Abstract
Background
The goals of this study were to examine the trajectory of pediatric chronic postsurgical pain (CPSP) over the first year after surgery and to identify acute postsurgical predictors of CPSP.
Methods
Eighty-three children aged 8–18 years (mean 13.8, standard deviation 2.4) who underwent major orthopedic or general surgery completed pain and pain-related psychological measures at 48–72 hours, 2 weeks (pain anxiety and pain measures only), and 6 and 12 months after surgery.
Results
Results showed that 1 year after surgery, 22% of children developed moderate to severe CPSP with minimal functional disability. Children who reported a Numeric Rating Scale pain-intensity score ≥ 3 out of 10 two weeks after discharge were more than three times as likely to develop moderate/severe CPSP at 6 months and more than twice as likely to develop moderate/severe CPSP at 12 months than those who reported a Numeric Rating Scale pain score < 3 (6-month relative risk 3.3, 95% confidence interval 1.2–9.0 and 12-month relative risk 2.5, 95% confidence interval 0.9–7.5). Pain unpleasantness predicted the transition from acute to moderate/severe CPSP, whereas anxiety sensitivity predicted the maintenance of moderate/severe CPSP from 6 to 12 months after surgery.
Conclusions
This study highlights the prevalence of pediatric CPSP and the role played by psychological variables in its development/maintenance. Risk factors that are associated with the development of CPSP are different from those that maintain it.
Background
Chronic postsurgical pain (CPSP) is defined as pain attributable to the surgical procedure and lasting for more than 2 months after surgery.Citation1 The 1-year prevalence of adult CPSP typically ranges between 1.5% and 10%, although the prevalence can reach up to 70% in amputees and after thoracotomy.Citation2 CPSP may be accompanied by moderate to severe pain intensity and pain-related disability.Citation3 The variability in prevalence rates is due not only to the nature of the surgical procedure and time since surgery but also to the criteria used to classify patients: rates are typically higher in studies that include any report of pain (ie, regardless of intensity) and lower when an upper limit on intensity is specified.
In contrast to the adult literature, there are not a lot of data on CPSP in children and/or adolescents. Moreover, the issue of how best to define pediatric CPSP has not been addressed. To date, only retrospective studies of pediatric CPSP have been reported. These studies estimate the prevalence of CPSP to be between 13% more than 15 years after surgery to 50% 3 months after surgery.Citation4–Citation7 Pain intensity reported in these studies also ranged from mild to severe. Retrospective studies are subject to recall bias and do not permit identification of risk factors for the development of CPSP.
Biopsychosocial models of chronic pain highlight the important role played by biomedical, social/environmental, and psychological factors in the development and maintenance of CPSP in various surgical populations.Citation8–Citation10 Recent reviews of adult CPSP have identified different categories of risk factors, including patient-related (eg, previous or concurrent pain experience, acute postsurgical pain [APSP] intensity, younger age, female sex, analgesic consumption during the first week after surgeryCitation11–Citation13), social/environmental (eg, social supportCitation8), and psychological (eg, anxiety, pain catastrophizing, surgery-related fears, psychological vulnerabilities, emotional numbing).Citation11,Citation12,Citation14–Citation16
Factors associated with the development and maintenance of CPSP have yet to be examined in pediatric populations.Citation17 The focus of the present study is on patient-related factors, including age, sex, acute pain intensity, and unpleasantness (how horrible, yucky, or unpleasantness the feeling of pain is), and analgesic consumption, as well as pain-related psychological factors, including pain anxiety, pain catastrophizing, and anxiety sensitivity. Pain anxiety refers to cognitive, emotional, physiological, and behavioral reactions that one exhibits in association to an actual or anticipated pain experience.Citation18,Citation19 Pain catastrophizing refers to the rumination, feeling of helplessness, and magnification of the experience of pain.Citation20,Citation21 Anxiety sensitivity refers to the degree to which one interprets anxiety-related symptoms as being associated with potentially harmful somatic, psychological, or social consequences.Citation22 Research has shown that these pain-related psychological constructs are associated with pain severity and disability in community and clinical pediatric populationsCitation23–Citation27 and are important factors in empirically validated models of chronic pain, including the diathesis–stress model of chronic pain and disabilityCitation28 and the cognitive–behavioral fear-avoidance model of chronic pain.Citation29–Citation31 However, the role of these constructs in the development and maintenance of pediatric CPSP has yet to be examined.
Findings from the adult literature indicate that the factors predicting the transition to chronicity (ie, from the acute postoperative phase to ~6 months after surgery) are different from those involved in the maintenance of CPSP (ie, from 6 months onward),Citation32,Citation33 and this has also been found in at least one pediatric study of patients with widespread body pain.Citation34 These findings point to the importance of examining in children the factors that predict the transition to chronicity separately from those that predict its persistence, since different mechanisms and risk factors might be involved at each stage.Citation33 They also highlight the importance of assessing outcomes at more than one time point after surgery,Citation35 since the transition to CPSP and related disability is a dynamic process that evolves over time.Citation33
The goals of this study were to (1) describe the course of pediatric CPSP by modeling pain intensity and pain unpleasantness trajectories over the first year after surgery, (2) identify risk factors for the transition from APSP to CPSP (ie, development of CPSP), and (3) identify risk factors for the maintenance of CPSP. It is hypothesized that (1) CPSP of moderate to severe intensity will have a prevalence rate similar to the adult literature (~10%), (2) pain anxiety, pain catastrophizing, and anxiety sensitivity will predict the development of CPSP, and (3) pain anxiety, pain catastrophizing, and anxiety sensitivity will predict the maintenance of CPSP. APSP was measured 48–72 hours and approximately 2 weeks after surgery, and CPSP was measured 6 and 12 months after surgery. Children were classified as having moderate/severe CPSP if they reported a Numeric Rating Scale (NRS) for pain intensity score ≥ 4Citation36,Citation37 6 and/or 12 months after surgery; otherwise, they were classified as not having or having mild CPSP.
