Abstract
Advances in intrathecal analgesia and intrathecal drug delivery systems have allowed for a range of medications to be used in the control of pain and spasticity. This technique allows for reduced medication doses that can decrease the side effects typically associated with oral or parenteral drug delivery. Recent expert panel consensus guidelines have provided care paths in the treatment of nociceptive, neuropathic, and mixed pain syndromes. While the data for pain relief, adverse effect reduction, and cost-effectiveness with cancer pain control are compelling, the evidence is less clear for noncancer pain, other than spasticity. Physicians should be aware of mechanical, pharmacological, surgical, and patient-specific complications, including possible granuloma formation. Newer intrathecal drug delivery systems may allow for better safety and quality of life outcomes.
Introduction
In 1999, the Joint Commission on Accreditation of Healthcare Organizations issued comprehensive standards of care for pain management. It stated, “No cancer patient should live or die with unrelieved pain”.Citation1 The World Health Organization established a simple three-step “ladder” approach in 1986, beginning with nonopioid drugs and progressing to stronger opioids as necessary. Adjuvant drugs, such as anticonvulsants and antidepressants, may also be used at each step, as follows: patients who are receiving no analgesic therapy should receive nonopioid analgesic drugs, including nonsteroidal anti-inflammatory drugs (step 1); if the pain is not adequately treated, persists, or increases despite therapy with nonopioid medications, the dose of the nonopioids should be increased and mild opioid analgesic drugs may be added (step 2); and for increasing or persistent pain, or moderate-severe pain at the outset, strong opioids such as morphine may be used (step 3).
Few medical guidelines have made such an impact on health care as the World Health Organization pain ladder, so much so that it has been unofficially applied for use in other pain syndromes, including chronic nonmalignant pain. Patients receiving adjuvant therapy and oral or transdermal opioids achieve adequate pain control in approximately 80% of cases. However, in 20% of patients, some form of alternative or invasive therapy is needed to control recalcitrant pain despite aggressive titration of these medications along the World Health Organization ladder. Adverse effects of opioids may also prevent patients from achieving maximal benefit.Citation2 For example, gastrointestinal side effects, including both constipation and nausea, are estimated to range as much as 10%–40%.Citation3 In 1994, as part of the Mayo Clinic symposium on pain management, the World Health Organization ladder was extended to include interventional pain therapies, such as spinal analgesia, nerve stimulation, and neurolytic blocks.Citation4 This “fourth step” of the analgesic ladder has provided practitioners with an indispensable means of pain control for patients suffering from refractory pain. This article focuses on one of these interventional treatments, namely intrathecal drug delivery.
History of intrathecal analgesia and intrathecal drug delivery systems
In 1898, soon after the discovery of cocaine as a local anesthetic, August Bier documented the first spinal analgesia by injecting cocaine into his own intrathecal space as well as that of six patients who were to undergo lower extremity surgery, thus creating immense interest in this technique.Citation5 Soon after, Rudolph Matas showed that mixing morphine with cocaine was able to mitigate the adverse symptoms associated with intrathecal cocaine.Citation6 Continuous spinal analgesia was first used in the 1940s, but it was the discovery of opiate receptors in the spinal cord in 1973 that provided a scientific rationale for this form of treatment.Citation7 The development of infusion pumps in the early 1970s allowed for greater flexibility in administering intrathecal opiates.Citation8 For example, Wang et al first reported the successful use of intrathecal morphine for the treatment of intractable cancer pain in 1979.Citation9 Two years later, the first use of an implantable intrathecal drug delivery system (IDDS) was reported.Citation6 During the 1980s, this form of drug delivery provided a fixed continuous infusion rate, allowing physicians to use lower doses of analgesics and thus decreasing adverse effects such as sedation and constipation. Changing the dose could only be performed by aspirating the medication from the pump reservoirs and refilling them with a different concentration of the medication.Citation10 These dose changes introduced the inherent risks associated with frequent aspiration and injection of these solutions, such as infection and inadvertent deposition of the solution outside the reservoir, leading to potential systemic overdose. In 1991, externally programmable, battery-powered IDDS pumps were introduced, allowing for noninvasive dose changes of these medications using an external programmer.Citation10 This allowed for an easier means of changing patients’ analgesic therapies in response to the dynamic changes in pain.Citation11
Indications for intrathecal drug delivery and patient selection criteria
There are no universally accepted guidelines or recommendations for patient selection. In general, patient selection for IDDS therapy can be divided into cancer and noncancer pain. In both groups, patients suffering from significant side effects of oral, transdermal, or intravenous opioids that inhibit adequate titration of these medications or those patients who cannot achieve adequate analgesia despite high doses of opioids should be considered for intrathecal therapy. Several other factors should be included when considering the patient for intrathecal drug delivery, such as previous adherence to treatment, ability/willingness to attend for follow-up, and anatomical changes that may affect implantation of either the pump or catheter. The patient’s disease state, failure of conservative therapy, psychological state, adherence to treatment, and candidacy for a surgical procedure all reflect important considerations prior to possible implantation.Citation12
Indications for the use of IDDS in chronic noncancer pain typically include pain originating from the spine and, specifically, patients with failed back surgery syndrome primarily, followed by compression fractures, spondylosis, spondylolisthesis, and spinal stenosis. Other conditions include spinal cord injury-induced spasticity, complex regional pain syndrome, chronic pancreatitis, neuropathies, and rheumatoid arthritis.Citation13 Patients with chronic noncancer pain being considered for IDDS must undergo a thorough evaluation for psychiatric comorbidities such as depression, anxiety, addiction, suicidal ideation, or personality disorders, since the presence of these have been associated with a poor response to intrathecal therapy.Citation14
Several studies have shown the benefits of IDDS in patients with cancer pain.Citation15,Citation16 Previous studies and reviews have considered including only patients who have a life expectancy of at least 3 months.Citation17 However, the Polyanalgesic Consensus Committee (PACC) revised their recommendations in 2012, stating that previous recommendations for the placement of pumps only in patients with greater than 3 months of life expectancy was based on antiquated data.Citation18 Deer et al feel that life expectancy may be increased with intrathecal therapy because of reduced side effects, suggesting that, in the absence of impending death, IDDS should be considered even if a patient’s prognosis falls short of 3 months.Citation19 With improved pain control and fewer adverse effects, an enhanced survival seems probable. However, at 6 months post-implantation, Smith et al showed that IDDS only trended toward significance (53.9% for IDDS versus 37.2% for control, P=0.06).Citation15 Patients undergoing chemotherapy or radiation should also be considered for IDDS. Smith et al further showed that patients could endure more aggressive chemotherapy/radiation treatment due to the decreased adverse effects associated with IDDS medications when compared with conventional forms of pain management.Citation15 Indications with reasonable evidence for support of IDDS therapy include failed back surgery syndrome, and select patients with diabetic peripheral neuropathy, complex regional pain syndrome, and spasticity. Patients with these conditions can benefit the most from IDDS therapy.
