Abstract
Background
The majority of surgical patients experience significant levels of pain after a procedure. While opioid analgesics have been a mainstay of postsurgical analgesic regimens, recent evidence has supported the use of multimodal therapy as a way to decrease opioid usage with its concomitant opioid-related adverse events. The goal of multimodal therapy is to minimize the negative effects of these events on clinical and economic outcomes. The purpose of this study was to assess the opioid burden and health economic outcomes in patients undergoing open colectomy who received a liposomal bupivacaine-based multimodal analgesic regimen as compared with a standard opioid-based regimen for postsurgical pain.
Methods
In this open-label, single-center, sequential-cohort study, adults undergoing open colectomy were assigned to treatment via patient-controlled analgesia with opioids (first cohort) or multimodal analgesia therapy including a single administration of liposomal bupivacaine (second cohort). Both treatment groups were offered rescue analgesia as needed. The main outcome measures were total mg amount of opioids consumed after surgery, total hospital costs, and length of hospital stay. Adverse events, including opioid-related adverse events, were recorded.
Results
Thirty-nine patients were enrolled, 18 in the opioid-based analgesia group and 21 in the multimodal analgesia group. Mean total amount of postsurgical opioids consumed was significantly less in the multimodal analgesia group (57 mg) compared with the opioid analgesia group (115 mg; P = 0.025). The average total cost of hospitalization in the multimodal group was $8766 versus $11,850 in the opioid group (P = 0.027), and the median length of hospital stay was 2.0 days versus 4.9 days, respectively (P = 0.004).
Conclusion
This study confirmed that a liposomal bupivacaine–based multimodal analgesic regimen resulted in less opioid consumption, lower hospital costs, and a shorter length of stay than a standard opioid-based analgesic regimen for postsurgical pain in patients undergoing open colectomy.
Introduction
During the past two decades, there has been heightened awareness about the importance of perioperative pain management, and several clinical guidelines have been published with a focus on the treatment of perioperative pain.Citation1–Citation4 However, a large proportion of patients continue to experience significant pain following both inpatient and outpatient surgeries.Citation5–Citation8 Balancing the need for adequate postsurgical pain relief while minimizing treatment-related adverse effects is a key factor for timely patient recovery and ambulation, as well as patient satisfaction after surgery.Citation4,Citation9–Citation12 While opioids are effective analgesics and continue to be a mainstay of postsurgical pain management regimens, opioid-related adverse events are common and the clinical and economic burden associated with these events is significant.Citation11 Data from previous studies suggest a reduction in opioid-related adverse events may result in shorter length of hospital stay and lower hospital costs.Citation11
Liposomal bupivacaine (Exparel®, Pacira Pharmaceuticals Inc, Parsippany, NJ) is an extended-release formulation of bupivacaine designed to allow drug diffusion to occur for up to 72 hours following a single administration at the end of surgery. It is indicated for single-dose administration into the surgical site to produce postsurgical analgesia.Citation13 The objective of this study was to assess total opioid burden and health economic outcomes in adult patients undergoing open colectomy with general anesthesia who received a multimodal regimen that included liposomal bupivacaine for postsurgical pain compared with those who received patient-controlled analgesia (PCA) with opioids.
Materials and methods
This single-center, Phase IV, prospective, open-label, sequential study evaluated the pharmacoeconomic outcomes associated with a multimodal analgesia regimen including a single administration of liposomal bupivacaine 266 mg given via wound infiltration at the end of surgery, compared with an analgesia regimen including PCA with intravenous morphine or hydromorphone. The study was conducted by the author at Southern Regional Medical Center, Riverdale, GA, from December 2011 to June 2012 (US National Institutes of Health Clinical Trials Identifier NCT01207246). The protocol was approved by a central institutional review board, and the study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines.Citation14 Written informed consent was obtained from all patients before any study procedures were conducted.
Patients
Adults ≥ 18 years of age undergoing open segmental colectomy with anastomosis (cecectomy, right or left hemicolectomy, resection of transverse colon, or sigmoidectomy) were included in the study. Patients were excluded from participation in the study if they were pregnant, had a history of drug/alcohol abuse, or had severe hepatic impairment or any other concomitant condition that, in the opinion of the investigator, could make the patient an inappropriate candidate for the study. Those who required unplanned multiple resections of the large intestine or unplanned colostomies placed during surgery were excluded, as well as those who required administration of intraoperative analgesics (other than fentanyl), nonsteroidal anti-inflammatory drugs, local anesthetics (other than liposome bupivacaine), or alvimopan.
