a type of SCS, as the peripheral nerves or the dorsal root ganglion are stimulated rather than the spinal cord. However, these techniques share most of the indications and use much of the same hardware. Therefore, they should be mentioned in the context of SCS.
Peripheral nerve field stimulation
Stimulation of a single peripheral nerve has been employed for more than 30 years in pain treatment.Citation48 With this technique, the affected peripheral nerve (ie, N. ulnaris) is exposed and an electrode (ie, a cuff-electrode) is placed directly adjacent to the nerve. Alternatively, a percutaneous-type electrode can be inserted under the epineurium.Citation49
Peripheral nerve field stimulation (PNFS) is a different method. Here the electrode is placed subcutaneously, with no direct relation to a particular peripheral nerve. One of the first applications of this method was occipital nerve stimulation (ONS) for chronic migraineCitation50 or chronic cluster headache.Citation51–Citation54 Here, however, there are different opinions about whether ONS is a peripheral nerve stimulation or a PNFS. In fact, the electrode is mostly placed adjacent to the occipital nerve in ONS, but as this is not done under visual control, this ONS is probably a form of PNFS rather than peripheral nerve stimulation.Citation55
PNFS in a way closes a therapeutic gap in neuromodulative pain treatment, as it can possibly reach the trunk more effectively than SCS. First publications on PNFS dealt with chronic abdominal pain.Citation56,Citation57 Later, studies concerning chronic thoracic pain,Citation58,Citation59 chronic low back pain,Citation60–Citation63 failed back surgery syndrome (FBSS),Citation64,Citation65 and sacroiliac joint painCitation66 also were published.
PNFS has been described in a couple of case reports,Citation58,Citation59,Citation67 smaller cases series,Citation56,Citation62,Citation65 and some studies with higher case numbers.Citation61,Citation64,Citation68 A retrospective multicentre study on 111 patients with chronic low back pain, FBSS, cervical pain, postherpetic neuralgia, tension headache, and some patients with trigeminal neuralgia or thoracic pain showed that the relatively best pain relief was obtained in patients with thoracic back pain, whereas the least pain relief was seen in patients with tension headache. Pain scores in total decreased from 8.2 without stimulation to 4.0 with stimulation. The mean medication use was significantly reduced.Citation69 A recent prospective multicenter study on 118 patients of PFNS on chronic low back pain showed similar results.Citation70
Interestingly, pain relief by TENS does not seem to be a predictor for the success of PFNS,Citation71 and pain relief elicited by PNFS seems to be stronger than in TENS.Citation69 A so-called “hybrid simulation”, combining SCS and PFNS, has also been proposed for various pain syndromes.Citation61,Citation62,Citation65,Citation67,Citation72–Citation74
A randomized study on PFNS showed that the stimulation under standard settings is significantly more effective than at subthreshold intensity or low frequency.Citation75
The advantages of PNFS are that it is less invasive and that it offers the opportunity to treat the pain syndromes that cannot be treated with SCS. Some pain syndromes, such as postherpetic neuralgia, can be treated by both PFNS and SCS, and it remains to be determined which of the two techniques offers the higher efficiency. The comparison of the effects of SCS and TENS, as a peripheral stimulation, on evoked potentials showed that the SCS effect was nearly twice as strong as that of TENS.Citation76
Dorsal root ganglion stimulation
In this technique, the electrode is placed directly adjacent to the spinal ganglion. With one electrode, paresthesia is only achieved within a single dermatome. Greater pain areas can be covered with the use of more than one electrode. The implantation technique is more challenging than that of SCS. A pilot study on 10 patients showed a mean pain relief of 70% in the first days after implantation. Energy consumption with this technique seems to be significantly less compared with SCS.Citation77 A recent multicenter study on 32 patients with a 6-month follow-up showed a mean pain relief of 58%. As expected, mean pain relief was strongest in the feet and weakest in the low back.Citation78 A series of eight patients with complex regional pain syndrome (CRPS) showed a decrease of mean pain scores of 62% under dorsal root ganglion stimulation.Citation79 Dorsal root ganglion stimulation can be considered particularly in monoradicular pain syndromes or pain syndromes affecting a very limited number of dermatomes.