IDDS is contraindicated in patients who: are unable/unwilling to have the pump refilled, have significant coagulopathies, require therapeutic anticoagulation, are hemodynamically unstable, have spinal cord pathology with cerebrospinal fluid outflow obstruction, have intracranial hypertension, have sepsis, have an infection at the site of the catheter or pump insertion, have significant emaciation preventing implantation of the device, or have significant psychiatric comorbidities.Citation20
Intrathecal medications
Currently there are only three medications approved by the US Food and Drug Administration (FDA) for use via the intrathecal route, ie, morphine, ziconotide, and baclofen. Morphine targets opioid receptors within the dorsal horn. It is considered by many to be the gold standard intrathecal opioid agonist, against which all other opioids are compared. Morphine binds to receptors on the primary afferent neurons (presynaptic) and cells within the dorsal horn of the spinal cord (postsynaptic) to inhibit the release of neurotransmitters like substance P and calcitonin gene-related peptide and hyperpolarize postsynaptic neurons, respectively. Ziconotide provides analgesia by blocking specific N-type calcium channels found at presynaptic terminals in the dorsal horn.
Baclofen is a gamma-aminobutyric acid (GABA)-B agonist acting on both presynaptic sites (decreasing calcium conduction resulting in decreased excitatory amino acid release) and postsynaptic sites (increasing potassium conductance leading to neuronal hyperpolarization). Baclofen inhibits sensory input to the motor neurons of the spinal cord. It is used to treat muscle spasms, spasticity, and neuropathic pain. While these three medications are the only FDA-approved drugs to be used intrathecally, in practice, other medications, such as clonidine, bupivacaine hydromorphone, fentanyl, and sufentanil are also used. In fact, the PACC outlined their most recent recommendations on drug choice, concentration limits, and recommended starting dosages in their 2012 guidelines.Citation18 Their medication recommendations are based on the type of pain that the patient exhibits. For instance, presents recommendations based on neuropathic pain management while presents recommendations based on nociceptive pain management. For patients with mixed pain states, the PACC left the decision-making process to the managing physician based upon the clinical scenario and adaptation of the appropriate algorithm. presents the recommended daily starting dosages for these medications.
Table 1 2012 Polyanalgesic Consensus Committee recommendations for intrathecal medication in neuropathic pain management
Table 2 2012 Polyanalgesic Consensus Committee recommendations for intrathecal medication in nociceptive pain management
Table 3 Recommended starting dosage ranges of intrathecal medications
Trialing protocol
Trialing of intrathecal agents was previously considered to be the standard of care in evaluating a patient’s response and potential side effects to therapy. Patients’ responses to neuraxial medications were used to determine whether an IDDS pump should be implanted and to satisfy requirements for insurance company reimbursement. In 2012, however, the PACC revised their recommendations on trialing for intrathecal drug delivery, stating that trialing was debatable, specifically in patients with cancer pain.Citation12 They acknowledged that a trial may underestimate potential side effects and the failure rate of IDDS therapy. Despite the opinion shift, it is generally recommended to consider a trial prior to implantation because it is currently the most demonstrative method of emulating a system that would eventually be implanted. Although trialing has been used for more than a decade, there is a dearth of studies that demonstrate the best method.Citation12 The PACC 2012 expert panel provided a potential algorithm for intrathecal trials. After determining that a patient is a candidate for intrathecal pump implantation, the next step is to determine the trialing method based upon the type of pain the patient exhibits. If it is predominantly nociceptive pain, then consider using a single-shot intrathecal trial (single-shot epidural trials are not recommended by the panel). If the patient exhibits either neuropathic pain or a mixed pain state, then the clinician must determine whether or not the patient is able to tolerate a prolonged infusion. If the answer is yes, then consider either a morphine trial via epidural/intrathecal infusion route or a ziconotide/baclofen intrathecal infusion trial. If the patient cannot tolerate a continuous infusion trial, then one may administer a bolus injection trial of morphine epidurally/intrathecally or ziconotide/baclofen intrathecally. It is important to note, however, that manifestations of opioid-induced hyperalgesia or disease progression are difficult to assess from either a single-shot or infusion trial.Citation12 presents the recommended doses for intrathecal bolus trialing based upon the PACC 2012 recommendations. Pain relief, functional status, and adverse effects are observed during the trial. A 50% decrease in pain, as well as a favorable side effect profile, are considered by many to be predictive of a successful IDDS implantation.Citation21
Table 4 Recommended doses for intrathecal (IT) bolus trialing
IDDS devices
There are four methods of delivering medications intrathecally: two include the use of an external pump while the other two represent fully implantable devices. First, an external pump with a percutaneous catheter (tunneled or not tunneled) is less invasive to place and can be beneficial for patients with a limited life expectancy. Second, for patients with a short life expectancy, totally implanted catheters with a subcutaneous injection port connected to an external pump may be more suitable. These may also be used to conduct a prolonged trial.