Study procedures
Patients were enrolled in sequential cohorts, and those assigned to the opioid-based treatment group were enrolled first. Screening procedures, including obtaining informed consent, medical history, physical examination, documentation of American Society of Anesthesiologists (ASA) physical status classification, and pregnancy testing, were performed in the 2 weeks prior to surgery (study day 1). Patient eligibility was reconfirmed on the day of surgery and the surgical procedure was performed as per the surgeon’s standard technique.
For patients assigned to the opioid-based treatment group, the study drug was initiated as soon as possible after surgery and administered via an intravenous PCA pump as needed, according to the institutional protocol at the study site. PCA treatment was continued until patient discharge, or until the patient was converted to orally administered pain medication (oxycodone, either alone or in combination with acetaminophen). Patients were converted from intravenous administration to orally administered drugs as per the clinical judgment of the investigator. Those assigned to the multimodal therapy group received a single administration of liposomal bupivacaine 266 mg in 40 mL of 0.9% normal saline administered into the surgical site prior to wound closure. Administration of the drug was divided equally between the left and right side of the surgical wound; approximately 75% was infiltrated in the perifascial region and 25% was infiltrated around the junction between the subcutaneous and dermal regions. A moving needle technique with frequent aspirations was used for infiltration of the study drug to reduce the risk of accidental intravascular injection. Patients in the multimodal therapy group also received a single administration of intravenous ketorolac 30 mg (or alternative intravenous nonsteroidal anti-inflammatory drug) at the end of surgery followed by orally administered acetaminophen 1000 mg and ibuprofen 600 mg given every 6 hours for 72 hours after surgery, once patients were no longer non per os. Patients in both treatment groups were offered rescue analgesia with intravenous opioid and/or orally administered oxycodone 5 mg/acetaminophen 325 mg every 6 hours as needed (maximum daily dose of acetaminophen was 4000 mg) until patient discharge.
Adverse events and total amounts of postsurgical opioids consumed during the hospital stay were recorded. Timing of patient discharge was at the discretion of the surgeon, and only after resolution of the postoperative ileus. Patients were examined in the surgeon’s office between 7 and 10 days after discharge, and were contacted by telephone 30 days after surgery for administration of follow-up surveys regarding postsurgical complications, satisfaction with postsurgical analgesia, and adverse events.
Outcome measures
Three alternative primary outcome measures were used to determine the efficacy of the intervention: total mg amount of opioids (in morphine equivalents) consumed after surgery until discharge or through day 30, whichever occurred first; total cost of hospitalization, calculated using UB-40 and/or similar claim forms as appropriate; and length of hospital stay after surgery, defined as time from wound closure until discharge or through day 30, whichever occurred first. Secondary outcome measures included patient satisfaction with postsurgical analgesia assessed at day 30 using a five-point Likert scale (-2, extremely dissatisfied; +2, extremely satisfied); patient responses to a follow-up survey regarding hospital readmission, unplanned medical visits, and health-related problems during recovery (assessed at day 30); and postsurgical adverse events, including opioid-related adverse events, through day 30. Opioid-related adverse events were defined as somnolence, respiratory depression, hypoventilation, hypoxia, dry mouth, nausea, vomiting, constipation, sedation, confusion, pruritus, urinary retention, and postoperative ileus.
Data analysis
The safety population included all patients who underwent the planned surgery. The efficacy population included all patients who underwent the planned surgery and did not meet any of the intraoperative exclusion criteria. Patients in the multimodal therapy group had to receive liposome bupivacaine in order to be included in the efficacy population. Between-group comparisons for continuous measures of efficacy were conducted using a one-way analysis of variance model. Between-group time to event comparisons were conducted using a log-rank test, and comparisons for categorical measures of efficacy were conducted using a Fisher’s Exact test. For total amount of opioid consumption, all opioid medication amounts were converted to equianalgesic opioid amounts and log-transformed for comparison. The significance level was set at 0.05 for all comparisons, with no adjustments made for multiple tests.
