Abstract
Paracetamol remains the recommended first-line option for mild-to-moderate acute pain in general population and particularly in vulnerable populations. Despite its wide use, debate exists regarding the analgesic mechanism of action (MoA) of paracetamol. A growing body of evidence challenged the notion that paracetamol exerts its analgesic effect through cyclooxygenase (COX)-dependent inhibitory effect. It is now more evident that paracetamol analgesia has multiple pathways and is mediated by the formation of the bioactive AM404 metabolite in the central nervous system (CNS). AM404 is a potent activator of TRPV1, a major contributor to neuronal response to pain in the brain and dorsal horn. In the periaqueductal grey, the bioactive metabolite AM404 activated the TRPV1 channel‐mGlu5 receptor‐PLC‐DAGL‐CB1 receptor signaling cascade. The present article provides a comprehensive literature review of the centrally located, COX-independent, analgesic MoA of paracetamol and relates how the current experimental evidence can be translated into clinical practice. The evidence discussed in this review established paracetamol as a central, COX-independent, antinociceptive medication that has a distinct MoA from non-steroidal anti-inflammatory drugs (NSAIDs) and a more tolerable safety profile. With the establishment of the central MoA of paracetamol, we believe that paracetamol remains the preferred first-line option for mild-to-moderate acute pain for healthy adults, children, and patients with health concerns. However, safety concerns remain with the high dose of paracetamol due to the NAPQI-mediated liver necrosis. Centrally acting paracetamol/p-aminophenol derivatives could potentiate the analgesic effect of paracetamol without increasing the risk of hepatoxicity. Moreover, the specific central MoA of paracetamol allows its combination with other analgesics, including NSAIDs, with a different MoA. Future experiments to better explain the central actions of paracetamol could pave the way for discovering new central analgesics with a better benefit-to-risk ratio.
Plain Language Summary
The mechanisms of how paracetamol works are still subject to debate despite its widespread use. Previous scientific research questions the idea that paracetamol relieves pain by inhibiting cyclooxygenases (COX). Now, it is more apparent that paracetamol’s pain-relieving effect occurs in the brain and is independent of the COX enzyme. This article reviewed the central paracetamol actions and how the current experimental evidence can be used in clinical practice. Based on the evidence discussed in this review, paracetamol works differently than NSAIDs and has a safer safety profile. With the central MoA for paracetamol in place, we think paracetamol will still be the first choice for acute pain management. When the COX-independent mechanism of action and stable pharmacokinetic properties of paracetamol are considered, it is safe to say that paracetamol should be preferred over NSAIDs for analgesia, especially in special populations. Newer painkillers, based on the central action of paracetamol, are being studied and show promising results in relieving pain without increasing the risk of liver damage.
Introduction
Pain represents substantial healthcare and financial burden despite the available pain management approaches, highlighting the urgent need for innovative medications and, possibly, indications of established analgesics.Citation1 Non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol (also known as acetaminophen or N-acetyl-p-aminophenol) are commonly prescribed for pain management in a wide range of conditions. Globally, paracetamol is also at the apex of the most commonly utilized over-the-counter (OTC) analgesic and the preferred first-line option for patients with mild-to-moderate acute pain.Citation2 The good tolerability at the recommended dosage (eg, lack of gastrointestinal adverse effects) is a major contributor to the preference for paracetamol by general practitioners, specialists, and pharmacists.Citation3 Although it was introduced in clinics more than 100 years ago,Citation4 paracetamol remains the recommended first-line option for pain management by several scientific societies, and particularly in vulnerable populations such as geriatrics, children, patients with peptic ulcers, and pregnant women.Citation5,Citation6 Despite its wide use, debate exists regarding the analgesic mechanism of action (MoA) of paracetamol.Citation7
Historically, it was believed that the paracetamol-mediated analgesia stems solely from its cyclooxygenase (COX)-dependent inhibitory effect on prostaglandin (PG) synthesisCitation8; however, it is now evident that the analgesic MoA of paracetamol is multidimensional and involves several pathways within the central nervous system (CNS), such as the endocannabinoid, serotonergic, and nitric oxide pathwaysCitation9–14 or for some authors opioid pathways.Citation15 The notion that we already know concerning the metabolic pathways for paracetamol has been challenged in the past two decades following the 2005 study by Högestätt et al; in this report, the authors demonstrated that, following hepatic deacetylation to p-aminophenol, this paracetamol metabolite crosses the blood–brain barrier (BBB) and is converted to N-arachidonoylphenolamine (AM404).Citation16 Subsequent experimental evidence found that this new metabolite pathway was involved in its analgesic MoA.Citation17–19 Moreover, several molecular actors targeted by the bioactive AM404, including the Cav3.2 calcium channel, the cannabinoid CB1 receptors and TRPV1 receptors, would participate in the MoA.Citation10,Citation20,Citation21
Paracetamol is generally considered safe for many patients, particularly in comparison with NSAIDs and opioids. The growing evidence of the centrally located COX-independent actions of paracetamol can pave the way for optimizing the use of an old drug in a more widened patients’ profile. More importantly, the development of paracetamol–based derivatives can maximize the central effects of paracetamol while reducing the risk of toxicity.Citation22–25 The present article provides a comprehensive literature review of the centrally located, COX-independent, analgesic MoA of paracetamol and how the current experimental evidence can be translated into clinical practice. This review highlighted the advantages of the centrally located MoA and the benefit/risk of paracetamol in mild-to-moderate pain conditions. Consequently, we discussed the potential clinical applications of paracetamol analogs. Besides, we review the safety aspects of paracetamol administration and how it can be preferred over NSAIDs due to its more tolerable safety profile.
Review Development
A bibliographic literature search was performed on Medline via PubMed to collect relevant articles from their inception to May 2022. The following keywords were employed during the literature search: (paracetamol[MeSH Terms]) OR (acetaminophen[MeSH Terms])) AND (AM404). The online bibliographic search was complemented by a manual screening of the relevant references.