Third, a fully implanted fixed-rate (or constant flow) IDDS may be beneficial for long-term delivery of analgesia. The Codman 3000 (Codman and Shurtleff, Inc., Raynham, MA, USA) is an example of such a system (). Fixed-rate delivery systems are less expensive than variable-rate delivery systems and do not require a battery to operate, so should theoretically last the lifetime of the patient. They generally possess a larger drug reservoir accommodating larger volumes and permitting longer time periods between refills. However, due to the constant flow nature of the system, it lacks the flexibility to change medication delivery or allow for a patient-controlled bolus.
Figure 1 Codman 3000 intrathecal pump with progressively increasing reservoir sizes. Courtesy of Codman Neuro.

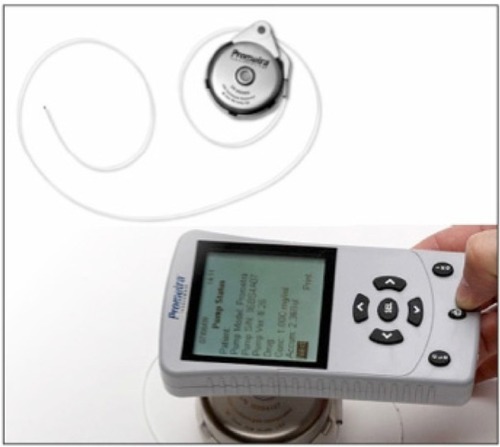
The fourth method of spinal medication delivery consists of a fully implanted programmable IDDS, such as the Medtronic SynchroMed II infusion system (Medtronic Inc., Minneapolis, MN, USA) (). These programmable devices deliver either an intermittent or continuous amount of medication intrathecally. Drug dosages can be changed without intervention such as the aspiration and refilling of a different medication concentration as seen in fixed-rate delivery systems. Programmable dose changes are quite useful for conditions such as opioid tolerance or dynamic changes in pain that necessitate frequent dose alterations for patients with cancer. The pump can be interrogated or deactivated without emptying the drug reservoir in cases of suspected malfunction. However, because the system is battery driven, the life span of a pump is typically 4–7 years and requires surgical revision. Also, the refill interval is shorter (ie, more frequent) due to a smaller reservoir than those found in fixed-rate systems. The newest iteration of the SynchroMed II system includes the patient therapy manager. This is a handheld device that patients use to self-administer a dose of drug intrathecally, and thereby helps to address breakthrough pain. The software includes additional features, such as registering the visual analog pain scale before and after a bolus. These numbers are stored, analyzed, and used to reprogram the system for optimum dosing. A 2008 study looking at 168 patients found that 85% of patients were satisfied with this technology.Citation22 The FDA recently approved a new programmable IDDS, the Prometra programmable pump system (Flowonix Medical Inc., Mount Olive, NJ, USA) in February 2012 (). This pump uses a valve-gated dose regulation system rather than the typical peristaltic pump roller system found in the Medtronic SynchroMed II. Rauck et al published the results of a trial evaluating the efficacy of this new system in administering intrathecal morphine in 110 patients with chronic pain.Citation23 They found the mean accuracy of the pump to be 97.1% (90% confidence interval 96.2–98.0), which offers slightly improved accuracy when compared with the Medtronic SynchroMed II. Both the SynchroMed II and Prometra pumps are magnetic resonance imaging (MRI) compatible. However, the Prometra pump is only MRI compatible after complete removal of medication from the reservoir, whereas there is no need to remove medication from the SynchroMed II device. It is recommended to interrogate the SynchroMed II pump before and after MRI to ensure that the pump has properly reactivated following suspension of drug infusion during the MRI.
Figure 2 Medtronic SynchroMed II intrathecal pump, programmer, and patient therapy manager. Reprinted with the permission of Medtronic, Inc. © 2012.

Other innovative infusion devices include the Medstream programmable infusion system (Codman and Shurtleff, Inc.), and the Medallion (Alfred Mann Foundation, Los Angeles, CA, USA). The Medstream system is currently only FDA-approved for the infusion of baclofen to treat spasticity. Its catheter design resists kinking and tearing, and uses compressed gas as the driving force as well as a ceramic drive flow valve system to maintain the infusion. Currently, Codman has stopped commercialization of the Medstream pump worldwide to focus on their core products within Codman Neurosurgery. The Medallion system awaits FDA approval. It incorporates a negative pressure reservoir, thus drawing medication from the syringe during a pump refill, and reducing the risk of inadvertent deposition of medication outside the reservoir into the pump pocket. Moreover, the Medallion may offer a unique pressure sensor at the catheter tip, thus alerting the patient/physician to unanticipated changes in flow. Medtronic has also developed a new intrathecal catheter with improved resistance to kinks and breaks, a sutureless pump connector, and a new catheter anchor.