Indications
In general, SCS is indicated in refractory neuropathic pain (). Early experiences have suggested that SCS is less effective in nociceptive pain. Nonetheless, in some cases it might be difficult to differentiate between the neuropathic and the nociceptive components of pain, and an SCS trial can be justified even if there are also nociceptive components in the pain syndrome. The most frequent and best studied indication is radicular pain secondary to lumbar disc surgery (FBSS). According to a recent review, FBSS evolves in approximately 30% of patients after lumbar disc surgery.Citation80 Two randomized controlled trials and numerous retrospective studies have been published for FBSS. In a crossover design, North et alCitation81 studied 50 patients with FBSS who had either SCS or a revision operation. Forty-five persons were followed for 3 years. Nine of 19 patients with SCS and three of 26 patients with the reoperation had more than 50% pain relief. In the SCS group, the crossover rate was lower than in the reoperation group (5/24 versus 14/26).Citation81 A study on 100 patients with FBSS and predominantly neuropathic leg pain was conducted by Kumar et al.Citation82 They randomized the patients into two groups; one group had conventional analgetic therapy alone and the other group had conventional analgetic plus SCS. The earliest time for a crossover was 6 months. At that time, 48% of the patients who had been treated with SCS had more than 50% pain relief, whereas this was the case in the conservative treatment group in only 9% of the patients. Again, the crossover rate was significantly lower in the SCS group than in the conservative group (5/50 versus 32/50).Citation82 Several guidelines recommend a trial of SCS in FBSS. A systematic review found class IIb evidence for efficacy of SCS in FBSS.Citation83
Table 1 Overview of indications and contraindications for spinal cord stimulation
Another good and well-studied indication for SCS is CRPS (CRPS I and II). In this potentially devastating neuro-orthopedic pain syndrome the use of SCS has been studied in several observational studies and one randomized controlled trial. In this study, the effectivity of SCS combined with a 6-month physiotherapeutic treatment protocol was compared with a physiotherapeutic treatment protocol alone. Thirty-six patients were included in the SCS group, whereas 18 patients had solely physiotherapeutic treatment. Test stimulation was successful in 24 of the 36 patients. Patients in the SCS group had a mean pain relief of 2.4 points (on an 11-point scale), whereas patients with solely physiotherapeutic treatment had pain relief of 0.2 points. These differences were statistically significant and remained stable after 1 and 2 years.Citation84 After 3 and 5 years, however, significant differences in the treatment groups were no longer seen. In these studies, as in the primary study, an intention-to-treat analysis was carried out that included the 12 patients with the negative trial in the SCS group. Moreover, four patients in the control group had received an SCS system.Citation85,Citation86 Interestingly, a study by Harke et alCitation87 had a kind of internal control. This internal control, in which the stimulator was switched off for 45 minutes, was performed every 3 months during the 35-month follow-up. Pain scores for depth pain and allodynia during these inactivity tests were 7.1/10 and 4.0/10, respectively. Under stimulation, these scores decreased to 1.7/10 and 0.03/10, respectively.Citation87 in addition to these studies, several case series regarding the use of SCS for the treatment of CRPS have been published. A meta-analysis showed that 67% of the patients experienced pain relief of more than 50%.Citation88
SCS has also been successfully employed in a number of neuropathic pain syndromes. Excellent results have been achieved in the treatment of postherpetic neuralgia. In 23/28 patients, pain relief from a median 9/10 on the visual analog scale to 1/10 was achieved. Complete pain relief was observed in 23 of 28 patients (82%) after 3–66 months.Citation89 Moreover, postherpetic neuralgia has been studied in a number of larger studies with miscellaneous indications.Citation90–Citation92 A meta-analysis showed a success rate of 82%.Citation93 Interestingly, SCS has also been proposed as a short-term therapy for subacute herpes zoster or beginning postherpetic neuralgia. Here, a 14-day short-term therapy with an externalized electrode led to complete and lasting pain relief in some patients.Citation94,Citation95
One indication for SCS that is not yet general practice is diabetic polyneuropathy. In 1996, a case series was published in which 8 of 10 patients tested were treated with SCS. Mean pain scores decreased in these patients from 7.7/10 to 2.3/10. However, pallhypesthesia and electrophysiological tests remained unchanged.Citation96 A systematic review of SCS for diabetic polyneuropathy lists three prospective case series and one retrospective cohort study. At 1-year follow-up, 63% of the patients had more than 50% pain reduction. In addition, analgetic medication could be stopped in 60% of the patients.Citation97 Recently, long-term effects for SCS for diabetic polyneuropathy were shown over a follow-up of 3 years.Citation98
Successful pain treatment with SCS has been described in a single case report in a vast variety of neuropathic pain syndromes such as syringomyelia,Citation99 post-thoracotomy neuralgia,Citation100 recurrent neuroma,Citation101 tethered cord syndrome,Citation102 meralgia paresthetica,Citation103 or even multiple sclerosis.Citation104 Favorable outcomes have also been reported in mixed pain syndromes regarding shoulder painCitation105 and knee pain.Citation106
An overview of randomized controlled studies is given in .Citation36,Citation39,Citation46,Citation47,Citation75,Citation81,Citation82,Citation84,Citation85,Citation107–Citation111
Table 2 Overview of randomized studies for SCS (and PNFS)
Safety