Results
A total of 42 patients were enrolled in the study. Of these, 39 underwent the planned surgery and were evaluable for safety and efficacy. The three excluded patients were discontinued from the study due to unplanned colostomy (n = 2) or loop ileostomy (n = 1) procedures. Patient demographics and baseline characteristics for the opioid (n = 18) and multimodal (n = 21) analgesic treatment groups are summarized in .
Table 1 Demographics and baseline characteristics
Graphical representations of results for the three main outcomes are shown in –C. The mean total amount of opioid medications consumed postsurgery (), mean total cost of hospitalization (), and median length of hospital stay () were significantly less (P < 0.05) in the multimodal analgesic group compared with the opioid group. In the multimodal analgesic group, 80% of patients reported being “satisfied” or “extremely satisfied” with their postsurgical analgesia compared with 89% in the group that received an opioid-based regimen (P = 0.38). There were no statistically significant differences observed in the proportion of patients who were readmitted to the hospital or had unplanned visits/contact with their physician due to postsurgical complications ().
Figure 1 (A) Mean total amount of opioid medications consumed after surgery was 50% less in the group receiving a multimodal analgesic regimen (57 mg) versus the opioid group (115 mg). (B) Total average costs of hospitalization were 26% lower in the multimodal analgesic group ($8766) versus the opioid group ($11,850). (C) Median length of hospital stay after surgery was 59% shorter in the multimodal analgesic group (2.0 days) versus the opioid group (4.9 days).
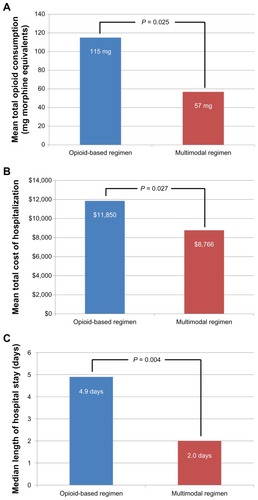
Table 2 Survey results regarding hospital readmission, unplanned medical visits, and health-related problems during recovery
Overall, 11 patients (28%) experienced 18 postsurgical adverse events (). The most frequently reported adverse events were abdominal pain and ileus. Serious adverse events occurred in 17% (three of 18) of the patients in the group receiving an opioid regimen (one incident each of small bowel obstruction, wound evisceration, and wound drainage) and 14% (three of 21) of the group receiving multimodal analgesic therapy (two incidents of abdominal pain and one incident each of ileus and intestinal obstruction). None of these events were considered to be related to the study drug by the investigator. No opioid-related adverse events were observed.
Table 3 Adverse events
Discussion
A liposomal bupivacaine-based multimodal analgesic regimen for postsurgical pain was associated with a 50% reduction in the average amount of opioid medications consumed after surgery (57 mg versus 115 mg), a median length of hospital stay that was 59% shorter (2.0 days versus 4.9 days), and total average hospital costs that were 26% lower ($8766 versus $11,850) compared with an opioid-based analgesic regimen in this single-center study of patients undergoing open colectomy. These differences occurred without any apparent compromise in patients’ postsurgical recovery experiences. The vast majority of patients (≥80% in each treatment group) were satisfied with their analgesia after surgery. Also, there were few patient-reported problems during recovery and the rates of return for care through one month after surgery were not statistically different between the two groups. It is surprising that no opioid-related adverse events were observed in either treatment group, especially given the higher amounts of opioids consumed by the group receiving an opioid-based analgesic regimen. Reasons for the lack of association between opioid usage and opioid-related adverse events in this study are unclear, but may be related to difficulties in differentiating opioid-related adverse events from normal recovery after gastrointestinal surgery. It should also be noted that this small, single-center study was not powered to assess opioid-related adverse events as one of the coprimary outcome measures.
While patients were sequentially assigned (not randomized) to their treatment arm, the two groups were well matched for age and ethnicity. There was a slightly higher proportion of females included in the multimodal analgesic group. This group also had a higher proportion of patients with comorbidities (95%) versus the opioid-based analgesic group (83%), based on ASA physical classifications. It is possible that this higher proportion of “sicker” patients may have contributed to the slightly higher adverse event rate observed in the multimodal analgesic regimen group.