The Analgesic MoA is Independent of Central COX Inhibition
Historically, Vane and Flower demonstrated that paracetamol reduced brain PGE2 with a preferred action in the CNS than in the periphery.Citation8
PGs are produced by COX enzymes which need to be oxidized by hydroperoxides to be catalytically active. Paracetamol, as reducing agent,Citation26 reduces the hydroperoxide tone and thus inactivates COX.Citation27–30 The selectivity of paracetamol for CNS was hypothesized to be based on cellular concentration of peroxide and arachidonic acid. High levels of peroxide (found especially in the periphery, eg, in an inflammatory context) or arachidonic acid, thwart paracetamol COX inhibition.Citation30–33
Another proposed COX-dependent MoA of paracetamol is the involvement of another COX isoenzyme, named COX-3. Flower and Vane demonstrated that the COX inhibitory effect of paracetamol was more potent in the brain than in the spleen, suggesting another COX isoenzyme as a sensitive site for paracetamol action.Citation8 COX-3 is an exon splice variant from COX-1 that was identified in the canine cerebral cortex and exhibited selective sensitivity to paracetamol.Citation34 However, further genomic and kinetic studies showed that a full-length, catalytically active form of COX-3 does not exist in humansCitation35,Citation36 and that the COX-3 selective interaction is unlikely to be clinically relevant.Citation31
However, the question remained whether the analgesic MoA of paracetamol is primarily mediated by COX inhibition. In mice, administering a weekly dose of paracetamol, which induced an analgesic effect, did not reduce PGE2 content in the brain.Citation37 Besides, the antinociceptive profile of paracetamol was found to be different from that of the competitive COX inhibitor, ibuprofen, with no observed antinociceptive action of ibuprofen, in the first phase of the formalin test, despite the proposed shared MoA.Citation37 Altogether, these results indicate that the analgesic action of paracetamol cannot be attributed to the inhibition of central COX, however data from the literature would suggest such an involvement.Citation38 Furthermore, the inhibitory effect of paracetamol on COX observed by some authors seems more closely related to its hypothermic/antipyretic effects than to its analgesic action.Citation39–42
AM404: The Active Metabolite of Paracetamol
Research experiments have shown that the analgesic MoA of paracetamol is primarily COX-independent and mediated by the formation of the bioactive AM404 metabolite in the CNS, highlighting that paracetamol is a pro-drug of a potent analgesic metabolite. The first description of the central conversion of paracetamol was reported in 2005. In this report, Högestätt et al demonstrated that the deacetylated form of paracetamol, the p-aminophenol, is converted mainly in the brain to AM404 by the fatty acid amide hydrolase (FAAH) enzyme ().Citation16 These findings were then supported by Muramatsu et al’s experiment.Citation20 The first human evidence of central conversion of paracetamol to AM404 was demonstrated by Sharma et al in 2017; 26 patients received 1g of paracetamol, followed by measurement of the AM404 in the cerebrospinal fluid (CSF) and blood. The study detected AM404 in the CSF of 17 patients, confirming the central conversion of paracetamol to the bioactive AM404 in humans.Citation43
Figure 1 Schematic representation of the central mechanism of action of paracetamol to its antinociceptive activity. Paracetamol is deacetylated in p-aminophenol in the liver, then metabolized in the brain by FAAH into AM404. AM404 activates the TRPV1 channel‐mGlu5 receptor‐PLC‐DAGL‐CB1 pathway and co-activates the Cav 3.2 T-type calcium channel, which in turn reinforces the activity of the descending serotonergic pathways.
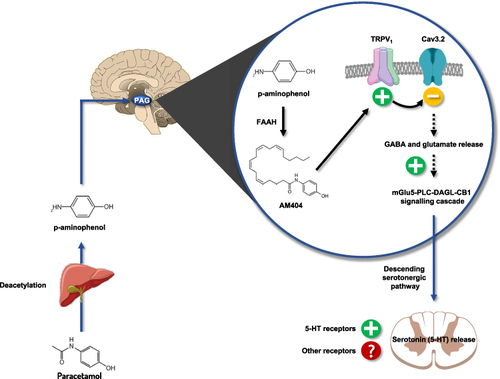
Importantly, it has been shown that 1) centrally injected AM404 induced analgesiaCitation37,Citation44,Citation45 and 2) the inhibition of FAAH (genetically and pharmacologically) suppressed the analgesic effect of paracetamol (and p-aminophenol), demonstrating the involvement of AM404 as the active metabolite in the analgesic effect of paracetamol.Citation17,Citation18,Citation37 In the setting of nociceptive pain, Dalmann et al observed the loss of paracetamol analgesic effect after supra-spinal administration of a FAAH inhibitor, which did not occur after peripheral FAAH inhibitor injection. This demonstrated that the FAAH-synthetized AM404 formation occurs mainly at supra-spinal level,Citation19 even if a study evoked a spinal level.Citation46
AM404-Modulated Molecular Targets
TRPV1 Channels
AM404 is a potent activator of TRPV1, a major contributor to neuronal response to pain. Contrary to the periphery, where activated TRPV1 induces nociception, hyperalgesia, and pain transduction modulation,Citation47 supraspinal TRPV1 activation induces anti-nociception.Citation37,Citation45,Citation48 In Zygmunt et al’s patch-clamp experiment, AM404 activated the vanilloid receptor TRPV1, an effect that was suppressed after administration of the TRPV1 antagonist, capsazepine.Citation49 Besides, experiments showed diminished analgesic action of paracetamol and p-aminophenol in TRPV1 knockout mice and mice pretreated with capsazepine.Citation18,Citation37,Citation45 Notably, a recent report by Barrière et alCitation50 demonstrated that paracetamol reduced the connectivity of brain structures in the pain pathway, particularly the periaqueductal grey, where local blockade of TRPV1 suppressed the analgesic action of paracetamol, this confirms a fully TRPV1-dependent mechanism and thus tends to exclude a mechanism involving central COX. As a confirmation of the role of TRPV1 in humans, Pickering et al demonstrated the involvement of a specific TRPV1 rs224534 variant in paracetamol antinociception in healthy volunteers.Citation51
Downstream Targets of TRPV1
The T-type calcium Cav3.2 isoform regulates the neuronal response to pain.Citation52,Citation53 Cav3.2 knockout models and mice pretreated with Cav3.2 blockers showed impaired nociceptive response to pain stimuli.Citation54 Previous reports indicated that the antinociceptive action of arachidonic compounds involves inhibition of Cav3.2 channels, suggesting a potential interaction between Cav3.2 and AM404.Citation11 In 2014, Kerckhove et al demonstrated that Cav3.2 knockout mice had impaired response to the analgesic effect of a systemic administration of paracetamol or intracerebroventricular injection of AM404.Citation55 Interestingly, an experiment aimed to establish the location of Cav3.2 involved in the analgesic effect of paracetamol found that, contrary to supra-spinal channels, spinal Cav3.2 blockade did not affect the analgesic profile of paracetamol, highlighting that the involvement of Cav3.2 in the analgesic activity of paracetamol is mainly supra-spinal.Citation45
In Cav3.2 knockout mice, the analgesic effect of the TRPV1 agonist (capsaicin) was diminished, despite the analgesic action of an inhibitor of Cav3 channels (TTA-A2) persisted in TRPV1 knockout mice suggesting that Cav3.2 and TRPV1 support paracetamol’s analgesic activity sequentially, Cav3.2 acting downstream of TRPV1.Citation45
In the setting of paracetamol injection, CB1 knockout models or mice pretreated with selective CB1 antagonists showed no analgesic response to paracetamol.Citation17,Citation56 The expected synergism between paracetamol and opiates was also attenuated in mice pretreated with selective CB1 antagonist.Citation57 Notably, two previous reports showed that paracetamol injection did not alter the brain endocannabinoid content, suggesting that other studies are needed to know how CB1 receptors are involved. Recently, periaqueductal grey-located CB1 receptors were found to be essential for the MoA of paracetamol through a AM404-activated TRPV1 channel‐mGlu5 receptor‐PLC‐DAGL‐CB1 receptor signaling.Citation50
Serotonin (5-HT) and norepinephrine are engaged in pathways of physiological nociception, both of which can be produced via descending pain pathways to alter nociceptive transmission in the spinal cord. Serotonin appears to have both inhibitory and facilitative effects on pain.Citation58 The involvement of the serotonergic pathway in the analgesic effect of paracetamol was described even before the 2005 work by Högestätt et al.Citation59 Earlier reports described the pharmacological blockade of the serotonergic pathway decreased the antinociceptive effect of paracetamol.Citation60 The involvement of the serotonergic pathway in paracetamol action was reported to stem from increasing 5-TH levels in the central structures and activating the spinal 5-TH receptors.Citation60–71 Nonetheless, the evidence was not conclusive regarding the contribution of serotonergic receptors to the paracetamol action. As an example, the involvement of 5-HT3 receptors has been discussed in humans,Citation62,Citation70,Citation72–74 and experimental work in rodents with 5-HT3 receptor antisense oligonucleotides showed that they were not involved in the analgesic effect of paracetamol, while a tropisetron-sensitive receptor was.Citation74 However, investigations showed that p-aminophenol analgesic action was mediated by the bulbospinal serotonergic system,Citation18 which may suggest an involvement of this serotonergic system in the AM404 antinociceptive activity, as recently suggested.Citation63 Finally, if bulbospinal serotoninergic pathways seem to be involved in the analgesic effect of paracetamol or its metabolites, the exact nature of the spinal tropisetron-sensitive receptor involved in this effect remains to be elucidated.