SCS can be considered a safe technique. The probability of sustaining permanent damage caused by an SCS trial is extremely low. The puncture of the spinal canal comprises the inherent risk of spinal bleeding and permanent neurological deficit; however, the literature lists only six reported cases of neurological deficits resulting from damage of the spinal cord. In 2010, Smith et al reported a series of four patients with paraparesis occurring after a spinal cord stimulator trial.Citation112 Two of these patients had spinal bleeding, one patient had spinal cord injury, and in one patient a preexisting thoracic disc herniation was encountered. To rule out thoracic disc herniation or spinal stenosis, the authors advocated magnetic resonance imaging (MRI) before SCS lead insertion. In the same article, the authors quoted two additional cases of paraparesis secondary to SCS lead insertion: one case of dural puncture and intramedullary lead placementCitation113 and another case of needle penetration in the cervical cord.Citation114 However, neurological deficit developing in the course of time, as a consequence of scarring,Citation115–Citation117 of an epidural mass,Citation118 or of a foreign body reactionCitation119 has also been reported in case reports. Given the fact that several hundred thousand SCS devices have been implanted worldwide since its advent, even admitting that neurological deficits after SCS might be underreported, this hints at a low probability of permanent neurologic deficit to SCS electrode implantation.
Infection can occur after SCS implantation. The incidence is reported between 3.4%Citation92 and 6%.Citation120 Usually, cases of infection have an uneventful benign course when the electrodes are removed and antibiotic therapy is initiated.
The probability of unscheduled additional revision operations caused by hardware problems, such as lead dislocation or lead breakage, is apparently high. A systematic review of SCS for FBSS or chronic back and leg pain found that 43% of the patients had one or more complications with SCS. Most of these were lead problems (lead dislocation). Infections were encountered in 6% of the patients and cerebral spinal fluid leaks in 7%.Citation120 A monocentric study on 707 patients found no patient with permanent neurologic deficit. However, hardware failures in this series occurred in 38% of the patients (22.6% lead migration, 9.5% lead connection failure, and 6% lead breakage).Citation121 Another large study on hardware failure modes found a significantly higher revision rate in cervical than in thoracolumbar SCS systems. Again, lead migration was the most frequent cause of repeat surgeryCitation122 ().
Table 3 Possible complications of spinal cord stimulation
The use of paddle leads that have to be implanted surgically via laminotomy has been advocated for patients with a history of lead migration. A recent study showed that the initial complication rate was higher with paddle leads compared with percutaneous leads, but the longer-term reoperation rate was significantly lower.Citation123
Interaction of SCS with diathermy, cardiac pacemakers, ultrasound, or MRI is possible and can lead to unwanted paresthesia or dysfunction of the system. In particular, MRI may exert forces on the implanted material and therefore may induce the risk of, for example, intraspinal bleeding. Last year a MRI-compatible SCS system was newly introduced. However, a recent study showed that as with conventional SCS systems using an adapted protocol, a MRI (1.5 T) can be performed safely.Citation124
Long-term outcomes and place in therapy
Outcomes of SCS therapy have considerably improved over the course of the last 30 years. Although in the 1970s about 40% of the patients achieved pain relief of more than 50%, this percentage in recent studies is more than 70%. Proper patient selection and meticulous preoperative diagnostics, including psychological assessment, are crucial for the success of the method. Moreover, more knowledge regarding promising indications has been gathered over the course of the years. SCS therapy today is not necessarily seen as a therapy of last resort, but as an effective possibility to overcome pain that would otherwise be difficult to treat. Unsuccessful conventional medical management is regarded as a criterion in favor of an SCS trial. However, most of the guidelines do not state whether or not opioids, particularly strong opioids, should be employed. Today, many pain physicians probably would not insist on a prior therapeutic attempt with highly potent opioids, and SCS would be ranked in the therapeutic order between weak and strong opioids. At any rate, the disadvantages of long-term opioid therapyCitation125 should be weighed against those of SCS therapy. SCS can certainly be used together with analgetic medications, but in an optimal setting, SCS can make pain medication redundant. Often, however, patients continue to take analgetic medications. For instance, in a study on 60 consecutive patients, 16% of the patients took no analgetics at all after SCS implantation, whereas more than half of the patients took two or more different analgetics.Citation31
Another important point is the duration of conservative therapy before SCS implantation. Kumar et al observed an inverse correlation between the long-term success rate and the time interval between the onset of the chronic pain syndrome and SCS implantation. They noted that the success rate in patients who received their SCS system within the first 2 years of their pain syndrome was 85%, whereas the success rate was 9% in patients with a more than 15-year history of chronic pain.Citation126,Citation127 As a consequence, an SCS trial should be performed within the first 2 years after onset of pain. In contrast, also less invasive therapies should have been tried before an SCS trial, so realistically, SCS will rarely be applied earlier than 6–12 months from the onset of pain. Anyway, also a recent review concluded that SCS for CRPS “should be considered earlier than last resort therapy”.Citation128
Conclusion
SCS is an effective treatment option for neuropathic pain. Technical advancement of SCS has led to improvement in stimulation patterns adapted to the patients’ needs. SCS for neuropathic pain is still underused. Careful preoperative diagnosis and proper patient selection is most important for the success of the methods.