Materials and methods
Participants and recruitment
Children between the ages of 8 and 18 years undergoing either orthopedic (ie, scoliosis, osteotomy, plate insertion tibial/femur, open hip reduction, hip capsulorrhaphy) or general surgical (ie, thoracotomy, thoracoabdominal surgery, Nuss or Ravitch surgical procedure to correct pectus excavatum, sternotomy, laparotomy) procedures were eligible to participate in this study. Surgical procedures were selected on the basis of expected postoperative pain. The authors chose procedures that are known to produce moderate to severe pain while in hospital. This was done to maximize the clinical relevance of the study results, and for reasons of sample size to maximize the proportion of patients expected to develop CPSP under the hypothesis that acute pain intensity impacts the development of chronic pain. Although there is variability in recovery time, depending on the procedure, it is generally known that acute pain improves over time after spinal fusion for scoliosisCitation38 (the usual indication for spinal fusion in this age-group).
Children were excluded if they had a developmental or cognitive delay, had cancer, or were not fluent in written and/or spoken English.
Questionnaires
Child Pain Anxiety Symptoms Scale
The Child Pain Anxiety Symptoms Scale (CPASS)Citation39 is a 20-item scale for children adapted from the adult PASS-20.Citation40 For each statement, children are asked to rate the extent to which they think, act, or feel that way on a scale from 0 (never think, act, or feel that way) to 5 (always think, act, or feel that way). Total scores range from 0 to 100, with higher scores indicating higher levels of pain anxiety. The CPASS is composed of four subscales: cognitive, escape/avoidance, fear, and physiological anxiety. The CPASS showed excellent internal consistency (α = 0.90) in a community sample of children,Citation39 as well as in the present study (α = 0.92–0.96).Citation41 In addition, the CPASS correlated more strongly with pain catastrophizing (r = 0.63) and anxiety sensitivity (r = 0.60) than with general anxiety (r = 0.44) (suggesting adequate construct validity), and was significantly associated with how often children reported pain.Citation39
Childhood Anxiety Sensitivity Index
The Childhood Anxiety Sensitivity Index (CASI)Citation42 assesses the extent to which the respondent interprets anxiety-related symptoms (eg, increased heart rate, feeling nauseated) as indicators of potentially harmful somatic, psychological, and/or social consequences.Citation22 The scale is composed of 18 items, such as “It scares me when my heart beats fast” and “It scares me when I feel like I’m going to throw up.” Items are rated on a scale ranging from 1 (none) to 3 (a lot). Total scores range from 18 to 54, with higher scores indicating higher levels of anxiety sensitivity. The CASI has good internal consistency (α = 0.87) and test–retest reliability (r = 0.76), as well as adequate convergent and discriminant validity.Citation42 Internal consistency for the present study was excellent (α = 0.87–0.93).
Pain Catastrophizing Scale – Children
The Pain Catastrophizing Scale – Children (PCS-C)Citation43 is a 13-item self-report measure assessing the extent to which children worry, amplify, and feel helpless about their current or anticipated pain experience.Citation43 The scale was modified for use with children based on the adult PCS.Citation44,Citation45 For each item, children are asked to rate, on a scale from 0 (not at all) to 4 (extremely), how strongly they experience a particular thought when they have pain. Total scores range from 0 to 52, with higher scores indicating higher levels of pain catastrophizing. The PCS-C comprises three subscales: rumination, magnification, and helplessness. Preliminary results suggest that the PCS-C has good internal consistency (α = 0.90) and correlates highly with pain intensity (r = 0.49) and disability (r = 0.50).Citation43 Internal consistency for the present study was excellent (α = 0.93).
Functional Disability Inventory
The Functional Disability Inventory (FDI)Citation46 is a 15-item scale that assesses the extent to which children experience difficulties in completing specific tasks (eg, walking to the bathroom, eating regular meals, and being at school all day). Typically, the FDI is used as a 5-point Likert scale and yields total scores ranging from 0 to 60. Inadvertently, the FDI in the present study was measured using a 4-point Likert scale and omitted the original 2 (some trouble). Children in this study rated each item on a scale from 0 to 3: (0, no trouble; 1, a little trouble; 2, a lot of trouble; and 3, impossible), yielding total scores ranging from 0 to 45. The FDI has excellent internal consistency (α = 0.86–0.91) and good test–retest reliability at 2 weeks (r = 0.74) and 3 months (r = 0.48). The FDI has been used with many pediatric populations, including children with chronic painCitation47–Citation49 and postsurgical pain.Citation50 Internal consistency for the present study was excellent (α = 0.83–0.89).
Numeric Rating Scale for pain intensity (NRSI) and pain unpleasantness (NRSU)
The NRS is a verbally administered scale that measures pain intensity (“How much pain do you feel right now?”). The NRS was also used to measure pain unpleasantness (“How unpleasant/horrible/yucky is the pain right now?”). The end points represent the extremes of the pain experience. Since there are no agreed-upon NRS anchors for measuring pain in children and adolescents,Citation51 the following anchors were used in the present study: for pain intensity, 0 = no pain at all to 10 = worst possible pain; for pain unpleasantness, 0 = not at all unpleasant/horrible/yucky to 10 = most unpleasant/horrible/yucky feeling possible. The NRS for pain intensity has been validated as an APSP measure in children aged 7–17 years; it correlated highly with the Visual Analog Scale (r = 0.89) and the revised Faces Pain Scale (r = 0.87).Citation52
Preoperative pain experience
Retrospectively (48–72 hours after surgery), children were asked to answer the following question: “Before surgery, how much pain did you have in general?” using one of four verbal descriptors: 0, no pain; 1, a little bit of pain; 2, a medium amount of pain; or 3, a lot of pain.