Altogether, the data collected draw the schematic MoA represented in .
Potential Clinical Consequences of the Centrally Located Analgesic Action of Paracetamol
Paracetamol, a COX-Independent, Centrally Acted as the First Choice for a Wide Range of Special Populations
Safety is the primary concern in choosing OTC analgesics, particularly for mild-to-moderate pain conditions in vulnerable groups. Patients with health concerns are frequently reluctant to take the appropriate analgesic dose due to safety concerns, leading to inadequate pain management.Citation6 Most NSAIDs have safety concerns related to the development of gastric ulcers and bleeding, bronchial asthma, acute kidney injury, and long-term adverse cardiovascular outcomes.Citation75 Initial reports classified paracetamol as a part of the NSAIDs class due to the presumably shared inhibitory effect on PG synthesis. One thing that is already accepted in the scientific community is that paracetamol has almost no anti-inflammatory activity and does not cause the same adverse effects as NSAIDs do.Citation5 So, it is sensible to assume that paracetamol is different from NSAIDs. The fact that paracetamol has a limited peripheral COX inhibitory effect and potent centrally located actions can help better understand the benefit–risk ratio of this old drug.
The maximum single dose of paracetamol for pain or fever treatment is 1 g every 4–6 hours as needed, up to a recommended maximum daily dose of 4 g. It is well established that paracetamol is clinically effective under the recommended daily dose and has a well-tolerable safety profile.Citation76 Paracetamol-related adverse events are rare in healthy adults receiving a daily dose of ≤4 g/day.Citation77 Thus, several guidelines recommend paracetamol as an optimal option for mild-to-moderate pain conditions, such as headache and migraine attacks, postoperative pain and chronic osteoarthritis for limited duration of treatment (however, with some discrepancies here, according to guidelines).Citation78–81
Interestingly, the analgesic effect of paracetamol has a distinct dose-response, according to experimental models and consumers’ experience.Citation82 The French National Agency for Medicines and Health Products Safety (ANSM) recommended initiating paracetamol with the minimum dose. In a recent focus review, studies comparing the analgesic response to higher (1 g) and lower (500–650 mg) doses of paracetamol were retrieved and summarized. Both direct and indirect comparisons demonstrated analgesic superiority of higher single dose compared to lower single doses, highlighting that a higher single dose of paracetamol for a short duration (4–5 days) poses more benefit than lower single doses.Citation80 This observation is consistent with the successful experimental demonstration in healthy volunteers of a lack of acetaminophen ceiling effect on R-III nociceptive flexion reflex.Citation83 In terms of safety, a Cochrane review concluded that the rate of adverse events was similar between the higher and lower single doses of paracetamol.Citation84 This was in line with another clinical trial showing similar rates of treatment-related adverse events between the higher and lower single doses of paracetamol.Citation85
Still, the safety of paracetamol is surrounded by controversy. Of course, hepatoxicity is well established and remains a major issue. Indeed, paracetamol produces variable amounts of the hepatotoxic compound, NAPQI. Hepatic metabolism of paracetamol via the P450 system (mainly CYP-2E1) leads to the synthesis of NAPQI normally conjugated with glutathione and eliminated in urine. However, when high doses of paracetamol are used or at usual doses, in some patients at risk (malnutrition, alcoholism, some combinations of drugs, etc), glutathione stores can be decreased and/or CYP activity increased, both leading to a high level of NAPQI and NAPQI-mediated liver necrosis, and thus doses must be adapted in this population. Interestingly, a recent work studying the role of acetaminophen on epitranscriptome suggests that its hepatotoxicity could be reduced by adequate strategy.Citation86 Otherwise, although some other safety issues associated with paracetamol remain inconclusive, some patients with comorbidities have concerns with the use of paracetamol for common pain conditions. Thus, it is imperative to consolidate the current literature and appropriately weigh the risk–benefit ratio of paracetamol for pain conditions in special populations until good scientific evidence of safety should prevail. Recently, Alchin et al reviewed the current evidence of paracetamol as the first-option analgesic in patients with kidney disease, cardiovascular diseases, gastrointestinal disorders, asthma, and the elderly.Citation6 In another context, a recent work suggests that acetaminophen would be a potential suppressor of antitumor immunity, suggesting that it should be used with caution in patients treated with immune checkpoint blockers.Citation87
Regarding kidney function, NSAIDs, inhibitors of COX, should be avoided in patients with renal impairment.Citation88 On the contrary, therapeutic doses of paracetamol are generally safe and do not lead to acute renal failure in patients with renal impairment.Citation89 It was previously hypothesized that paracetamol may cause renal injury due to the reports of nephropathy after phenacetin overuse; however, this hypothesis was then rejected by further evidence.Citation90 Data show no association between paracetamol and acute renal failure in patients with chronic kidney disease.Citation91 Thus, paracetamol remains the mainstay analgesic option in patients with chronic kidney disease.Citation92 It is unnecessary to modify the dose for dialytic patients; however, dose reduction or longer dose interval should be considered in patients with advanced kidney failure.Citation6
NSAIDs significantly increase the risk of cardiovascular adverse outcomes.Citation93 No robust evidence supports an association between paracetamol and adverse cardiovascular outcomes (stroke or myocardial infarction) in patients with atherosclerotic heart diseases.Citation94–96 On the other hand, the risk of worsening hypertension should be considered, with some observational studies showing an increase in blood pressure among chronic paracetamol users.Citation97,Citation98 Still, published studies failed to show an association between elevated blood pressure and the risk of serious cardiovascular events in paracetamol users.Citation96 Paracetamol remains probably safer than NSAIDs which showed a significant increase in adverse cardiovascular outcomes amongst patients receiving non-aspirin NSAIDs.Citation99 According to an American Heart Association position statement, paracetamol should be preferred over NSAIDs in patients with coronary artery disease.Citation100
Aspirin and other NSAIDs significantly increase the risk of gastrointestinal ulcers and bleeding. Unlike NSAIDs, paracetamol, which has a minimal peripheral COX inhibitory effect, does not cause damage to the gastrointestinal tract. The current evidence affirms the more tolerable gastrointestinal safety profile of paracetamol than NSAIDs.Citation101 Thus, paracetamol is the recommended first-line option for patients at risk of gastrointestinal ulcers or bleeding.Citation6
Finally, paracetamol is considered the preferred analgesic option for the elderly, a challenging group to manage due to the high frequency of comorbidities and polypharmacy. Paracetamol represents an effective and safe analgesic option; however, its use in the elderly with chronic pain is still questionable.Citation102
Based on the above, it can be concluded that the centrally acted paracetamol is a compelling first-line analgesic option even for special populations if a low dosage is used, and it should be preferred over NSAIDs due to its very limited COX action and tolerable safety profile.