Disclosure
The author reports no conflicts of interest in this work.
References
- FreynhagenRBaronRGockelUTölleTRpainDETECT: a new screening questionnaire to identify neuropathic components in patients with back painCurr Med Res Opin200622101911192017022849
- FinnerupNBOttoMMcQuayHJJensenTSSindrupSHAlgorithm for neuropathic pain treatment: an evidence based proposalPain2005118328930516213659
- KumarKRizviSCost-effectiveness of spinal cord stimulation therapy in management of chronic painPain Med201314111631164923710759
- VyawahareBHallasNBrookesMTaylorRSEldabeSImpact of the National Institute for Health and Care Excellence (NICE) guidance on medical technology uptake: analysis of the uptake of spinal cord stimulation in England 2008–2012BMJ Open201441e004182
- MelzackRWallPDPain mechanisms: a new theoryScience196515036999719795320816
- ShealyCNTaslitzNMortimerJTBeckerDPElectrical inhibition of pain: experimental evaluationAnesth Analg19674632993056067264
- ShealyCNMortimerJTReswickJBElectrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical reportAnesth Analg19674644894914952225
- ShealyCNMortimerJTHagforsNRDorsal column electroanalgesiaJ Neurosurg19703255605645438096
- NasholdBSJrFriedmanHDorsal column stimulation for control of pain. Preliminary report on 30 patientsJ Neurosurg19723655905975026545
- HosobuchiYAdamsJEWeinsteinPRPreliminary percutaneous dorsal column stimulation prior to permanent implantation. Technical noteJ Neurosurg19723722422455046094
- KrainickJUThodenUExperience with dorsal column stimulation (DCS) in the operative treatment of chronic intractable painJ Neurosurg Sci19741831871894377274
- TronnierVNeuromodulation for chronic pain conditionsBremenUni Med Verlag2003 German
- GrilloPJYuHCPattersonRHJrDelayed intraspinal hemorrhage after dorsal column stimulation for painArch Neurol19743011051064271318
- TaubACollinsWFVenesJPartial, reversible, functional spinal cord transection. A complication of dorsal column stimulation for the relief of painArch Neurol19743011071084808491
- NasholdBSomjenGFriedmanHParesthesias and EEG potentials evoked by stimulation of the dorsal funiculi in manExp Neurol19723622732875053355
- BarchiniJTchachaghianSShamaaFSpinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathyNeuroscience201221519620822548781
- YakhnitsaVLinderothBMeyersonBASpinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathyPain1999792–322323310068168
- StillerCOCuiJGO’ConnorWTBrodinEMeyersonBALinderothBRelease of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic ratsNeurosurgery19963923673748832675
- CuiJGO’ConnorWTUngerstedtULinderothBMeyersonBASpinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanismPain199773187959414060
- LinderothBMeyersonBASpinal cord stimulation: exploration of the physiological basis of a widely used therapyAnesthesiology201011361265126721042195
- CuiJGLinderothBMeyersonBAEffects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic ratPain1996662–32872958880852
- SchechtmannGSongZUlteniusCMeyersonBALinderothBCholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathyPain2008139113614518472215
- SongZMeyersonBALinderothBMuscarinic receptor activation potentiates the effect of spinal cord stimulation on pain-related behavior in rats with mononeuropathyNeurosci Lett2008436171218343581
- SongZUlteniusCMeyersonBALinderothBPain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathyPain20091471–324124819836134
- SaadéNETabetMSSoueidanSABitarMAtwehSFJabburSJSupraspinal modulation of nociception in awake rats by stimulation of the dorsal column nucleiBrain Res19863691–23073103697746
- SaadéNEAtwehSFPrivatAJabburSJInhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexesBrain Res19998461728610536215
- El-KhouryCHawwaNBalikiMAtwehSFJabburSJSaadéNEAttenuation of neuropathic pain by segmental and supraspinal activation of the dorsal column system in awake ratsNeuroscience2002112354155312074897
- GuanYWacnikPWYangFSpinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic ratsAnesthesiology201011361392140521068658