Device implantation
IDDS implantation consists of two parts requiring two separate incisions. The first entails placement of the catheter into the intrathecal space of the thoracolumbar region. Typically this incision occurs in the lower back for catheter insertion into the intrathecal space, and catheter adherence to the underlying fascia via anchoring devices (). The second part consists of placing the pump/reservoir into the abdominal region. Alternatively, the pump can be placed in the buttock region when abdominal pocket sites are unavailable (ie, multiple ostomies). Several aspects must be considered when deciding where to place the pump reservoir. First, the pump should be placed away from bony landmarks such as the lower thoracic ribs or the iliac crest, to avoid irritation. The implanting physician should discuss whether or not the patient sleeps on his/her side. If so, then the pump should be placed on the contralateral side. In the majority of patients, the pump is surgically placed on either the left or right lower quadrant of the abdomen ().
Figure 4 Patient in the lateral decubitus position for intrathecal drug delivery system implantation. The blue surgical mark indicates anticipated lumbar midline incision for intrathecal catheter placement. Photograph courtesy of MMB.
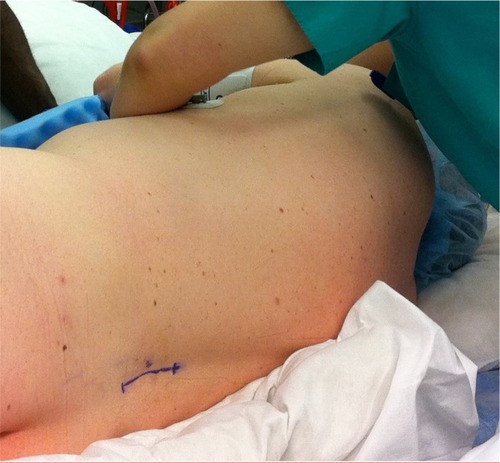
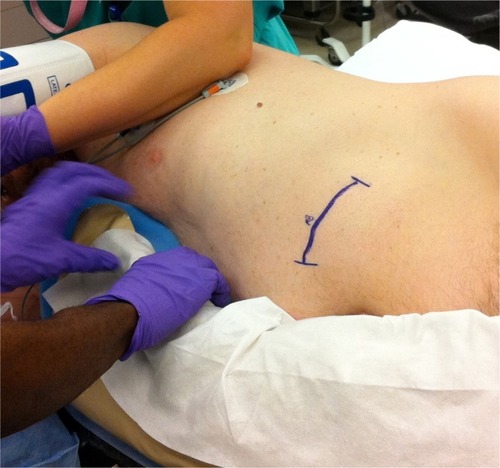
Figure 5 Patient in the lateral decubitus position for implantation of intrathecal drug delivery system. The blue surgical mark indicates anticipated incision in the left lower quadrant for the pump reservoir pocket. Note the importance of cushioning in this position to avoid complications associated with intraoperative nerve compression injuries. Photograph courtesy of MMB.
The procedure is carried out in an operating room using sterile technique which includes aseptic skin preparation, sterile drape, and full surgical attire. As placement of the pump is typically located in the abdomen, operating room positioning is usually in the lateral decubitus position to allow the physician access to the potential pump site without patient repositioning intraoperatively, which may increase both surgical time and infection risk. Fluoroscopy is used to identify needle placement for the intrathecal catheter. After the catheter is securely anchored, the surgeon then creates a subcutaneous pocket for the pump reservoir. The depth of the pocket is typically 1.5–2.5 cm to allow for appropriate needle length during pump refills in the future. Once the pocket is prepared, the catheter is tunneled subcutaneously from the posterior spine to the anterior lower abdomen. After securely connecting the catheter to the pump, the pump reservoir is anchored within the pocket and the incision sites are closed.
Pump refill procedure
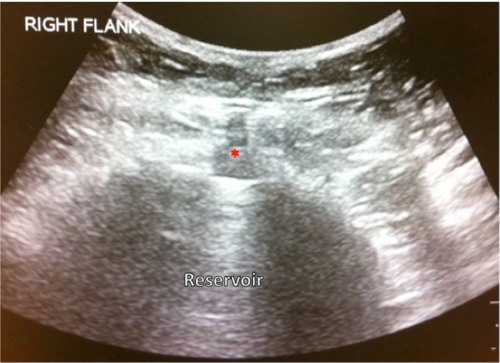
FDA regulations require that pumps be refilled at least every 6 months, even if the pump is not completely empty. Refill frequency varies between 1 and 6 months. A kit provided by the pump manufacturer is used during the refill procedure which typically contains an access needle and a template. First, the skin overlying the pump is cleansed in sterile fashion. Next, the template is placed on top of the skin overlying the pump, thus providing an outline that aids the detection of the reservoir fill port. The fill port lies in the center portion of the pump. This port may also be detected using ultrasound () or fluoroscopic guidance. The middle of the port contains a self-sealing silicone septum. The access needle is inserted through the skin and septum. Any remaining solution is withdrawn prior to refilling the pump with the new medication. The amount of withdrawn solution can be compared with the calculated amount based on the previously programmed rate of delivery to ensure that the correct amount of solution has been withdrawn and that the pump is functioning properly. Finally, the new medication is injected through the reservoir fill port followed by pump reprogramming with the new volume and/or new concentration of medication(s).
Complications
Complications of IDDS therapy can be classified into five categories, ie, mechanical system complications, pharmacological complications and side effects, surgical complications, patient-specific complications, and refill complications.