Hospital chart review
The following information was collected from children’s hospital charts: preoperative information (eg, ongoing pain problems, regular pain medications, previous surgery), perioperative information (eg, type of procedure, length of surgery), and postoperative information (eg, pain medication on the first day after surgery).
CPSP questionnaire
This questionnaire was designed to evaluate children’s pain experience postoperatively and included the following questions: “Do you ever feel pain in the area of your body where the surgery was done?” “If yes, how often did you feel pain?” “On average, how much pain do you feel on a scale from 0 to 10?” “When you feel pain, where exactly is the pain you are usually feeling?” “What kind of pain do you usually feel?” “Are you taking any medication for your pain?” “Are you using any alternative method to relieve your pain?” “Are you seeing a doctor specifically for your pain?”
We also administered the Center for Epidemiological Studies Depression Scale for ChildrenCitation53 and the Multidimensional Anxiety Scale for ChildrenCitation54 at 24–48 hrs, 6 months, and 12 months after surgery, but do not report these data here.
Procedure
The study was reviewed and approved by the research ethics boards of the Hospital for Sick Children and York University. Nurses who were not part of the research team approached potential participants to ask whether they would be interested in learning about the study. Children and one of their parents (who had expressed an interest in the study) were then approached by a research team member 48–72 hours after surgery. After obtaining informed written parental consent and consent or assent from children, a research team member read to the child the following questionnaires and recorded their responses to each item: CPASS, PCS-C, CASI, NRSI, NRSU, and preoperative experience. The order of administration of questionnaires was randomized (http://www.randomization.com) within participants to minimize potential order and fatigue effects. Telephone follow-ups were conducted approximately 2 weeks (CPASS, FDI, NRSI, and NRSU) and 6 and 12 months (CPASS, PCS-C, CASI, NRSI, NRSU, FDI, and CPSP questionnaire) after surgery by a research assistant who verbally administered the questionnaires to the children. A verbal mode of administration of questionnaires was chosen because some participants could not move their arms or sit up 48–72 hours after surgery and thus could not complete the questionnaires themselves. Verbally administering the questionnaires to all participants minimized response bias due to different types of administration of questionnaires. In addition, follow-ups were conducted over the telephone, and as such verbally administering the questionnaires 48–72 hours after surgery ensured that the same method of administration was preserved throughout the study.
Parents also completed measures; these results will be presented in a separate manuscript.
Data analysis
Data was screened for univariate outliers on pain-related psychological predictor variables (pain anxiety, pain catastrophizing, and anxiety sensitivity), multivariate outliers (squared Mahalanobis distance as a probability [χ2] <0.001), as well as skewness and kurtosis.
Outlier analysis revealed that none of the data points was both a univariate and a multivariate outlier; as such, all participants were retained for the analyses. Visual examination of the predictor variable plots as well as skewness and kurtosis significance testing did not reveal the presence of nonnormality. Skewness and kurtosis significance testing (estimate/standard error > 3) did reveal, however, nonnormality of two outcome variables: pain intensity and pain unpleasantness at 2 weeks, 6 months, and 12 months after surgery. A Poisson distribution best represents the variability of the outcome variables, given that at all three time points, approximately half of the sample did not report experiencing CPSP on the NRSI and NRSU, whereas scores for the other half of the participants ranged between 1 and 10, with a greater proportion reporting lower levels of pain. Nonnormality of the outcome variables was addressed either through square-root transformation (NRSI[2]t, NRSU[2]t, NRSI[6]t, NRSU[6]t, NRSI[12]t, and NRSU[12]t) or by using a Poisson (loglinear) distribution in the analyses whenever possible.
Description of moderate/severe pediatric CPSP and modeling of pain intensity and pain unpleasantness trajectories
The Pearson Chi-squared test was used to examine the presence of sex differences in children’s chronic pain status (moderate/severe CPSP or no/mild CPSP [moderate to severe CPSP is defined as NRS ≥ 4]) 6 and 12 months after surgery.
CPSP intensity and unpleasantness trajectories across time
A generalized estimating equation (GEE) using Poisson log-linear distribution was used to compare the pain intensity (model 1) and pain unpleasantness (model 2) trajectories (pattern of pain scores over time) of children with moderate/severe CPSP to those with no/mild CPSP. In addition to the linear time variable, time was also transformed to generate an asymptotic curve to model the decline of pain scores over time. This asymptotic function better characterizes the pattern by which pain scores decrease over time; namely, a more rapid decline in pain over the first days/weeks and a slower decline from 2 weeks to 6 and 12 months after surgery. The following variables (and their interactions) were sequentially entered in each model and retained when statistically significant: time (in months, with 48–72 hours as the starting point – zero), asymptotic time (time transformed), previous surgical experience, and pain status (moderate/severe CPSP vs no/mild CPSP).
Risk factors for the transition from APSP to CPSP
Binary logistic regression analyses were used to examine if sex, age, analgesic consumption of day 1 after surgery, and 48- to 72-hour pain intensity, pain unpleasantness, pain anxiety, pain catastrophizing, and anxiety sensitivity predicted the development of moderate/severe CPSP 6 months (model 1) and 12 months (model 2) after surgery. Predictors were entered in the models using a forward conditional method.