Paracetamol in Combination with Other Analgesics
Multimodal analgesia has become a principal approach for the management of acute postoperative pain conditions that offers additive or synergistic analgesia and lower opioid dose requirements. Theoretically, combining two analgesics with different mechanisms of action potentiates their analgesic actions without increasing their doses and compromising the safety outcomes.Citation103–106 Paracetamol has linear and consistent pharmacokinetics, and its tolerable profile makes it a good option when a combination is required.Citation107
As previously elucidated, paracetamol has a central, COX-independent, analgesic action that can synergize other analgesics with different central or peripheral MoA. Thus, it can potentiate the analgesic effect of opioids without increasing the risk of adverse events (). The paracetamol plus opioids combination can benefit from the rapid onset of action of paracetamol and the sustained analgesic effect of opioids.Citation108 Several reports demonstrated that paracetamol plus opioids led to superior pain relief and lower dose requirements than individual components without increasing the risk of adverse events in a wide range of pain conditions, such as osteoarthritis and postoperative pain.Citation109,Citation110 However, we do not have good evidence that paracetamol spares the opioid adverse effects when a high dose of opioid is used.Citation111 Recently, a comparative trial showed that the oxycodone/paracetamol combination was more effective than celecoxib in terms of reducing postoperative pain and rescue analgesia consumption amongst patients undergoing arthroscopic knee surgery. The oxycodone/paracetamol combination exhibited well tolerable safety profile, with no difference between the two groups.Citation112
Table 1 Selected Clinical Studies Using Paracetamol Plus Opioids for Acute Post-Operative Pain
Paracetamol/NSAIDs combinations have regained much attention following the establishment of the COX-independent MoA of paracetamol. Combining paracetamol with NSAIDs can represent an alternative for opioids-based analgesics in patients with moderate pain and no contraindication for NSAIDs. Paracetamol can theoretically potentiate the analgesic and morphine-sparing effects of NSAIDs in patients with acute pain without the need to increase NSAIDs doses.Citation113,Citation114 In a previous systematic review, combining paracetamol and NSAIDs was more effective than either agent alone in acute postoperative pain.Citation115 The antinociceptive effects derived from paracetamol/NSAIDs combinations appear to be additive rather than synergistic; in 2012, Moore et al demonstrated that paracetamol plus NSAIDs led to additive analgesic rather than supra-additive (ie, synergism) effects.Citation116
Previous pharmacokinetic reports demonstrated the lack of significant interactions in the pharmacokinetic profiles of ibuprofen and paracetamol when administered in a fixed-dose combination tablet, highlighting that the effect of this combination is primarily a pharmacodynamic one.Citation117,Citation118 Thus, the paracetamol/ibuprofen combination has been studied in several trials over the recent two decades. In a 2013 Cochrane review over three trials (n = 1647 patients), paracetamol/ibuprofen combination demonstrated higher reductions in acute postoperative pain and fewer patients needed rescue analgesia than either drug alone in patients undergoing impacted third molars extraction.Citation119 These findings align with the 2010 systematic review showing that paracetamol/ibuprofen combination achieved superior analgesia than either drug alone.Citation115 Recently, the PANSAID trial showed paracetamol/ibuprofen combination achieved higher analgesia and morphine-sparing effect than paracetamol alone in the first 24 hours after total hip arthroplasty.Citation120 The benefit of the paracetamol/ibuprofen combination was not significantly influenced by age, sex, or type of anesthesia.Citation121 Notably, comparative trials also showed that the non-narcotic paracetamol/ibuprofen combination had a similar or higher analgesic effect compared to the acetaminophen/codeine approach.Citation122–124
According to the abovementioned studies performed in acute pain conditions, the analgesic paracetamol/ibuprofen combination appears to be well tolerable without a significant increase in safety concerns compared to either drug alone. In the PANSAID trial, patients on paracetamol/ibuprofen combination for postoperative pain at the first 24 hours after total hip arthroplasty did not experience a significant increase in the risk of serious adverse events compared to patients on either drug alone.Citation120 However, a 13-week trial showed patients on paracetamol/ibuprofen combination had a higher risk of decreases in hemoglobin level (≥1 g/dl) than monotherapy, suggesting a potential risk of bleeding on long-term use of paracetamol/ibuprofen combination.Citation122 A population-based retrospective cohort study, using data from the government of Quebec health insurance agency databases, suggested that combining traditional NSAIDs with acetaminophen increases the risk of upper GI events beyond that of either agent alone.Citation125 Even if one of the limitations of this study pertains to the use of administrative databases in which the indication for which a medication is prescribed and actual drug consumption is not known, the results of these two studies warrant performing further research to establish the beneficial effect of the paracetamol/NSAIDs combinations and to investigate their safety profile for patients in need of chronic therapy.
The combination of paracetamol and gabapentin is another combination that is currently under investigation. Experimental evidence showed that paracetamol had a synergistic effect on gabapentin.Citation126 In patients who underwent an abdominal hysterectomy, Durmus et al studied the analgesic effect of the preoperative paracetamol and gabapentin combination compared to gabapentin 1200 mg alone. The results showed that the paracetamol and gabapentin combination was superior to gabapentin alone on pain intensity scores.Citation127 Further investigations are warranted.
Paracetamol Perspective: Future Analogs
As we have elucidated in this article, paracetamol itself can be considered as a pro-drug of the active centrally located metabolite, AM404. Nonetheless, paracetamol must first undergo hepatic deacylation into p-aminophenol and also produces variable amounts of the hepatotoxic compound NAPQI. Thus, paracetamol analogs were proposed to improve the safety profile of the drug, while keeping the same or even higher analgesic activity.
The development of paracetamol analogs that target the brain TRPV1 receptors without the production of NAPQI and depletion of glutathione stores is a clinical perspective linked to the central action of paracetamol. It has been recently shown that paracetamol amine analogs undergo FAAH-dependent transformation into TRPV1 activators without producing hepatotoxicity at the same dose that paracetamol produces clinically relevant hepatotoxicity; these analogs exhibited dose-dependent antinociceptive effects without antipyretic action, liver cell necrosis, or elevation in liver enzyme levels.Citation18,Citation128 Adamantyl, a paracetamol analog that selectively inhibits the TRPA1 receptor, was also proposed.Citation129 These findings highlighted that developing centrally acted analgesics is possible; these compounds can exhibit a well-tolerable safety profile without significantly inducing hepatotoxicity.
Conclusion and Expert Opinion
Paracetamol remains among the most commonly prescribed analgesics. Nonetheless, the paracetamol MoA remained complex and not yet fully understood. It is now more evident that paracetamol analgesia has multiple pathways and is mediated by the formation of the bioactive AM404 metabolite in the CNS. The evidence discussed in this review established paracetamol as a central, COX-independent, analgesic medication that has a distinct MoA from NSAIDs and a more tolerable safety profile.
This leads to consider that paracetamol remains the preferred first-line option for mild-to-moderate acute pain management for healthy adults and patients with health concerns, such as the elderly, patients with gastrointestinal, renal impairments and/or cardiovascular disease. Nonetheless, to avoid hepatotoxicity, healthcare practitioners should consider appropriate paracetamol dosing individually in special populations and weigh the potential risk–benefit ratio after considering the patient-specific factors.
The central COX-independent MoA of paracetamol warrants to use it for multimodal analgesic approaches. Paracetamol can potentiate the analgesic effect of opioids without increasing the risk of adverse events; still, the evidence is limited, and further studies are needed on the morphine-sparing effect of combining paracetamol and opioids. Paracetamol/NSAIDs combination resulted in higher analgesia and no increase in the risk of adverse events than NSAIDs alone. Although the interest in paracetamol-based combinations is growing, the evidence is still limited, and further high-quality evidence is warranted.