Mechanical system complications include a variety of errors that can result in either a sudden reduction in medication administration leading to potential withdrawal, or a potential significant overdose leading to hemodynamic instability, respiratory depression, and potentially death.Citation19 Mechanical errors can be further categorized beginning with the catheter and progressing to the pump: intrathecal catheter displacement causing cerebrospinal fluid leakage and potential local hygroma, intrathecal catheter kinking, catheter pump disconnection leading to leakage of administered agent, loss of pump propellant leading to altered rate of drug delivery, and gear shaft wear/motor stall causing drug underinfusion.Citation24 During pump implantation, manually testing for a leak by injecting saline through the catheter access port while occluding the catheter tip distally can aid in detecting a leak at the catheter connection site. After pump implantation, fluoroscopy is commonly used to assess catheter integrity if concerns arise. For example, the physician can aspirate 2–3 mL of fluid from the accessory port to fully clear the catheter volume in order to avoid delivering a bolus of residual medication contained inside the catheter during assessment. Radiographic contrast can then be injected safely through the accessory port and catheter under fluoroscopic guidance. This permits examination of tip placement or any disruptions in the integrity of the catheter itself. Rarely, a pump may “flip” 180 degrees within the abdomen, thus preventing reservoir access for refills and necessitating surgical intervention. The annual rate for mechanical complications requiring a surgical procedure is reported to be 10.5%, the majority being catheter-related (65%) versus pump-related (35%).Citation25 However, catheter complications may decrease with the advent of advanced catheter designs that resist occlusions and breaks.
Pharmacological adverse effects are those inherent to the drugs administered intrathecally. For example, a retrospective evaluation showed that among IDDS-related complications, the most common complication was associated with a patient’s adverse reaction to a drug.Citation26 Serious complications include anaphylaxis, respiratory depression or arrest, and/or meningitis from a contaminated solution. More specifically, intrathecal opioids may cause centrally mediated respiratory depression, nausea, vomiting, sedation, pruritus, constipation, urinary retention, cognitive impairment, and headache.Citation27 Intrathecal ziconotide may cause dizziness, nausea, vomiting, urinary retention, gait imbalance, nystagmus, and confusion. Rarer side effects include psychosis, suicide, and rhabdomyolysis.Citation24 Intrathecal baclofen has been shown to potentially cause nausea, vomiting, dizziness, urinary retention, constipation, headache, fatigue, hypotonia, and paresthesias. Life-threatening withdrawal can occur in patients in whom baclofen is abruptly discontinued, and should be treated promptly with oral baclofen supplementation and resumption of baclofen infusion. Side effects of intrathecal clonidine include hypotension, bradycardia, and sedation. Sudden discontinuation of clonidine may cause paradoxical hypertension due to a rebound in sympathetic flow. Local anesthetics can cause autonomic dysfunction, motor impairment, sensory deficits, and potential neurotoxicity, although these are not typically seen at doses of less than 15 mg per day. At higher doses, weakness, fatigue, somnolence, paresthesias, and urinary retention have all been observed.Citation27 Using device registration and social security analyses, Coffey et al found that patients with noncancer pain treated with intrathecal opioid therapy had an increased mortality (0.088% at 3 days after implantation, 0.39% at 1 month, and 3.89% at 1 year) compared with similar patients who were using other therapies. While the exact mechanism was not fully elucidated, they hypothesized that respiratory depression due to intrathecal drug overdose or mixed intrathecal and systemic drug interactions could be a possibility.Citation28
Surgical complications include bleeding, infection, cerebrospinal fluid leakage, seroma formation, neurological injury, shredded catheters, and malpositioned subcutaneous pockets.Citation24 Infection has been shown to be decreased with the use of strict sterile technique, preoperative antibiotic administration, and postoperative monitoring of the incision sites. Most infections do not necessitate removal of the implanted device, but may be treated with appropriate antibiotics after culture. Infections involving the epidural or intrathecal space require immediate removal of the IDDS and administration of intravenous antibiotics. The incidence of superficial or deep infection after placement of IDDS ranges from 2% to 5% based on case series with results from more than 100 patients.Citation29–Citation31 The risk of deep infections including epidural abscess and meningitis ranges from 0% to 0.5% in the same series. Because placement of an IDDS necessitates a postdural puncture for the placement of an intrathecal catheter, persistent cerebrospinal fluid leak can occur in as many as 20% of patients, including a postdural puncture headache.Citation32 Severe symptoms may require an epidural blood patch or surgical closure of the dural tear.
Patient-specific complications include possible hormonal fluctuations with opioid therapy. For instance, follicle-stimulating hormone, luteinizing hormone, testosterone, and growth hormone levels may decrease, inducing symptoms such as fatigue, reduced libido, and sexual dysfunction. Therefore, physicians should monitor serum levels of the aforementioned hormones during intrathecal therapy and treat accordingly.Citation33 While a physician may wean a patient from intrathecal opioids under these conditions, patients may not tolerate intrathecal opioid reduction or elimination; therefore, hormone replacement therapy may be more appropriate.Citation18
Complications may occur during the pump refill related to either medication error (ie, wrong medication or wrong concentration), reprogramming error (ie, wrong rate), or inadvertent deposition of medication outside the reservoir into the pump pocket, leading to systemic toxicity and potential overdose or death.