Risk factors for the maintenance of moderate/severe CPSP
Binary logistic regression analysis was used to examine if age, sex, and 6-month (model 2) pain anxiety (CPASS), pain catastrophizing (PCSC), anxiety sensitivity (CASI), pain intensity (NRSI), and pain unpleasantness (NRSU) predicted the maintenance of moderate/severe CPSP 12 months after surgery. Predictors were entered in the model using a forward conditional method. All statistical analyses were conducted using SPSS v.19 (2010, IBM Corporation, Armonk, NY, USA).
Sample-size estimation
Sample size was estimated a priori using G*Power version 3.1 (Franz Faul, Universitat Kiel, Germany).Citation55
Description of pediatric moderate/severe CPSP and modeling of pain intensity and pain unpleasantness trajectories
Sample-size estimation for the Pearson Chi-squared test showed that a sample size of 50 participants would be required to detect a medium to large effect size (w = 0.40) with α = 0.05, power = 80%, and degrees of freedom = 1. Sample-size estimation for the mixed-effects models, including GEE and generalized linear mixed-effects models, is difficult to perform, given the multiple combinations of mixed and random effects involved. However, it has been shown that sample size required for a mixed-effects model is comparable to the sample size required for linear regression analysis for balanced designs.Citation56 For a multiple regression analysis with four predictors (time, asymptotic time, prior surgical procedure, and pain status), 65 participants would be required for α = 0.05, power = 80%, and a medium to large effect size (fCitation2 = 0.25).
Risk factor analyses of the transition from APSP to CPSP and maintenance of moderate/severe CPSP
Sample-size analysis showed that 60 participants would be required for a multiple logistic regression analysis Hsieh’s test procedure,Citation57 with odds ratio = 2.5, probability (y = 1 | x = 1) H0 = 0.5, α = 0.017, RCitation2 other x = 0.16, and power = 80%.
Taking into account an attrition rate of ~30% due to dropouts and losing patients to follow-up, we recruited 83 patients to ensure we would have sufficient power for our analyses at the various time points after surgery.
Results
Recruitment
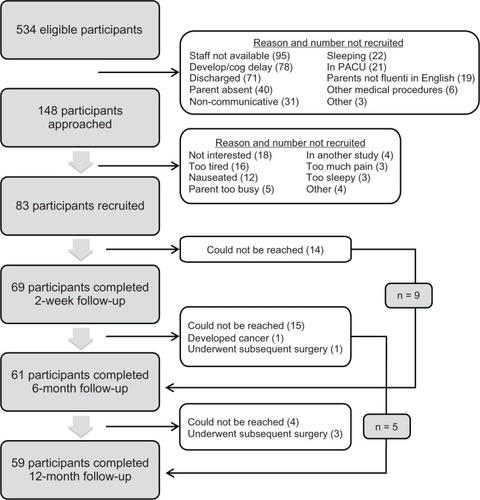
Recruitment took place between July 2008 and September 2010. Details of the recruitment process are presented in . Eighty-three children/parents provided assent/consent to participate, of whom 69 (83%) also completed the telephone follow-up approximately 2 weeks after discharge from hospital (mean = 15.6 days, standard deviation [SD] = 2.15). Sixty-one (73%) and 59 (71%) participants completed the 6- and 12-month follow-ups, respectively.
There were no significant differences between children who completed vs those who did not complete the 6-month follow-up on measures of pain intensity (P = 0.087), pain unpleasantness (P = 0.347), pain anxiety (P = 0.741), pain catastrophizing (P = 0.864), anxiety sensitivity (P = 0.363) measured 48–72 hours after surgery, analgesic consumption on day 1 (P = 0.335), age (P = 0.920), or sex (P = 0.934). There were no significant differences between children who completed vs those who did not complete the 12-month follow-up on measures of pain intensity (P = 0.412), pain unpleasantness (P = 0.090), pain anxiety (P = 0.668), pain catastrophizing (P = 0.555), anxiety sensitivity (P = 0.601) measured 48–72 hours after surgery, analgesic consumption on day 1 (P = 0.255), age (P = 0.572) or sex (P = 0.677).
Descriptive statistics
The final sample comprised 83 children (female = 56 [67.5%]) aged between 8 and 18 years (mean age in years for total sample = 13.8, SD = 2.4; girls = 14.0, SD = 2.3; boys = 13.5, SD = 2.6). The majority of children (or parents) in the sample self-identified as Caucasian (n = 53, 64%); 12% self-identified as Asian, 8.4% as African-Caribbean/African-Canadian, 4.8% as Middle Eastern, 3.6% as Hispanic, and 7.2% were categorized as “Other.” Eighty-nine percent of children spoke English as their first language at home. The majority of children underwent surgery for scoliosis (spinal fusion) (n = 42, 50.6%) or osteotomy (n = 25, 30.1%); eight children (9.6%) underwent Nuss (n = 5) or Ravitch (n = 3) procedures, seven children (8.4%) had a laparotomy, and one child had a thoracotomy. The present surgery was the first for 44 children (53%); 39 others had previously undergone other surgical procedures (mean = 2.0, SD = 1.6, range = 1–7). When asked to rate the level of presurgical pain they had experienced the majority of children (80.7%) reported no pain or a little bit of pain. Two children who developed CPSP at 6 months and one child who developed CPSP at 12 months had reported moderate to severe pain before surgery. Eighteen out of 20 children with baseline pain underwent surgery for scoliosis or osteotomy which corrected the source of their baseline pain.