- KumarKWilsonJRFactors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rateActa Neurochir Suppl200797Pt 1919917691362
- SparkesERaphaelJHDuarteRVLeMarchandKJacksonCAshfordRLA systematic literature review of psychological characteristics as determinants of outcome for spinal cord stimulation therapyPain2010150228428920603026
- WolterTFaulerIKieselbachKThe impact of psychological factors on outcomes for spinal cord stimulation: an analysis with long-term follow-upPain Physician201316326527523703413
- BrandwinMAKewmanDGMMPI indicators of treatment response to spinal epidural stimulation in patients with chronic pain and patients with movement disordersPsychol Rep1982513 Pt 2105910646220420
- BurchielKJAndersonVCBrownFDProspective, multicenter study of spinal cord stimulation for relief of chronic back and extremity painSpine19962123278627948979327
- WolterTWinkelmüllerMContinuous versus intermittent spinal cord stimulation: an analysis of factors influencing clinical efficacyNeuromodulation20121511319 discussion 2022151660
- De RidderDVannesteSPlazierMvan der LooEMenovskyTBurst spinal cord stimulation: toward paresthesia-free pain suppressionNeurosurgery201066598699020404705
- De RidderDPlazierMKamerlingNMenovskyTVannesteSBurst spinal cord stimulation for limb and back painWorld Neurosurg201380564264923321375
- Van BuytenJPAl-KaisyASmetIPalmisaniSSmithTHigh-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical studyNeuromodulation2013161596523199157
- Al-KaisyAVan BuytenJPSmetIPalmisaniSPangDSmithTSustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter studyPain Med201415334735424308759
- PerruchoudCEldabeSBatterhamAMAnalgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled studyNeuromodulation2013164363369 discussion 36923425338
- BarolatGZemeSKetcikBMultifactorial analysis of epidural spinal cord stimulationStereotact Funct Neurosurg1991562771031947505
- CameronTAloKMEffects of posture on stimulation parameters in spinal cord stimulationNeuromodulation19981417718322151029
- OlinJCKiddDHNorthRBPostural changes in spinal cord stimulation perceptual thresholdsNeuromodulation19981417117522151028
- HolsheimerJBarolatGStruijkJJHeJSignificance of the spinal cord position in spinal cord stimulationActa Neurochir Suppl (Wien)199564119124
- AbejonDFelerCAIs impedance a parameter to be taken into account in spinal cord stimulation?Pain Physician200710453354017660851
- HolsheimerJden BoerJAStruijkJJRozeboomARMR assessment of the normal position of the spinal cord in the spinal canalAJNR Am J Neuroradiol19941559519598059666
- SchadeCMSchultzDMTamayoNIyerSPankenEAutomatic adaptation of neurostimulation therapy in response to changes in patient position: results of the Posture Responsive Spinal Cord Stimulation (PRS) Research StudyPain Physician201114540741721927044
- SchultzDMWebsterLKosekPDarUTanYSunMSensor-driven position-adaptive spinal cord stimulation for chronic painPain Physician201215111222270733
- LawJDSwettJKirschWMRetrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulationJ Neurosurg1980524482485
- BuschmannDOppelFPeripheral nerve stimulation for pain relief in CRPS II and phantom-limb painSchmerz1999132113120 German12799940
- WeinerRLReedKLPeripheral neurostimulation for control of intractable occipital neuralgiaNeuromodulation19992321722122151211
- BurnsBWatkinsLGoadsbyPJTreatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patientsLancet200736995671099110617398309
- BurnsBWatkinsLGoadsbyPJTreatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patientsNeurology200972434134519171831
- MagisDAllenaMBollaMDe PasquaVRemacleJMSchoenenJOccipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot studyLancet Neurol20076431432117362835
- MagisDGerardyPYRemacleJMSchoenenJSustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headacheHeadache20115181191120121848953
- TronnierVRascheDSubcutaneous peripheral stimulation of the greater occipital nerve for the treatment of chronic headache syndromesSchmerz2010245441448 German20872125
- PaiciusRMBernsteinCALempert-CohenCPeripheral nerve field stimulation in chronic abdominal painPain Physician20069326126616886036