As our understanding of the AM404-mediated activation of the TRPV1 channel and CB1 receptor signaling evolves, several investigations are being conducted to test the benefit of paracetamol analogs, with promising results. Paracetamol analogs can activate the TRPV1 channel signaling without the hepatic production of the toxic metabolite, NAPQI; hence, they can induce a higher analgesic effect than that of paracetamol without increasing the risk of hepatoxicity. Thus, future experiments to better explain the central actions of paracetamol could pave the way for discovering analgesics with a better benefit-to-risk ratio as genetic studies could help to predict efficacy and tolerability of paracetamol.
Abbreviations
5-HT, serotonin (5-hydroxytryptamine); AM404, N-arachidonoylphenolamine; ANSM, Agence Nationale de Sécurité des Médicaments (French national agency for medicines and health products safety); CNS, central nervous system; COX, cyclooxygenase; CSF, cerebrospinal fluid; CYP, cytochrome; DAGL, diacylglycerol lipases; FAAH, fatty acid amide hydrolase; MoA, mechanism of action; NAPQI, N-acetyl-p-benzoquinone imine; NSAIDs, nonsteroidal anti-inflammatory drugs; OTC, over-the-counter; PG, prostaglandin; PLC, phospholipase C; POX, peroxidase; TRP, transient receptor potential; TRPV1, transient receptor potential vanilloid.
Disclosure
Alain Eschalier and Jules Desmeules received honoraria from UPSA for the development of this manuscript. Rassa Pegahi is an employee of UPSA. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Nagakura Y. The need for fundamental reforms in the pain research field to develop innovative drugs. Expert Opin Drug Discov. 2017;12(1):39–46. doi:10.1080/17460441.2017.1261108
- Ennis ZN, Dideriksen D, Vægter HB, Handberg G, Pottegård A. Acetaminophen for chronic pain: a systematic review on efficacy. Basic Clin Pharmacol Toxicol. 2016;118(3):184–189. doi:10.1111/BCPT.12527
- O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–342. doi:10.1016/j.amjopharm.2012.09.004
- Mallet C, Eschalier A. The rediscovery of paracetamol, In: Farquhar-Smith P, Beaulieu P, Jaggar S, editors. Landmark Papers in Pain: Seminal Papers in Pain with Expert Commentaries. Vol. 1. 1st ed. Oxford University Press; 2018. doi:10.1093/MED/9780198834359.003.0004
- Freo U, Ruocco C, Valerio A, Scagnol I, Nisoli E. Paracetamol: a review of guideline recommendations. J Clin Med. 2021;10(15). doi:10.3390/jcm10153420
- Alchin J, Dhar A, Siddiqui K, Christo PJ. Why paracetamol (Acetaminophen) is a suitable first choice for treating mild to moderate acute pain in adults with liver, kidney or cardiovascular disease, gastrointestinal disorders, asthma, or who are older. Curr Med Res Opin. 2022;1–15. doi:10.1080/03007995.2022.2049551
- Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12(3–4):250–275. doi:10.1111/j.1527-3458.2006.00250.x
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature. 1972;240(5381):410–411. doi:10.1038/240410A0
- Esh CJ, Chrismas BCR, Mauger AR, Taylor L. Pharmacological hypotheses: is Acetaminophen selective in its cyclooxygenase inhibition? Pharmacol Res Perspect. 2021;9(4):e00835. doi:10.1002/prp2.835
- Ohashi N, Kohno T. Analgesic effect of acetaminophen: a review of known and novel mechanisms of action. Front Pharmacol. 2020;11:580289. doi:10.3389/fphar.2020.580289
- Przybyła GW, Szychowski KA, Gmiński J. Paracetamol - An old drug with new mechanisms of action. Clin Exp Pharmacol Physiol. 2020. doi:10.1111/1440-1681.13392
- Mallet C, Eschalier A, Daulhac L. Paracetamol: update on its analgesic mechanism of action. Pain Reli. 2017. doi:10.5772/66649
- Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21(3):201–232. doi:10.1007/s10787-013-0172-x
- Toussaint K, Yang XC, Zielinski MA, et al. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35(6):617–638. doi:10.1111/J.1365-2710.2009.01143.X
- Raffa RB, Walker EA, Sterious SN. Opioid receptors and acetaminophen (paracetamol). Eur J Pharmacol. 2004;503(1–3):209–210. doi:10.1016/j.ejphar.2004.08.055
- Högestätt ED, Jönsson BAG, Ermund A, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system*. J Biol Chem. 2005;280(36):31405–31412. doi:10.1074/JBC.M501489200
- Mallet C, Daulhac L, Bonnefont J, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139(1):190–200. doi:10.1016/J.PAIN.2008.03.030
- Barrière DA, Mallet C, Blomgren A, et al. Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One. 2013;8(8). doi:10.1371/JOURNAL.PONE.0070690
- Dalmann R, Daulhac L, Antri M, Eschalier A, Mallet C. Supra-spinal FAAH is required for the analgesic action of paracetamol in an inflammatory context. Neuropharmacology. 2015;91:63–70. doi:10.1016/J.NEUROPHARM.2014.11.006
- Muramatsu S, Shiraishi S, Miyano K, et al. Metabolism of AM404 From Acetaminophen at Human Therapeutic Dosages in the Rat Brain. Anesthesiol Pain Med. 2016;6(1). doi:10.5812/AAPM.32873
- Saliba SW, Marcotegui AR, Fortwängler E, et al. AM404, paracetamol metabolite, prevents prostaglandin synthesis in activated microglia by inhibiting COX activity. J Neuroinflammation. 2017;14(1). doi:10.1186/S12974-017-1014-3
- Sodano F, Lazzarato L, Rolando B, et al. Paracetamol-galactose conjugate: a novel prodrug for an old analgesic drug. Mol Pharm. 2019;16(10):4181–4189. doi:10.1021/acs.molpharmaceut.9b00508
- Parashar A. Pharmacological screening of glycine amino acid prodrug of acetaminophen. Indian J Pharmacol. 2015;47(2):202–205. doi:10.4103/0253-7613.153431
- Wu Z, Patel A, Dave R, Yuan X. Development of acetaminophen proline prodrug. Bioorg Med Chem Lett. 2010;20(13):3851–3854. doi:10.1016/J.BMCL.2010.05.050
- Fadl TA, Omar FA. Paracetamol (acetaminophen) esters of some non-steroidal anti-inflammatory carboxylic acids as mutual prodrugs with improved therapeutic index. Inflammopharmacology. 1998;6(2):143–157. doi:10.1007/S10787-998-0031-3
- Hanel AM, Lands WEM. Modification of anti-inflammatory drug effectiveness by ambient lipid peroxides. Biochem Pharmacol. 1982;31(20):3307–3311. doi:10.1016/0006-2952(82)90565-2
- Schildknecht S, Daiber A, Ghisla S, Cohen RA, Bachschmid MM. Acetaminophen inhibits prostanoid synthesis by scavenging the PGHS-activator peroxynitrite. FASEB J. 2008;22(1):215–224. doi:10.1096/FJ.06-8015COM
- Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387(2):273–280. doi:10.1006/ABBI.2000.2232
- Lucas R, Warner TD, Vojnovic I, Mitchell JA, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J. 2005;19(6):1–15. doi:10.1096/FJ.04-2437FJE
- Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc Natl Acad Sci U S A. 2002;99(10):7130–7135. doi:10.1073/PNAS.102588199/ASSET/682AB754-3CF6-4EC9-B4FB-18483C903C21/ASSETS/GRAPHIC/PQ1025881008.JPEG
- Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12(1):46–55.
- Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79(1):9–19. doi:10.1016/j.clpt.2005.09.009
- Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275(42):32783–32792. doi:10.1074/JBC.M003505200
- Chandrasekharan NV, Dai H, Roos KLT, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–13931. doi:10.1073/pnas.162468699
- Schwab JM, Beiter T, Linder JU, et al. COX-3--A virtual pain target in humans? FASEB J. 2003;17(15):2174–2175. doi:10.1096/FJ.03-0595LTE
- Dinchuk JE, Liu RQ, Trzaskos JM. COX-3: in the wrong frame in mind. Immunol Lett. 2003;86(1):121. doi:10.1016/S0165-2478(02)00268-7
- Mallet C, Barrière DA, Ermund A, et al. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One. 2010;5(9):1–11. doi:10.1371/journal.pone.0012748
- Ayoub SS. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature. 2021;8(4): 351–371. doi:10.1080/23328940.2021.1886392
- Engström Ruud L, Wilhelms DB, Eskilsson A, et al. Acetaminophen reduces lipopolysaccharide-induced fever by inhibiting cyclooxygenase-2. Neuropharmacology. 2013;71:124–129. doi:10.1016/J.NEUROPHARM.2013.03.012
- Feldberg W, Gupta KP. Pyrogen fever and prostaglandin-like activity in cerebrospinal fluid. J Physiol. 1973;228(1):41–53. doi:10.1113/JPHYSIOL.1973.SP010071
- Ayoub SS, Botting RM, Goorha S, Colville-Nash PR, Willoughby DA, Ballou LR. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein. Proc Natl Acad Sci U S A. 2004;101(30):11165–11169. doi:10.1073/PNAS.0404185101
- Mirrasekhian E, Nilsson JLÅ, Shionoya K, et al. The antipyretic effect of paracetamol occurs independent of transient receptor potential ankyrin 1-mediated hypothermia and is associated with prostaglandin inhibition in the brain. FASEB J. 2018;32(10):5751–5759. doi:10.1096/fj.201800272R
- Sharma CV, Long JH, Shah S, et al. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J Pain Res. 2017;10:2703–2709. doi:10.2147/JPR.S143500
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi:10.1200/JCO.2007.15.0235
- Kerckhove N, Mallet C, François A, et al. Ca(v)3.2 calcium channels: the key protagonist in the supraspinal effect of paracetamol. Pain. 2014;155(4):764–772. doi:10.1016/J.PAIN.2014.01.015
- Ohashi N, Uta D, Sasaki M, Ohashi M, Kamiya Y, Kohno T. Acetaminophen metabolite N-acylphenolamine induces analgesia via transient receptor potential vanilloid 1 receptors expressed on the primary afferent terminals of C-fibers in the spinal dorsal horn. Anesthesiology. 2017;127(2):355–371. doi:10.1097/ALN.0000000000001700
- Ryskamp DA, Redmon S, Jo AO, Križaj D. TRPV1 and endocannabinoids: emerging molecular signals that modulate mammalian vision. Cells. 2014;3(3):914. doi:10.3390/CELLS3030914
- Liao HT, Lee HJ, Ho YC, Chiou LC. Capsaicin in the periaqueductal gray induces analgesia via metabotropic glutamate receptor-mediated endocannabinoid retrograde disinhibition. Br J Pharmacol. 2011;163(2):330. doi:10.1111/J.1476-5381.2011.01214.X
- Zygmunt PM, Chuang HH, Movahed P, Julius D, Högestätt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:1. 39–42. doi:10.1016/S0014-2999(00)00207-7
- Barrière DA, Boumezbeur F, Dalmann R, et al. Paracetamol is a centrally acting analgesic using mechanisms located in the periaqueductal grey. Br J Pharmacol. 2020;177(8):1773–1792. doi:10.1111/bph.14934
- Pickering G, Creveaux I, Macian N, Pereira B. Paracetamol and pain modulation by TRPV1, UGT2B15, SULT1A1 genotypes: a randomized clinical trial in healthy volunteers. Pain Med. 2020;21(4):661–669. doi:10.1093/PM/PNZ037
- Bourinet E, Francois A, Laffray S. T-type calcium channels in neuropathic pain. Pain. 2016;157:S15–S22. doi:10.1097/J.PAIN.0000000000000469
- Harding EK, Zamponi GW. Central and peripheral contributions of T-type calcium channels in pain. Mol Brain. 2022;15(1):1–13. doi:10.1186/S13041-022-00923-W
- Francois A, Kerckhove N, Meleine M, et al. State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain. 2013;154(2):283–293. doi:10.1016/J.PAIN.2012.10.023
- Barbara G, Alloui A, Nargeot J, et al. T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J Neurosci. 2009;29(42):13106–13114. doi:10.1523/JNEUROSCI.2919-09.2009
- Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531(1–3):280–281. doi:10.1016/J.EJPHAR.2005.12.015
- Hama AT, Sagen J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology. 2010;58(4–5):758–766. doi:10.1016/J.NEUROPHARM.2009.12.010
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–225. doi:10.1016/j.brainresrev.2008.12.009
- Tjølsen A, Lund A, Hole K. Antinociceptive effect of paracetamol in rats is partly dependent on spinal serotonergic systems. Eur J Pharmacol. 1991;193(2):193–201. doi:10.1016/0014-2999(91)90036-P
- Dogrul A, Seyrek M, Akgul EO, Cayci T, Kahraman S, Bolay H. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT 7 receptors. Eur J Pharmacol. 2012;677(1–3):93–101. doi:10.1016/j.ejphar.2011.12.016
- Courade JP, Caussade F, Martin K, et al. Effects of Acetaminophen on monoaminergic systems in the rat central nervous system. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(6):534–537. doi:10.1007/s002100100484
- Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79(4):371–378. doi:10.1016/J.CLPT.2005.12.307
- Nakamura S, Nonaka T, Komatsu S, Yamada T, Yamamoto T. Oral Acetaminophen-induced spinal 5-hydroxytryptamine release produces analgesic effects in the rat formalin test. Biomed Pharmacother. 2022;146. doi:10.1016/J.BIOPHA.2021.112578
- Alloui A, Pelissier T, Dubray C, Lavarenne J, Eschalier A. Tropisetron inhibits the antinociceptive effect of intrathecally administered paracetamol and serotonin. Fundam Clin Pharmacol. 1996;10(4):406–407. doi:10.1111/J.1472-8206.1996.TB00593.X
- Alloui A, Chassaing C, Schmidt J, et al. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol. 2002;443(1–3):71–77. doi:10.1016/S0014-2999(02)01578-9
- Bonnefont J, Alloui A, Chapuy E, Clottes E, Eschalier A. Orally administered paracetamol does not act locally in the rat formalin test: evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiology. 2003;99(4):976–981. doi:10.1097/00000542-200310000-00034
- Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114(3):482–490. doi:10.1016/J.PAIN.2005.01.019
- Bonnefont J, Daulhac L, Etienne M, et al. Acetaminophen recruits spinal p42/p44 MAPKs and GH/IGF-1 receptors to produce analgesia via the serotonergic system. Mol Pharmacol. 2007;71(2):407–415. doi:10.1124/MOL.106.025775
- Liu J, Reid AR, Sawynok J. Antinociception by systemically-administered acetaminophen (paracetamol) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett. 2013;536(1):64–68. doi:10.1016/J.NEULET.2012.12.052
- Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84(1):47–51. doi:10.1038/SJ.CLPT.6100403
- Yamaguchi C, Yamamoto D, Fujimaru Y, Asano T, Takaoka A. Acetaminophen exerts an analgesic effect on muscular hyperalgesia in repeated cold-stressed rats through the enhancement of the descending pain inhibitory system involving spinal 5-HT 3 and noradrenergic α 2 receptors. Biol Pharm Bull. 2021;44(8):1067–1074. doi:10.1248/BPB.B21-00178
- Pickering G, Faure M, Commun F, et al. Tropisetron and paracetamol association in post-operative patients. Fundam Clin Pharmacol. 2012;26(3):432–437. doi:10.1111/J.1472-8206.2011.00933.X
- Bandschapp O, Filitz J, Urwyler A, Koppert W, Ruppen W. Tropisetron blocks analgesic action of acetaminophen: a human pain model study. Pain. 2011;152(6):1304–1310. doi:10.1016/J.PAIN.2011.02.003
- Hamurtekin Y, Nouilati A, Demirbatir C, Hamurtekin E. The contribution of serotonergic receptors and nitric oxide systems in the analgesic effect of acetaminophen: an overview of the last decade. Turkish J Pharm Sci. 2020;17(1):119. doi:10.4274/TJPS.GALENOS.2018.35403
- Danelich IM, Wright SS, Lose JM, Tefft BJ, Cicci JD, Reed BN. Safety of nonsteroidal antiinflammatory drugs in patients with cardiovascular disease. Pharmacother J Hum Pharmacol Drug Ther. 2015;35(5):520–535. doi:10.1002/PHAR.1584
- Jozwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm Drug Res. 2014;71(1):11–23.