Significant differences were not found between moderate/severe CPSP and no/mild CPSP at 6 (χ2 = 3.61, P = 0.306) or 12 months (χ2 = 1.91, P ≤ 0.592) after surgery across the different types of surgical procedures. Mean ± SD of morphine equivalent analgesic consumption on the first day following surgery was 1.21 ± 1.02 mg/kg (62.81 ± 57.6 mg). Mean values for boys and girls on the relevant psychological and pain measures at 6 and 12 months after surgery are presented in .
Table 1 Means (standard deviations) of pain and related psychological variables for boys, girls, and the total sample measured 6 and 12 months after surgery
Description of moderate/severe pediatric CPSP and modeling of pain intensity and pain unpleasantness trajectories
Incidence and frequency of moderate/severe CPSP (NRSI ≥ 4) measured 6 and 12 months after surgery are presented in . Almost one-quarter of children reported experiencing moderate to severe pain at the surgical site at 6 (n = 14 out of a total of 61, 23%) and 12 (n = 13 out of a total of 59, 22%) months after surgery. Four children had CPSP at both 6 and 12 months. Three children who had pain at 6 months did not complete the 12-month follow-up, and as such their CPSP status at 12 months is unknown. Similarly, three children had CPSP at 12 months but did not complete the 6 month follow-up. Seven children with CPSP at 6 months no longer had pain at the 12-month follow-up. Lastly, six children who did not have CPSP at 6 months subsequently reported it at 12 months.
Table 2 Incidence and characteristics of pediatric CPSP 6 and 12 months after major orthopedic or general surgery
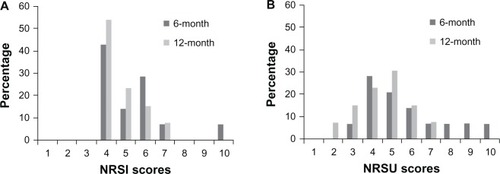
There were no significant sex differences in the presence/absence of moderate to severe CPSP 6 (P = 0.353) or 12 (P = 0.184) months after surgery. Six months after surgery, more than one-third of children (n = 5) with moderate/severe CPSP reported experiencing pain at least once a day, while only one child reported similar pain frequency 12 months after surgery. Average pain intensity and pain unpleasantness remained relatively constant over time. Distribution of pain intensity and pain unpleasantness scores for children with moderate/severe CPSP is presented in . Moderate to severe CPSP was accompanied by low levels of functional disability at both 6 (mean = 7.2, SD = 6.2) and 12 months (mean = 6.3, SD = 4.0).
Figure 2 Pain intensity (A) and pain unpleasantness (B) scores 6 and 12 months after surgery for children with moderate/severe chronic postsurgical pain.
Pain intensity trajectory
GEE using Poisson log-linear distribution was used to examine differences in pain intensity over time between children with no or mild CPSP versus children who developed moderate or severe CPSP (at 6 and/or 12 months after surgery). The final model revealed a significant interaction between pain status and asymptotic time (Wald χ2 = 38.9, P < 0.001) (see and ) in predicting pain intensity scores. That is, pain intensity scores declined nonlinearly and at a more rapid rate in the no/mild CPSP group compared to the moderate/severe CPSP group.
Figure 3 Differences in pain intensity and unpleasantness trajectories between children with moderate/severe CPSP and without or with mild CPSP.
Abbreviations: CPSP, chronic postsurgical pain; NRSI, Numeric Rating Scale for pain intensity; NRSU, Numeric Rating Scale for pain unpleasantness; CI, confidence interval.
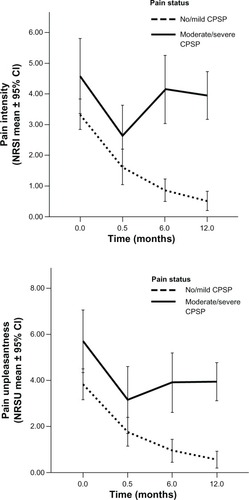
Table 3 Differences in CPSP and functional disability trajectories in children with moderate to severe CPSP or no/mild CPSP at 12 months (CPSP status) using generalized estimating equation
Based on the results of the GEE model (see ) a cutoff score of 3.0 on the NRSI 2 weeks after surgery separated children who reported moderate/severe CPSP from those who reported no/mild CPSP. shows the relative risks and 95% confidence intervals (CIs) for developing moderate/severe vs no/mild CPSP at 6 and 12 months after surgery, using a cutoff score of 3.0 on the NRSI and NRSU at 2 weeks after discharge from hospital. Children who reported higher pain intensity (NRSI ≥ 3.0) 2 weeks after discharge were 3.3 (95% CI 1.2–9.0) times more likely to report moderate/severe CPSP 6 months after surgery than children who reported lower pain intensity (NRSI < 3.0) and 0.7 (95% CI 0.4–1.1) times less likely to be pain-free or report mild pain intensity 6 months after surgery. Likewise, children who reported higher pain intensity (NRSI ≥ 3.0) 2 weeks after discharge were 2.5 (95% CI 0.9–7.5) times more likely to report moderate/severe CPSP 12 months after surgery as children who reported lower pain intensity (NRSI < 3.0) and 0.8 (95% CI 0.5–1.1) times less likely to be pain-free or report mild pain intensity 12 months after surgery.