- BarolatGPeripheral subcutaneous stimulation for intractable abdominal painProg Neurol Surg201124707621422777
- McJunkinTLBerardoniNLynchPJAmraniJAn innovative case report detailing the successful treatment of post-thoracotomy syndrome with peripheral nerve field stimulationNeuromodulation201013431131421992889
- GoyalGNGuptaDJainRKumarSMishraSBhatnagarSPeripheral nerve field stimulation for intractable post-thoracotomy scar pain not relieved by conventional treatmentPain Pract201010436636920230446
- PaiciusRMBernsteinCALempert-CohenCPeripheral Nerve Field Stimulation for the Treatment of Chronic Low Back Pain: Preliminary Results of Long-Term Follow-up: A Case SeriesNeuromodulation200710327929022150840
- BernsteinCAPaiciusRMBarkowSHLempert-CohenCSpinal cord stimulation in conjunction with peripheral nerve field stimulation for the treatment of low back and leg pain: a case seriesNeuromodulation200811211612322151044
- Hamm-FaberTEAukesHAde LoosFGültunaISubcutaneous stimulation as an additional therapy to spinal cord stimulation for the treatment of lower limb pain and/or back pain: a feasibility studyNeuromodulation201215210811621943376
- BurgherAHHuntoonMATurleyTWDoustMWStearnsLJSubcutaneous peripheral nerve stimulation with inter-lead stimulation for axial neck and low back pain: case series and review of the literatureNeuromodulation2012152100106 discussion 106–10721854499
- YakovlevAEReschBEYakovlevaVEPeripheral nerve field stimulation in the treatment of postlaminectomy syndrome after multilevel spinal surgeriesNeuromodulation2011146534538 discussion 53821854498
- ReverberiCDarioABarolatGSpinal cord stimulation (SCS) in conjunction with peripheral nerve field stimulation (PNfS) for the treatment of complex pain in failed back surgery syndrome (FBSS)Neuromodulation2013161788222985076
- PatilAAOttoDRaikarSPeripheral nerve field stimulation for sacroiliac joint painNeuromodulation20141719810123441931
- LepskiGVahediPTatagibaMSMorgallaMCombined spinal cord and peripheral nerve field stimulation for persistent post-herniorrhaphy painNeuromodulation20131618488 discussion 88–8922672211
- VerrillsPVivianDMitchellBBarnardAPeripheral nerve field stimulation for chronic pain: 100 cases and review of the literaturePain Med20111291395140521812906
- Sator-KatzenschlagerSFialaKKressHGSubcutaneous target stimulation (STS) in chronic noncancer pain: a nationwide retrospective studyPain Pract201010427928620230450
- KloimsteinHLikarRKernMPeripheral nerve field stimulation (PNFS) in chronic low back pain: a prospective multicenter studyNeuromodulation201417218018724320718
- GoroszeniukTKothariSHamannWSubcutaneous neuromodulating implant targeted at the site of painReg Anesth Pain Med200631216817116543103
- FalcoFJBergerJVrableAOnyewuOZhuJCross talk: a new method for peripheral nerve stimulation. An observational report with cadaveric verificationPain Physician200912696598319935981
- MironerYEHutchesonJKSatterthwaiteJRLaTourettePCProspective, two-part study of the interaction between spinal cord stimulation and peripheral nerve field stimulation in patients with low back pain: development of a new spinal-peripheral neurostimulation methodNeuromodulation2011142151154 discussion 15521992203
- LipovEG‘Hybrid neurostimulator’: simultaneous use of spinal cord and peripheral nerve field stimulation to treat low back and leg painProg Neurol Surg20112414715521422785
- McRobertsWPWolkowitzRMeyerDJPeripheral nerve field stimulation for the management of localized chronic intractable back pain: results from a randomized controlled studyNeuromodulation2013166565574 discussion 574–57523577773
- WolterTKieselbachKSircarRGierthmuehlenMSpinal cord stimulation inhibits cortical somatosensory evoked potentials significantly stronger than transcutaneous electrical nerve stimulationPain Physician201316440541423877457
- DeerTRGrigsbyEWeinerRLWilcoskyBKramerJMA prospective study of dorsal root ganglion stimulation for the relief of chronic painNeuromodulation20131616771 discussion 71–7223240657
- LiemLRussoMHuygenFJA multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic painNeuromodulation2013165471482 discussion 48223668228