Special consideration must be given to catheter tip granulomas. A granuloma is a noninfectious collection of inflammatory cells located at the catheter tip that can increase in size causing spinal cord compression. Although it is rare (0.04% incidence at 1 year and 1.16% after 6 years of therapy), a granuloma can induce devastating neurological complications.Citation34 While the etiology is unknown, theories include a reaction to an infused medication, a low-grade infection, or even a reaction to the catheter tip itself.Citation27 Previously, the low-flow state of cerebrospinal fluid in the thoracic region was implicated as an increased risk for granuloma formation; however, this has not been scientifically validated.Citation35 Granuloma formation has been consistently associated with opioids in the intrathecal space, either alone or with other agents, although there are no case reports of intrathecal inflammatory masses associated with lipophilic agents such as fentanyl or sufentanil.Citation35 In preclinical studies, catheter track granuloma formation was noted in three of 25 control drugs and two of 77 control monkeys treated with just saline infusions.Citation36 Granulomas are more common in patients with nonmalignant pain versus those with cancer pain and are more prevalent in younger patients. The most frequently reported symptom leading to diagnosis of a granuloma relates to a decrease in therapeutic response/inadequate pain relief (reported in 33.5% of cases associated with catheter tip granuloma), or the onset of new pain (eg, thoracic spine pain).Citation37 MRI with gadolinium contrast or computed tomography with myelography can both elucidate granuloma formation, the latter recommended when MRI is contraindicated. The majority of granulomas regress spontaneously when the offending agent is removed from the area.Citation38 The 2012 PACC provided an algorithm for the treatment of granulomas. For instance, if the imaging study confirms the presence of a granuloma and the patient reports a significant, progressive neurological deficit, then one should arrange for a surgical consult for possible laminectomy. If there is no neurological deficit, and this is not the first occurrence of a granuloma in this particular patient, then the practitioner should avoid intrathecal opioids and switch to ziconotide. Otherwise, the physician can consider moving the catheter inferiorly in the intrathecal space by 2–3 cm, reducing the drug concentration or dose, or changing to another opioid or ziconotide. If the symptoms resolve, then repeat imaging is recommended in 6 months. However, should the symptoms persist, the catheter should be replaced and the patient should discontinue intrathecal medications/opioids, use oral analgesics in lieu of intrathecal agents, and replace the intrathecal solution with saline.Citation35
Efficacy
With respect to cancer pain, a Cochrane review published in 2005 included one randomized controlled trial (RCT) comparing intrathecal morphine with conventional delivery of morphine in patients with cancer pain.Citation39 The success rate of intrathecal delivery was 85% compared with 71% for that of conventional drug delivery. Furthermore, patients receiving intrathecal morphine survived longer, had fewer side effects, and reported less pain.Citation15 In the Cochrane review, there were 28 cohort studies involving 722 patients using intrathecal opioid delivery, namely morphine. Good to excellent analgesic effect was achieved in 87% of this group. In those patients who fail to achieve sufficient pain relief with intrathecal morphine, one RCT and several cohort studies found that adding bupivacaine was effective in these patients.Citation40 Mercadante et al published a prospective observational study on opioid-tolerant cancer patients and the use of intrathecal morphine with levobupivacaine. There was significant improvement in drowsiness and confusion during the first month of intrathecal therapy as well as statistically significant differences in pain intensity at all time intervals studied until death.Citation41 In patients with neuropathic pain, clonidine was more effective than placebo (56% versus 5%) in one RCT,Citation42 and another RCT found intrathecal ziconotide to relieve pain in patients with cancer or acquired immune deficiency syndrome, although side effects were a major limitation of this drug.Citation43 In a study focusing on IDDS and cancer pain, Smith et al published a multicenter RCT including over 200 cancer patients, highlighting the efficacy of IDDS over comprehensive medical management in treating refractory cancer pain. For example, they compared IDDS (opioid ± bupivacaine) plus medical management (opioids ± adjuvant medications) with medical management alone.Citation15 At short-term, 4-month follow-up, the IDDS group showed a greater reduction in pain and drug toxicity, as well as fewer drug side effects (fatigue and decreased level of consciousness). Importantly, the study also demonstrated an improved 6-month survival in the IDDS group (54% versus 37%). Overall, using the United States Preventative Services Task Force criteria for evidence-based medicine, Hayek et al rated intrathecal therapy for cancer pain as level II-2 evidence, with a recommendation strength of moderate when specifically using Guayatt’s criteria.Citation44
The efficacy of IDDS for the treatment of chronic non-cancer pain is less clear and robust as that for cancer pain. For instance, in a systematic review, Patel et al reported level II-3 or level III (limited) evidence for the use of intrathecal infusion systems for chronic noncancer pain due to the paucity of literature, lack of quality evidence, and lack of randomized trials.Citation45 Moreover, a 2013 review of the literature identified 28 studies of which only seven nonrandomized studies met the inclusion criteria for methodological quality assessment, and no randomized trials met the inclusion requirements. Based on these data, Falco et al stated that the evidence for intrathecal infusion systems for long-term management of chronic noncancer pain was limited based on observational studies.Citation46
The data on intrathecal baclofen as an antispasmodic, however, are more compelling. In fact, intrathecal baclofen has been used to treat spasticity for almost 30 years. In a 2006 Cochrane review, Taricco et al concluded that only intrathecal baclofen proved significantly effective in treating spinal cord injury-induced spasticity.Citation47 Further, two studies totaling 14 spinal cord injury patients showed that intrathecal baclofen significantly reduced spasticity (eg, Ashworth score and performance of activities of daily living) without any adverse effects when compared with placebo.Citation48,Citation49
Cost-effectiveness
With the rising cost of health care, there has been intense scrutiny regarding the cost effectiveness of interventional therapies. There have been several studies published examining the cost-effectiveness of intrathecal therapy, and most have examined chronic nonmalignant pain. Review of the available evidence demonstrates that although there is an initial increased cost associated with intrathecal drug delivery, the maintenance cost over time is significantly lower than with conventional medical management. For example, de Lissovoy et al compared intrathecal morphine with medical management for patients with failed back surgery syndrome and showed that IDDS therapy was cost-effective at 12–22 months.Citation50 A cost analysis study by Kumar et al comparing intrathecal therapy versus conventional pain therapy for failed back surgery syndrome patients showed that the break-even point occurred at 28 months.Citation51 In 2013, the medical cost impact of intrathecal drug delivery for noncancer pain was studied in a retrospective database review of 555 patients with noncancer pain who received an IDDS between 2006 and 2009.Citation52 In the first year following implantation, IDDS was cumulatively $17,317 more expensive than conventional pain therapy. However, the authors found that the financial break-even point occurred soon after the second year of implantation. Importantly, the lifetime analysis indicated that IDDS resulted in a net savings of $3,111 compared with conventional pain therapy. IDDS was also compared with epidural morphine delivery using an external pump. This study demonstrated that the costs of therapy were equivalent to a break-even point of approximately 3 months.Citation53 Further, Mueller-Schwefe et al concluded that, for cancer patients, intrathecal drug delivery is most cost-effective between 3 and 6 months.Citation54 Most recently, Brogan et al published a cost utilization analysis of intrathecal therapy specific to cancer pain.Citation55 In this retrospective review of 36 patients with intractable cancer pain who survived beyond 4 weeks, they concluded that intrathecal drug delivery was more cost beneficial within 6 months of treatment. However, cost-effectiveness was only seen in the high cost conventional group (ie, using rapid-acting fentanyl products) rather than the low cost conventional group. The results were similar to those published by Mueller-Schwefe et al, suggesting that IDDS becomes cost-effective more quickly for cancer patients than for noncancer patients. This may be in part due to the dynamic changes associated with cancer pain and its progression compared with that of chronic nonmalignant pain.