Table 4 Relative risk of developing moderate/severe CPSP based on 2-week postdischarge pain intensity and unpleasantness scores
Pain unpleasantness trajectory
A similar model was run to examine the pain unpleasantness trajectory of children with moderate/severe CPSP versus children with no/mild CPSP. The final model revealed a significant interaction between pain status and asymptotic time (Wald χ2 = 21.4, P < 0.001) (see and ) in predicting pain unpleasantness scores. That is, pain unpleasantness scores declined nonlinearly and pain unpleasantness scores declined faster in the no/mild CPSP group compared to the moderate/severe CPSP group.
shows the risk of developing moderate/severe CPSP vs no/mild CPSP at 6 and 12 months after surgery, using a cutoff score of 3.0 on the NRSU at 2 weeks. Also shown are the relative risks and 95% CIs. Children who reported higher pain unpleasantness (NRSU ≥ 3.0) 2 weeks after discharge were 2.5 times more likely to report moderate/severe CPSP 6 months after surgery than children who reported lower pain unpleasantness (NRSI < 3.0), and 0.8 times less likely to be pain-free or report mild CPSP 6 months after surgery. Likewise, children who reported higher pain unpleasantness (NRSI ≥ 3.0) 2 weeks after discharge were 2.7 times more likely to report moderate/severe CPSP 12 months after surgery as children who reported lower pain unpleasantness (NRSI < 3.0), and 0.8 times less likely to be pain-free or report mild CPSP 12 months after surgery.
Risk factors for the transition from APSP to CPSP
Risk factors (48–72 hours) for 6-month moderate/severe CPSP
Results of the logistic regression analysis showed that pain unpleasantness (B = 0.272, P = 0.046) was the only significant predictor of 6-month chronic pain status (χ2 = 4.34, P = 0.037).
Risk factors (48–72 hours) for 12-month moderate/severe CPSP
Results of the logistic regression analysis showed that not one patient-related or psychological factor predicted the presence of moderate/severe CPSP 12 months after surgery.
Risk factors for the maintenance of moderate/severe CPSP
Six-month risk factors for 12-month moderate/severe CPSP
Results of the logistic regression analysis showed that anxiety sensitivity (B = 0.138, P = 0.043) was the only significant predictor of the maintenance of or recovery from moderate/severe CPSP 12 months after surgery (χ2 = 5.02, P = 0.025).
Discussion
The present study was designed to examine the course of pediatric moderate/severe CPSP and model the development and maintenance of moderate/severe CPSP from the acute postsurgical period.
Description of pediatric moderate/severe CPSP and modeling of pain intensity and unpleasantness trajectories
The results of the present study show that almost one-quarter of children who underwent major surgery developed CPSP of moderate to severe intensity and unpleasantness 1 year after surgery. About one-third of children with CPSP 6 months after surgery reported experiencing pain daily. Approximately 14% of children with CPSP reported severe (NRS ≥ 7 out of 10) levels of pain 6 months after surgery (n = 2). These statistics are similar to those found in adults; a review of CPSP suggests that 10% of patients report severe, intractable CPSP.Citation58 A recent retrospective study of pediatric CPSP found that 13% of children between 3 and 18 months following surgery report current pain at the surgery site.Citation6 The 12-month incidence of moderate/severe CPSP in the present study was higher (22%). This difference in prevalence estimates could be due to the type of surgical procedures studied; children in the present study all underwent major orthopedic or general surgery, and as such the magnitude of tissue damage might contribute to the higher prevalence rate found.
In general, pediatric chronic pain is prevalent but typically not accompanied by severe levels of disability.Citation59 Approximately 5%–15% of children with chronic pain report pain disability of a severity requiring professional attention.Citation59,Citation60 Results from the present study are consistent with this literature. The presence of moderate/severe CPSP was not accompanied by high levels of functional disability for the majority of children or with the use of pain medication in the first year following surgery. The absence of clinically relevant levels of functional disability might be due in part to the use of a measure of general disability and not one related specifically to pain. Participants are asked to rate items of the FDI based on the level of difficulty they had in completing specific tasks; it does not specify that these difficulties must have been related to the pain they were experiencing. As such, a participant might report higher levels of disability because of a cold/flu, for example. Alternatively, it could be that because of the way children cope with everyday problems, including pain,Citation61 they do not experience much interference from the pain. Children often have little experience coping with chronic pain on their own and instead rely on parental involvement and support to cope and function.Citation61 Consequently, the low levels of functional disability in the present study may indicate that parents pain are providing the necessary support so that even children with moderate to severe pain are protected from the interfering effects of pain.
Results indicate that while there is little difference in pain-intensity scores 48–72 hours after surgery between children who developed moderate/severe CPSP vs no/mild CPSP, the former reported significantly higher pain intensity and unpleasantness levels 2 weeks after discharge from hospital compared to the latter. Pain intensity and unpleasantness scores 2 weeks after discharge from hospital provided valuable information about the risk of developing moderate/severe CPSP. Children who reported pain intensity and/or pain unpleasantness scores of 3 or higher on the NRS two weeks after discharge were more than three times as likely to report moderate/severe CPSP 6 months later and more than twice as likely to report moderate/severe CPSP 12 months later than children who reported NRS scores lower than 3. We do not know whether intense acute pain is a causal risk factor for CPSP, but the present results raise the possibility that intervening with children and adolescents who present with NRSI scores greater than 3 out of 10 two weeks after major surgery might help prevent the transition from acute to CPSP. Future research might consider looking at whether pharmacological, psychological, social, or a combination of interventions in the acute postoperative period successfully prevent the development of CPSP or reduce the incidence and/or intensity of CPSP.