- Van BuytenJPSmetILiemLRussoMHuygenFStimulation of Dorsal Root Ganglia for the Management of Complex Regional Pain Syndrome: A Prospective Case SeriesPain Pract1201423
- van BuytenJPLinderothBThe failed back surgery syndrome’: Definition and therapeutic algorithms – An updateEur J Pain Suppl201044273286
- NorthRBKiddDHFarrokhiFPiantadosiSASpinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trialNeurosurgery200556198106 discussion 106–10715617591
- KumarKNorthRTaylorRSpinal Cord Stimulation vs Conventional Medical Management: A Prospective, Randomized, Controlled, Multicenter Study of Patients with Failed Back Surgery Syndrome (PROCESS Study)Neuromodulation20058421321822151547
- FreyMEManchikantiLBenyaminRMSchultzDMSmithHSCohenSPSpinal cord stimulation for patients with failed back surgery syndrome: a systematic reviewPain Physician200912237939719305486
- KemlerMADe VetHCBarendseGAVan Den WildenbergFAVan KleefMThe effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trialAnn Neurol2004551131814705107
- KemlerMAde VetHCBarendseGAvan den WildenbergFAvan KleefMSpinal cord stimulation for chronic reflex sympathetic dystrophy – five-year follow-upN Engl J Med2006354222394239616738284
- KemlerMAde VetHCBarendseGAvan den WildenbergFAvan KleefMEffect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trialJ Neurosurg2008108229229818240925
- HarkeHGretenkortPLadleifHURahmanSSpinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical studyEur J Pain20059436337315979016
- TaylorRSSpinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysisJ Pain Symptom Manage2006314(Suppl)S13S1916647590
- HarkeHGretenkortPLadleifHUKoesterPRahmanSSpinal cord stimulation in postherpetic neuralgia and in acute herpes zoster painAnesth Analg2002943694700 Table of contents11867400
- MeglioMCioniBRossiGFSpinal cord stimulation in management of chronic pain. A 9-year experienceJ Neurosurg19897045195242538588
- BroggiGServelloDDonesICarboneGItalian multicentric study on pain treatment with epidural spinal cord stimulationStereotact Funct Neurosurg1994621–42732787631081
- KumarKTothCNathRKSpinal cord stimulation for chronic pain in peripheral neuropathySurg Neurol19964643633698876718
- CameronTSafety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature reviewJ Neurosurg2004100Suppl Spine 325426715029914
- MoriyamaKEffect of temporary spinal cord stimulation on postherpetic neuralgia in the thoracic nerve areaNeuromodulation2009121394322151221
- IsekiMMoritaYNakamuraYIfukuMKomatsuSEfficacy of limited-duration spinal cord stimulation for subacute postherpetic neuralgiaAnn Acad Med Singapore200938111004100619956824
- TesfayeSWattJBenbowSJPangKAMilesJMacFarlaneIAElectrical spinal-cord stimulation for painful diabetic peripheral neuropathyLancet19963489043169817018973433
- PluijmsWASlangenRJoostenEAElectrical spinal cord stimulation in painful diabetic polyneuropathy, a systematic review on treatment efficacy and safetyEur J Pain201115878378821345703
- SlangenRPluijmsWAFaberCGDirksenCDKesselsAGvan KleefMSustained effect of spinal cord stimulation on pain and quality of life in painful diabetic peripheral neuropathyBr J Anaesth201311161030103124233316
- CamposWKAlmeida de OliveiraYSCiampi de AndradeDTeixeiraMJFonoffETSpinal cord stimulation for the treatment of neuropathic pain related to syringomyeliaPain Med201314576776823489298
- WiningerKLBesterMLDeshpandeKKSpinal cord stimulation to treat postthoracotomy neuralgia: non-small-cell lung cancer: a case reportPain Manag Nurs2012131525922341139
- MessinaGNazziVSinisiMDonesIPolloBFranziniASpinal cord stimulation for recurrent painful neuromas of the footNeurol Sci201132472372521678072
- MoensMDe SmedtAD’HaeseJDroogmansSChaskisCSpinal cord stimulation as a treatment for refractory neuropathic pain in tethered cord syndrome: a case reportJ Med Case Reports20104174
- BarnaSAHuMMBuxoCTrellaJCosgroveGRSpinal cord stimulation for treatment of meralgia parestheticaPain Physician20058331531816850089