- Hayward KL, Powell EE, Irvine KM, Martin JH. Can paracetamol (Acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol. 2016;81(2):210–222. doi:10.1111/bcp.12802
- Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. doi:10.1136/annrheumdis-2016-209707
- Haag G, Diener HC, May A, et al. Self-medication of migraine and tension-type headache: summary of the evidence-based recommendations of the Deutsche Migräne und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft für Neurologie (DGN), the Österreichische Kopfschmerzgesellschaft (Ö). J Headache Pain. 2011;12(2):201–217. doi:10.1007/s10194-010-0266-4
- Gaul C, Eschalier A. Dose can help to achieve effective pain relief for acute mild to moderate pain with over-the-counter paracetamol. Open Pain J. 2018;11(1):12–20. doi:10.2174/1876386301811010012
- Perrot S, Eschalier A, Desmeules J, Lanteri-Minet M, Attal N. Practice guidelines for the treatment of acute migraine and chronic knee osteoarthritis with paracetamol: an expert appraisal on evolution over time between scientific societies. Curr Med Res Opin. 2022. doi:10.1080/03007995.2022.2076475
- McQuay HJ, Moore RA. Dose-response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. Br J Clin Pharmacol. 2007;63(3):271–278. doi:10.1111/j.1365-2125.2006.02723.x
- Piguet V, Desmeules J, Dayer P. Lack of Acetaminophen ceiling effect on R-III nociceptive flexion reflex. Eur J Clin Pharmacol. 1998;53(5):321–324. doi:10.1007/S002280050386
- Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L. Non-prescription (OTC) oral analgesics for acute pain - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;2017(10). doi:10.1002/14651858.CD010794.pub2
- Qi DS, May LG, Zimmerman B, et al. A randomized, double-blind, placebo-controlled study of acetaminophen 1000 mg versus acetaminophen 650 mg for the treatment of postsurgical dental pain. Clin Ther. 2012;34(12). doi:10.1016/j.clinthera.2012.11.003
- Evke S, Lin Q, Melendez JA, Begley TJ. Epitranscriptomic reprogramming is required to prevent stress and damage from Acetaminophen. Genes. 2022;13(3). doi:10.3390/GENES13030421/S1
- Bessede A, Marabelle A, Guégan JP, et al. Impact of Acetaminophen on the efficacy of immunotherapy in cancer patients. Ann Oncol off J Eur Soc Med Oncol. 2022;33(9):909–915. doi:10.1016/J.ANNONC.2022.05.010
- Davison SN. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol. 2019;14(6):917–931. doi:10.2215/CJN.05180418
- Hiragi S, Yamada H, Tsukamoto T, et al. Acetaminophen administration and the risk of acute kidney injury: a self-controlled case series study. Clin Epidemiol. 2018;10:265–276. doi:10.2147/CLEP.S158110
- Evans M, Fored CM, Bellocco R, et al. Acetaminophen, aspirin and progression of advanced chronic kidney disease. Nephrol Dial Transplant. 2009;24(6):1908–1918. doi:10.1093/ndt/gfn745
- Graham G, Graham R, Day R. Comparative analgesia, cardiovascular and renal effects of celecoxib, rofecoxib and acetaminophen (paracetamol). Curr Pharm Des. 2005;8(12):1063–1075. doi:10.2174/1381612023394917
- Davison SN, Rathwell S, George C, Hussain ST, Grundy K, Dennett L. Analgesic use in patients with advanced chronic kidney disease: a systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;(7). doi:10.1177/2054358120910329
- Martín Arias LH, Martín González A, Sanz Fadrique R, Vazquez ES. Cardiovascular risk of nonsteroidal anti-inflammatory drugs and classical and selective cyclooxygenase-2 inhibitors: a meta-analysis of observational studies. J Clin Pharmacol. 2019;59(1):55–73. doi:10.1002/JCPH.1302
- White WB, Kloner RA, Angiolillo DJ, Davidson MH. Cardiorenal Safety of OTC Analgesics. J Cardiovasc Pharmacol Ther. 2018;23(2):103–118. doi:10.1177/1074248417751070
- Dawson J, Fulton R, Mcinnes GT, et al. Acetaminophen use and change in blood pressure in a hypertensive population. J Hypertens. 2013;31(7):1485–1490. doi:10.1097/HJH.0b013e328360f6f8
- Fulton RL, Walters MR, Morton R, et al. Acetaminophen use and risk of myocardial infarction and stroke in a hypertensive cohort. Hypertension. 2015;65(5):1008–1014. doi:10.1161/HYPERTENSIONAHA.114.04945
- Turtle EJ, Dear JW, Webb DJ. A systematic review of the effect of paracetamol on blood pressure in hypertensive and non-hypertensive subjects. Br J Clin Pharmacol. 2013;75(6):1396–1405. doi:10.1111/BCP.12032
- MacIntyre IM, Turtle EJ, Farrah TE, Graham C, Dear JW, Webb DJ. Regular acetaminophen use and blood pressure in people with hypertension: the PATH-BP trial. Circulation. 2022;145(6):416–423. doi:10.1161/CIRCULATIONAHA.121.056015
- Schjerning AM, McGettigan P, Gislason G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol. 2020;17(9):574–584. doi:10.1038/s41569-020-0366-z
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. doi:10.1161/CIRCULATIONAHA.106.181424
- Ishitsuka Y, Kondo Y, Kadowaki D. Toxicological property of acetaminophen: the dark side of a safe antipyretic/analgesic drug? Biol Pharm Bull. 2020;43(2):195–206. doi:10.1248/bpb.b19-00722
- BMJ Publishing Group Limited. What dose of paracetamol for older people? Drug Ther Bull. 2018;56(6):69–72. doi:10.1136/dtb.2018.6.0636
- Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215–2225. doi:10.1016/S0140-6736(11)60245-6
- Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–264. doi:10.1046/j.1365-2710.2001.00355.x
- Patel R, Dickenson AH. Neuropharmacological basis for multimodal analgesia in chronic pain. Postgrad Med. 2022;134(3):245–259. doi:10.1080/00325481.2021.1985351
- Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12(11):1084–1095. doi:10.1016/S1474-4422(13)70193-5
- Bannwarth B, Péhourcq F. [Pharmacologic basis for using paracetamol: pharmacokinetic and pharmacodynamic issues]. Drugs. 2003;63(Special Issue 2):5–13. French. doi:10.2165/00003495-200363992-00003