Risk factors for the transition from APSP to CPSP and maintenance of moderate/severe CPSP
Factors that predict the transition to chronicity differ from those that predict the maintenance or persistence of adult chronic pain.Citation32,Citation33 Although research has not yet examined CPSP in children, studies of pediatric widespread pain suggests that factors predicting the onset of widespread pain (conduct and emotional problems and other somatic symptoms) differ somewhat from those that predict the maintenance of pain (behavioral characteristics and somatic symptoms).Citation34 The present results are consistent with these findings. Anxiety sensitivity emerged as a significant predictor of moderate/severe CPSP between 6 months and 1 year after surgery (but not earlier), and as such might be an important marker that maintains CPSP once it has developed. In contrast, pain unpleasantness appears to be involved in the transition from APSP to CPSP but interestingly not in its maintenance.
Anxiety sensitivity has been found to play an important role in the pain experience of individuals due to the negative interpretations of pain-related sensations.Citation62 A recent meta-analytic review of anxiety sensitivity in clinical and nonclinical pain populations found that anxiety sensitivity is strongly associated with fear of pain, which can lead to avoidance behaviors and the maintenance of chronic pain; as such, fear of pain has been suggested as a bridge to explain the association between anxiety sensitivity and pain (see Ocanez et alCitation62 for a review). Consistent with the diathesis–stress model of chronic pain and disabilityCitation28,Citation63 and the fear-avoidance models of adultCitation64 and childCitation65 chronic pain, by amplifying pain-related fears, anxiety sensitivity is associated with avoidance behaviors and ultimately the maintenance of chronic pain.Citation64 Results from the present study are consistent with the theoretical models and supportive empirical evidence suggesting that this construct may play an important role in the maintenance of the pediatric chronic pain experience.Citation65 It has yet to be determined if fear of pain is a mediator in the observed relationship between anxiety sensitivity and the maintenance of pediatric moderate/severe CPSP.
Results from the present study contrast with those found in the adult literature suggesting that patient-related factors (particularly APSP intensity) predict the development of CPSP.Citation66 Pain unpleasantness, but not pain intensity, was found to predict the development of moderate/severe CPSP. These results suggest that the affective dimension of the pain experience might play a particularly important role in the development of pediatric chronic pain.
These results are not consistent either with adult studies demonstrating the important role of psychological factors (particularly pain catastrophizing) in predicting the development of CPSP.Citation66 Pain catastrophizing is also an important component of the pediatric fear-avoidance model of chronic pain and has received empirical support for its role in pediatric chronic pain and disability.Citation65 Researchers have recently questioned the content validity of the pain-catastrophizing construct for use in children, in part because it lacks developmental and social contextualization (eg, parental psychological factors might have a direct impact on children’s response to painCitation65) in this patient population and because it fails to capture social and cognitive milestones associated with youth development.Citation61 Additional research is needed to examine the role of pain catastrophizing in the development of CPSP within a social, cognitive, and developmental context.
Pain anxiety did not emerge as a significant predictor of the development or maintenance of CPSP. It is possible that pain anxiety, while not directly predicting the development or maintenance of chronic pain, is indirectly related to pain outcomes through its influence on certain pain behaviors, such as escape and avoidance behaviors. Future research should examine the role of pain anxiety as a potential moderator of the relationship between pain behaviors and pain outcomes.
Limitations
All measures were collected postoperatively, and as such preoperative baseline data were not collected regarding the participants’ pain-related psychological distress. Second, children recruited in this study all underwent a major surgical procedure, and as such results cannot be generalized to children who are undergoing less invasive surgery. Third, the response rate for this study was 55%. It is possible that children with higher APSP or higher postoperative anxiety refused to participate. Fourth, 25% of participants did not complete the 6- and 12-month follow-ups, and consequently analyses of CPSP are based on 75% of the sample. Fifth, this study focused on psychological risk factors for the development of CPSP and did not consider biological factors that can influence the development and/or maintenance of CPSP. It is possible that, for example, the type of analgesic regimen administered perioperatively influenced the development of CPSP through central sensitization. Additional research is needed to understand the biopsychosocial factors involved in the development and maintenance of CPSP. Finally, our use of a 4-point FDI scale instead of the typical; 5-point scale may have led to inaccurate levels of functional disability.
Conclusion
In conclusion, this study is the first to prospectively examine the development and persistence of pediatric CPSP. Results suggest that moderate/severe CPSP is a frequent occurrence, despite low levels of associated functional disability. Importantly, a cutoff score of 3.0 on the NRS for pain intensity or pain unpleasantness 2 weeks after discharge is associated with more than twice the risk of developing moderate/severe CPSP 1 year after surgery. The factors that predict the development of moderate/severe CPSP appear to differ from those that predict its persistence. Future studies with additional time points would help to differentiate risk factors associated with the development and maintenance of moderate/severe CPSP. Pain unpleasantness is involved in the development of moderate/severe CPSP, while anxiety sensitivity appears to play a significant role in its persistence. This line of research has the potential to provide valuable information for the development of preventive interventions that can reduce the occurrence of moderate/severe CPSP in this patient population.
Acknowledgments
MGP is supported by a Canada Graduate Scholarship – Doctoral Award from the Canadian Institutes of Health Research (CIHR). MGP is a recipient of a Lillian-Wright Maternal-Child Health Scholarship from York University, a trainee member of Pain in Child Health and a CIHR Strategic Training Fellow in Pain: Molecules to Community. JS is supported by a Ministry of Health and Long-Term Care Career Scientist Award. JK is supported by a CIHR Canada Research Chair in Health Psychology at York University.
Disclosure
The authors have no conflicts of interest.