- BurkeyARAbla-YaoSSuccessful treatment of central pain in a multiple sclerosis patient with epidural stimulation of the dorsal root entry zonePain Med201011112713220447296
- WilliamsKABabadeMCohenSPSpinal cord stimulation for severe degenerative joint disease of the shoulder in a patient with severe chronic obstructive pulmonary disease: a new indication?Anesth Analg2010110122022119897798
- LowryAMSimopoulosTTSpinal cord stimulation for the treatment of chronic knee pain following total knee replacementPain Physician201013325125620495589
- KumarKTaylorRSJacquesLSpinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndromePain20071321–217918817845835
- NorthRBKiddDHLeeMSPiantodosiSA prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: initial resultsStereotact Funct Neurosurg1994621–42672727631080
- NorthRBKiddDHPiantadosiSSpinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study designActa Neurochir Suppl (Wien)199564106108
- KemlerMABarendseGAvan KleefMSpinal cord stimulation in patients with chronic reflex sympathetic dystrophyN Engl J Med2000343961862410965008
- WolterTKiemenAPorzeliusCKaubeHEffects of sub-perception threshold spinal cord stimulation in neuropathic pain: a randomized controlled double-blind crossover studyEur J Pain201216564865522337509
- SmithCCLinJLShokatMDosanjhSSCasthelyDA report of paraparesis following spinal cord stimulator trial, implantation and revisionPain Physician201013435736320648204
- MeyerSCSwartzKJohnsonJPQuadriparesis and spinal cord stimulation: case reportSpine20073219E565E56817762800
- BarolatGPeacockWStaudtLPain and spasticityBenzelESpine Surgery: Techniques, Complication Avoidance, and Management22nd edPhiladelphiaElsevier Churchill Livingstone200512391252
- Dam-HieuPMagroESeizeurRSimonAQuinioBCervical cord compression due to delayed scarring around epidural electrodes used in spinal cord stimulationJ Neurosurg Spine201012440941220367377
- WadaEKawaiHLate onset cervical myelopathy secondary to fibrous scar tissue formation around the spinal cord stimulation electrodeSpinal Cord201048864664820065981
- CicuendezMMunarrizPMCastaño-LeonAMParedesIDorsal myelopathy secondary to epidural fibrous scar tissue around a spinal cord stimulation electrodeJ Neurosurg Spine201217659860123062176
- WlochACapelleHHSaryyevaAKraussJKCervical myelopathy due to an epidural cervical mass after chronic cervical spinal cord stimulationStereotact Funct Neurosurg201391426526923652576
- LennarsonPJGuillenFTSpinal cord compression from a foreign body reaction to spinal cord stimulation: a previously unreported complicationSpine20103525E1516E151921102282
- TaylorRSVan BuytenJPBuchserESpinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factorsSpine (Phila Pa 1976)200530115216015626996
- MekhailNAMathewsMNageebFGuirguisMMekhailMNChengJRetrospective review of 707 cases of spinal cord stimulation: indications and complicationsPain Pract201111214815321371254
- RosenowJMStanton-HicksMRezaiARHendersonJMFailure modes of spinal cord stimulation hardwareJ Neurosurg Spine20065318319016961078
- BabuRHazzardMAHuangKTOutcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costsNeuromodulation2013165418426 discussion 426–42723647789
- MutterUMBellutDPorchetFSchuknechtBSpinal magnetic resonance imaging with reduced specific absorption rate in patients harbouring a spinal cord stimulation device – A single-centre prospective study analysing safety, tolerability and image qualityActa Neurochir (Wien)2013155122327233224078115
- IvanovaJIBirnbaumHGYushkinaYSorgRAReedJMerchantSThe prevalence and economic impact of prescription opioid-related side effects among patients with chronic noncancer painJ Opioid Manag20139423925424353017
- KumarKHunterGDemeriaDSpinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experienceNeurosurgery2006583481496 discussion 481–49616528188
- KumarKRizviSNguyenRAbbasMBishopSMurthyVImpact of Wait times on Spinal Cord Stimulation Therapy OutcomesPain Pract Epub10252013
- PoreeLKramesEPopeJDeerTRLevyRSchultzLSpinal cord stimulation as treatment for complex regional pain syndrome should be considered earlier than last resort therapyNeuromodulation201316212514123441988