To the author’s knowledge, this is the first published report of data regarding effects of a multimodal analgesic regimen involving a locally administered, prolonged-release local anesthetic formulation compared with an opioid-based analgesic regimen in the setting of open colectomy. Polglase et alCitation15 investigated the efficacy and safety of a continuous wound infusion with ropivacaine 1% administered adjunctively with intravenous morphine PCA, as needed, and intravenous tramadol 50 mg and intravenous or rectally administered acetaminophen 1000 mg, each given every 6 hours (n = 143). This was compared with the same regimen without ropivacaine (placebo, n = 167) in patients undergoing colorectal surgery. In the study by Polglase et al,Citation15 there were no significant between-group differences observed in opioid usage or hospital length of stay (costs were not assessed).
Conclusion
Given that this was a nonrandomized, open-label, single-center study conducted by only one surgeon in a small cohort of patients, the observed results should be interpreted with caution. Despite these limitations, a liposomal bupivacaine-based multimodal analgesic regimen for postsurgical pain, which also included ketorolac, acetaminophen, and ibuprofen, was well tolerated in the setting of open colectomy. This regimen resulted in a statistically significant difference, demonstrating less opioid use, a shorter hospital stay, and lower mean hospitalization costs compared with an opioid-based regimen. Although the standard of care for postsurgical analgesia is evolving, opioids remain a key component of analgesic regimens for most surgical patients. Future investigations should aim for larger, randomized, and well-controlled studies to confirm these results and to define further the potential health and economic benefits of a liposomal bupivacaine-based multimodal analgesic regimen. Nevertheless, liposomal bupivacaine appears to be a useful therapeutic option for postsurgical analgesia with the potential to reduce opioid burden during patient recovery.
Disclosure
The author is a consultant and a member of the speakers bureau for Pacira Pharmaceuticals, Inc. This study was funded by Pacira Pharmaceuticals, Inc. Editorial and writing assistance was provided by Peloton Advantage, LLC, and supported by Pacira Pharmaceuticals, Inc.
References
- Clinicians’ quick reference guide to postoperative pain management in adultsPain Management Guideline Panel. Agency for Health Care Policy and Research, US Department of Health and Human ServicesJ Pain Symptom Manage199272142281517644
- The Joint CommissionFacts about pain management Available from: http://www.jointcommission.org/pain_management/Accessed August 21, 2012
- RowlingsonJCRawalNPostoperative pain guidelines – targeted to the site of surgeryReg Anesth Pain Med20032826526712945017
- ApfelbaumJLPractice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain ManagementAnesthesiology201211624827322227789
- WarfieldCAKahnCHAcute pain management. Programs in US hospitals and experiences and attitudes among US adultsAnesthesiology199583109010947486160
- ApfelbaumJLChenCMehtaSSGanTJPostoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanagedAnesth Analg20039753454012873949
- SommerMde RijkeJMVan KleefMThe prevalence of postoperative pain in a sample of 1490 surgical inpatientsEur J Anaesthesiol20082526727418053314
- GramkeHFde RijkeJMVan KleefMThe prevalence of postoperative pain in a cross-sectional group of patients after day-case surgery in a university hospitalClin J Pain20072354354817575496
- BeauregardLPompAChoiniereMSeverity and impact of pain after day-surgeryCan J Anaesth1998453043119597202
- WhitePFMultimodal analgesia: its role in preventing postoperative painCurr Opin Investig Drugs200897682
- OderdaGMSaidQEvansRSOpioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stayAnn Pharmacother20074140040617341537
- AdamsonRTLewIBeyzarovEAmaraSReitanJClinical and economic implications of postsurgical use of opioid therapyHosp Pharm2011466 Suppl 1S4S11
- Exparel (bupivacaine liposome injectable suspension) [package insert]San Diego, CAPacira Pharmaceuticals Inc2011
- International Conference on Harmonisation Working GroupICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1)International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human UseJune 10, 1996Washington, DC Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdfAccessed April 19, 2012
- PolglaseALMcMurrickPJSimpsonPJContinuous wound infusion of local anesthetic for the control of pain after elective abdominal colorectal surgeryDis Colon Rectum2007502158216717914653