Conclusion
Since morphine was first injected into the spinal fluid to treat cancer pain in 1979, there have been innovations in IDDS as well as the breadth of medications delivered to the intrathecal space for control of pain and spasticity. Delivering medications intrathecally allows for significantly reduced doses compared with oral therapy, along with a reduction in the adverse effects associated with oral or parenteral delivery of analgesics. Recent consensus guidelines provide lines of therapy with agents such as morphine, ziconotide, clonidine, and bupivacaine based on nociceptive, neuropathic, or mixed pain conditions. Most experts believe that IDDS can be quite effective for a smaller subset of patients with cancer, noncancer, and spasticity-induced pain. Further, trialing patients prior to implantation is generally recommended, but may be less necessary for patients suffering from cancer pain. The data emphasize the value of intrathecal therapy for cancer pain in terms of pain relief, reduction in adverse effects, and cost-effectiveness. The evidence is less clear for long-term relief in noncancer pain other than spasticity, although the cost-effectiveness data support its use within approximately 12–24 months compared with traditional therapies. Implanting physicians should be mindful of the need to monitor fluctuations in selected serum hormones, especially with intrathecal opioids as well as the potential for development of granuloma. Studies in progress with novel intrathecal agents coupled with advanced IDDS technology offer promise for more complete pain relief, enhanced safety, and better long-term outcomes in terms of quality of life.
Disclosure
MMB has no financial interests or disclosures regarding the content of this paper. PJC has no financial interest in the content of the paper. PJC has received grant support from Medtronic, served on advisory boards for Ameritox and Purdue Pharma, and receives support for media work via Algiatry, LLC.
References
- LevyMHPain control in patients with cancerOncology1999135 Suppl 291410356692
- ChernyNIFoleyKMNonopioid and opioid analgesic pharmacotherapy of cancer painOtolaryngol Clin North Am1997302793069052671
- PappagalloMIncidence, prevalence, and management of opioid bowel dysfunctionAm J Surg2001182Suppl 5A11S18S11755892
- LamerTTreatment of cancer-related pain: when orally administered medications failMayo Clin Proc1994694734808170201
- BierAAttempts over Cocainisirung of the RuckenmarkersLangenbecks Arch Klin Chir Ver Dtsch Z Chir189951361369 German
- OnofrioBMYakshTLArnoldPGContinuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant originMayo Clin Proc1981565165206894954
- PertCBSnyderSHOpiate receptor: demonstration in nervous tissueScience1973179101110144687585
- BlackshearPJRohdeTDProslFBuchwaldHThe implantable infusion pump: a new concept in drug deliveryMed Prog Technol197930146149
- WangJKNaussLAThomasJEPain relief by intrathecally applied morphine in manAnesthesiology197950149151373503
- WallaceMYakshTLLong-term spinal analgesic delivery: a review of the preclinical and clinical literatureReg Anesth Pain Med20002511715710746527
- PragerJPNeuraxial medication delivery: the development and maturity of a concept for treating chronic pain of spinal originSpine2002272593260512435999
- DeerTRPragerJLevyRPolyanalgesic Consensus Conference 2012: recommendations on trialing for intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panelNeuromodulation20121543646422748024
- TurnerJASearsJMLoeserJDProgrammable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complicationsClin J Pain20072318019517237668
- BrownJKlapowJDoleysDLoweryDTutakUDisease specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patientsClin J Pain19991512213110382926
- SmithTJStaatsPSDeerTRandomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain, drug related toxicity, and survivalJ Clin Oncol2002204040404912351602
- RauckRLCherryDBoyerMFKosekPDunnJAloKLongterm intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer painJ Pain2003444144714622664
- ChristoPJMazloomdoostDInterventional pain treatments for cancer painAnn N Y Acad Sci2008113829932818837908
- DeerTRPragerJLevyRPolyanalgesic Consensus Conference 2012: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panelNeuromodulation201215543646622748024
- DeerTRSmithHSBurtonAWComprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer painPain Physician201114E283E31221587338
- SmithTJCoynePJHow to use implantable intrathecal drug delivery systems for refractory cancer painJ Support Oncol20031737615352650
- HassenbuschSJStanton-HicksMCovingtonECWalshJGGuthreyDSLong-term intraspinal infusions of opioids in the treatment of neuropathic painJ Pain Symptom Manage1995105275438537695
- IliasWle PolainBBuchserEDemartiniLoPTiMa Study GroupPatient-controlled analgesia in chronic pain patients: experience with a new device designed to be used with implanted programmable pumpsPain Pract2008816417018384501
- RauckRDeerTRosenSAccuracy and efficacy of intrathecal administration of morphine sulfate for treatment of intractable pain using the Prometra programmable pumpNeuromodulation20101310210821992782