- Schug SA. Combination analgesia in 2005—a rational approach: focus on paracetamol–tramadol. Clin Rheumatol. 2006;25(SUPPL 7):16–21. doi:10.1007/S10067-006-0202-9/FIGURES/5
- Dhillon S. Tramadol/paracetamol fixed-dose combination: a review of its use in the management of moderate to severe pain. Clin Drug Investig. 2010;30(10):711–738. doi:10.2165/11205830-000000000-00000
- Jebaraj B, Maitra S, Baidya DK, Khanna P. Intravenous paracetamol reduces postoperative opioid consumption after orthopedic surgery: a systematic review of clinical trials. Pain Res Treat. 2013;2013. doi:10.1155/2013/402510
- Freo U. Paracetamol for multimodal analgesia. Pain Manag. 2022;12(6):737–750. doi:10.2217/pmt-2021-0116
- Liu J, Di J, Zhang Y, Xing E. Oxycodone–paracetamol tablet exhibits increased analgesic efficacy for acute postoperative pain, higher satisfaction and comparable safety profiles compared with celecoxib in patients underwent arthroscopic knee surgery. Inflammopharmacology. 2021;29(4):1091–1099. doi:10.1007/s10787-021-00828-5
- Dahl JB, Nielsen RV, Wetterslev J, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1165–1181. doi:10.1111/AAS.12382
- Miranda HF, Puig MM, Prieto JC, Pinardi G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain. 2006;121(1–2):22–28. doi:10.1016/J.PAIN.2005.11.012
- Ong CKS, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179. doi:10.1213/ANE.0B013E3181CF9281
- Moore RA, Derry CJ, Derry S, Straube S, McQuay HJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. Eur J Pain. 2012;16(4):585–591. doi:10.1016/J.EJPAIN.2011.08.009
- Tanner T, Aspley S, Munn A, Thomas T. The pharmacokinetic profile of a novel fixed-dose combination tablet of ibuprofen and paracetamol. BMC Clin Pharmacol. 2010;10(1):10. doi:10.1186/1472-6904-10-10
- Atkinson HC. A pharmacokinetic analysis of a novel fixed dose oral combination of paracetamol and ibuprofen, with emphasis on food effect. J Bioequiv Availab. 2015;7(3). doi:10.4172/JBB.1000230
- Derry CJ, Derry S, Moore RA. Single dose oral ibuprofen plus paracetamol (Acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 2013;2013(6). doi:10.1002/14651858.CD010210.PUB2
- Thybo KH, Hägi-Pedersen D, Dahl JB, et al. Effect of combination of paracetamol (acetaminophen) and ibuprofen vs either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty: the PANSAID randomized clinical trial. JAMA. 2019;321(6):562–571. doi:10.1001/JAMA.2018.22039
- Thybo KH, Hägi‐Pedersen D, Wetterslev J, et al. Benefits and harm of paracetamol and ibuprofen in combination for post-operative pain: pre-planned subgroup analyses of the multicenter, randomized PANSAID trial. Acta Anaesthesiol Scand. 2020;64(2):245–253. doi:10.1111/AAS.13496
- Daniels SE, Goulder MA, Aspley S, Reader S. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain. 2011;152(3):632–642. doi:10.1016/J.PAIN.2010.12.012
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37(7):1007–1013. doi:10.1111/J.1524-4725.2011.02022.X
- Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19(12):3792–3800. doi:10.1245/S10434-012-2447-7
- Rahme E, Barkun A, Nedjar H, Gaugris S, Watson D. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103(4):872–882. doi:10.1111/j.1572-0241.2008.01811.x
- Mititelu Tartau L, Popa EG, Lupusoru RV, Lupusoru CE, Stoleriu I, Ochiuz L. Synergic effects of pregabalin-acetaminophen combination in somatic and visceral nociceptive reactivity. Pharmacology. 2014;93(5–6):253–259. doi:10.1159/000362649
- Durmus M, But AK, Saricicek V, Toprak HI, Ersoy MO. The post-operative analgesic effects of a combination of gabapentin and paracetamol in patients undergoing abdominal hysterectomy: a randomized clinical trial. Acta Anaesthesiol Scand. 2007;51(3):299–304. doi:10.1111/J.1399-6576.2006.01237.X
- Johan JL, Mallet C, Shionoya K, et al. Paracetamol analogues conjugated by FAAH induce TRPV1-mediated antinociception without causing acute liver toxicity. Eur J Med Chem. 2021:213. doi:10.1016/J.EJMECH.2020.113042
- Fresno N, Pérez-Fernández R, Goicoechea C, et al. Adamantyl analogues of paracetamol as potent analgesic drugs via inhibition of TRPA1. PLoS One. 2014;9(12):e113841. doi:10.1371/journal.pone.0113841
- Fricke JR, Karim R, Jordan D, Rosenthal N. A double-blind, single-dose comparison of the analgesic efficacy of tramadol/acetaminophen combination tablets, hydrocodone/acetaminophen combination tablets, and placebo after oral surgery. Clin Ther. 2002;24(6):953–968. doi:10.1016/S0149-2918(02)80010-8
- Macleod AG, Ashford B, Voltz M, et al. Paracetamol versus paracetamol-codeine in the treatment of post-operative dental pain: a randomized, double-blind, prospective trial. Aust Dent J. 2002;47(2):147–151. doi:10.1111/J.1834-7819.2002.TB00319.X
- Smith AB, Ravikumar TS, Kamin M, Jordan D, Xiang J, Rosenthal N. Combination tramadol plus acetaminophen for postsurgical pain. Am J Surg. 2004;187(4):521–527. doi:10.1016/j.amjsurg.2003.12.038
- Rawal N, Macquaire V, Catalá E, Berti M, Costa R, Wietlisbach M. Tramadol/paracetamol combination tablet for postoperative pain following ambulatory hand surgery: a double-blind, double-dummy, randomized, parallel-group trial. J Pain Res. 2011;4:103. doi:10.2147/JPR.S16760
- Aweke Z, Seyoum F, Shitemaw T, Doba DN. Comparison of preemptive paracetamol, paracetamol-diclofenac & paracetamol-tramadol combination on postoperative pain after elective abdominal surgery under general anesthesia, Ethiopia: a randomized control trial study, 2018. BMC Anesthesiol. 2020;20(1). doi:10.1186/S12871-020-01115-6
- Singh AM, Kirsch JM, Patel MS, et al. Effect of perioperative acetaminophen on pain management in patients undergoing rotator cuff repair: a prospective randomized study. J Shoulder Elb Surg. 2021;30(9):2014–2021. doi:10.1016/J.JSE.2021.03.132