- UpadhyaySPMallickPNIntrathecal drug delivery system (IDDS) for cancer pain management: a review and updatesAm J Hosp Palliat Care20122938839822089523
- SteamsLBoortz-MarxRDu PenSIntrathecal drug delivery for the management of cancer pain: a multidisciplinary consensus of best clinical practicesJ Support Oncol2005339940816350425
- BhatiaGLauMEGulurPKouryKMIntrathecal drug delivery (ITDD) systems for cancer pain. Version 3F1000Res201329624555051
- Britishpainsociety.orgIntrathecal drug delivery for the management of pain and spasticity in adults; recommendations for best clinical practiceThe British Pain Society2008 Available from: http://www.britishpainsociety.org/Accessed March 7, 2014
- CoffeyRJOwensMLBrosteSKMortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer painAnesthesiology200911188189120029253
- KumarKNathRWyantGMTreatment of chronic pain by epidural spinal cord stimulation: a 10-year experienceJ Neurosurg1991754024071869942
- LangPThe treatment of chronic pain by epiduralspinal cord stimulation – a 15 year follow up; present statusAxon19971871739295480
- MeglioMCioniBRossiGFSpinal cord stimulation in management of chronic pain. A 9-year experienceJ Neurosurg1989705195242538588
- KnightKHBrandFMMchaourabASVenezianoGImplantable intrathecal pumps for chronic pain: highlights and updatesCroat Med J200748223417309136
- DeerTRPragerJLevyRPolyanalgesic Consensus Conference 2012: recommendations to reduce morbidity and mortality in intrathecal drug delivery in the treatment of chronic painNeuromodulation20121543646422748024
- YakshTLHassenbuschSBurchielKHildebrandKRPageLMCoffeyRJInflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human dataPain Med20023299312
- DeerTRPragerJLevyRPolyanalgesic Consensus Conference 2012: consensus on diagnosis, detection, and treatment of catheter-tip granulomas (inflammatory masses)Neuromodulation20121548349522494332
- ButtMTMorphologic changes associated with intrathecal catheters for direct delivery to the central nervous system in preclinical studiesToxicol Pathol20113921321921147930
- Professional.medtronic.comMedtronic Neuromodulation: intrathecal distal end catheter occlusions as a result of pH and salt concentration gradients between delivery solution and cerebrospinal fluid (CSF) Available from: https://professional.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/inflammatory_mass_letter.pdfAccessed June 1, 2014
- JourdainVCantinLPrud’HommeMFournier-GosselinMIntrathecal morphine therapy-related granulomas: faster to grow than thoughtNeuromodulation20091216416822151292
- BallantyneJCCarwoodCMComparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancerCochrane Database Syst Rev20051CD00517815654707
- VissersKCBesseKWagemansMPain in patients with cancerPain Pract20111145347521679293
- MercadanteSIntravaiaGVillariPIntrathecal treatment in cancer patients unresponsive to multiple trials of systemic opioidsClin J Pain20072379379818075407
- Landelijke richtlijnwerkgroep Pijn bij kankerPijn bij kanker. Landelijke richtlijnLandelijke richtlijnwerkgroep Pijn bij kanker20081168978-90-8523-168-4
- StaatsPSYearwoodTCharapataSGIntrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trialJAMA2004291637014709577
- HayekSMDeerTRPopeJEPanchalSJPatelVBIntrathecal therapy for cancer and noncancer painPain Physician20111421924821587327
- PatelVBManchikantiLSinghVSchultzDMHayekSMSmithHSSystematic review of intrathecal infusion systems for long-term management of chronic non-cancer painPain Physician20091234536019305484
- FalcoFJPatelVBHayekSMIntrathecal infusion systems for long-term management of chronic non-cancer pain: an update of assessment of evidencePain Physician201316Suppl 2SE185SE21623615891
- TariccoMPagliacciMCTelaroEAdoneRPharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic reviewEura Medicophys20064251516565680
- PennRDSavoySMCorcosDLatashMGottliebGParkeBIntrathecal baclofen for severe spinal spasticityN Engl J Med1989320151715212657424
- HugenholtsHNelsonRFDehouxEBickertonRIntrathecal baclofen for intractable spinal spasticity-a double blind crossover study in eight patientsCan J Neurol Sci1992191881951623444
- de LissovoyGBrownREHalpernMHassenbuschSJRossECost-effectiveness of longterm intrathecal morphine therapy for pain associated with failed back surgery syndromeClin Ther199719961129083712
- KumarKHunterGDemeriaDDTreatment of chronic pain by using intrathecal drug therapy compared with conventional pain therapies: a cost-effectiveness analysisJ Neurosurg20029780381012405366
- GuillemetteSWitzkeSLeierJHinnenthalJPragerJPMedical cost impact of intrathecal drug delivery for noncancer painPain Med20131450451523480485
- BedderMDBurchielKLarsonACost analysis of two implantable narcotic delivery systemsJ Pain Symptom Manage199163683731908884
- Mueller-SchwefeGHassenbuschSJReigECost effectiveness of intrathecal therapy for painNeuromodulation19992778722151111
- BroganSEWinterNBAbiodunASafarpourRA cost utilization analysis of intrathecal therapy for refractory cancer pain: identifying factors associated with cost benefitPain Med20131447848623461787