References
- MacraeWADaviesHTOChronic postsurgical painCrombieIKLintonSCroftPVonKnorff MLeRescheLEpidemiology of PainSeattleIASP1999125142
- KatzJPagéMGIdentification of risk and protective factors in the transition from acute to chronic post surgical painLynchMECraigKDPengPWHClinical Pain Management: A Practical GuideOxford, UKWiley-Blackwell20103241
- CrombieIKDaviesHTMacraeWACut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinicPain1998761–21671719696470
- WongGTYuenVMChowBFIrwinMGPersistent pain in patients following scoliosis surgeryEur Spine J200716101551155617410382
- AasvangEKKehletHChronic pain after childhood groin hernia repairJ Pediatr Surg20074281403140817706504
- FortierMAChouJMaurerELKainZNAcute to chronic postoperative pain in children: preliminary findingsJ Pediatr Surg20114691700170521929977
- KristensenADPedersenTAHjortdalVEJensenTSNikolajsenLChronic pain in adults after thoracotomy in childhood or youthBr J Anaesth20101041757919915188
- HanleyMAJensenMPEhdeDMHoffmanAJPattersonDRRobinsonLRPsychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb painDisabil Rehabil20042614–1588289315497917
- HanleyMAJensenMPSmithDGEhdeDMEdwardsWTRobinsonLRPreamputation pain and acute pain predict chronic pain after lower extremity amputationJ Pain20078210210916949876
- JensenMPEhdeDMHoffmanAJPattersonDRCzernieckiJMRobinsonLRCognitions, coping and social environment predict adjustment to phantom limb painPain2002951–213314211790476
- KatzJSelterZTransition from acute to chronic postsurgical pain: risk factors and protective factorsExpert Rev Neurother20099572374419402781
- BruceJQuinlanJChronic post surgical painRev Pain20115232329
- NirajGRowbothamDJPersistent postoperative pain: where are we now?Br J Anaesth20111071252921610014
- PavlinDJSullivanMJFreundPRRoesenKCatastrophizing: a risk factor for postsurgical painClin J Pain2005211839015599135
- VoscopoulosCLemaMWhen does acute pain become chronic?Br J Anaesth2010105Suppl 1i69i8521148657
- Hinrichs-RockerASchulzKJärvinenILeferingRSimanskiCNeugebauerEAPsychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic reviewEur J Pain200913771973018952472
- AhnJCFortierMAKainZNAcute to chronic postoperative pain in children: does it exists?Pain Manage201225421423
- McCrackenLMDhingraLA short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validityPain Res Manage2002714550
- McCrackenLMZayfertCGrossRTThe Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of painPain199250167731513605
- CrombezGEcclestonCBaeyensFEelenPWhen somatic information threatens, catastrophic thinking enhances attentional interferencePain1998752–31871989583754
- SullivanMJLBishopSRPivikJThe pain catastrophizing scale: development and validationPsychol Assess199574524532
- ReissSMcNallyRJExpectancy model of fearReissSBootzinRRTheoretical Issues in Behavior TherapySan DiegoAcademic Press1985107121
- MartinALMcGrathPABrownSCKatzJAnxiety sensitivity, fear of pain and pain-related disability in children and adolescents with chronic painPain Res Manage2007124267272
- VervoortTGoubertLEcclestonCBijttebierPCrombezGCatastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic painJ Pediatr Psychol200631767468316093515
- TsaoJCLuQMyersCDKimSCTurkNZeltzerLKParent and child anxiety sensitivity: relationship to children’s experimental pain responsivityJ Pain20067531932616632321
- TsaoJCMeldrumMKimSCZeltzerLKAnxiety sensitivity and health-related quality of life in children with chronic painJ Pain200781081482317613277
- TsaoJCMyersCDCraskeMGBurschBKimSCZeltzerLKRole of anticipatory anxiety and anxiety sensitivity in children’s and adolescents’ laboratory pain responsesJ Pediatr Psychol200429537938815187176
- TurkDCA diathesis-stress model of chronic pain and disability following traumatic injuryPain Res Manag20027191916231063
- LethemJSladePDTroupJDBentleyGOutline of a fear-avoidance model of exaggerated pain perception – IBehav Res Ther19832144014086626110
- VlaeyenJWSKole-SnijdersAMBoerenRGvan EekHFear of movement/(re)injury in chronic low back pain and its relation to behavioral performancePain19956233633728657437
- VlaeyenJWSLintonSFear-avoidance and its consequences in chronic musculoskeletal pain: a state of the artPain200085331733210781906
- KatzJAsmundsonGJMcRaeKHalketEEmotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomyEur J Pain200913887087819027333
- KatzJOne man’s risk factor is another man’s outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transitionPain2012153350550622100359
- JonesGTSilmanAJMacfarlaneGJPredicting the onset of widespread body pain among childrenArthritis Rheum20034892615262113130481
- KehletHRathmellJPPersistent postsurgical pain: the path forward through better design of clinical studiesAnesthesiology2010112351451520124977
- GerbershagenHJRothaugJKalkmanCJMeissnerWDetermination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methodsBr J Anaesth2011107461962621724620
- JensenMPSmithDGEhdeDMRobinsinLRPain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe painPain200191331732211275389
- LandmanZOswaldTSandersJDiabMPrevalence and predictors of pain in surgical treatment of adolescent idiopathic scoliosisSpine (Phila Pa 1976)2011361082582921192302
- PagéMGFussSMartinALEscobarEMKatzJDevelopment and preliminary validation of the Child Pain Anxiety Symptoms Scale in a community sampleJ Pediatr Psychol201035101071108220430838
- McCrackenLMDhingraLA short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validityPain Res Manag200271455016231066
- PageMGCampbellFIsaacLStinsonJMartin-PichoraALKatzJReliability and validity of the Child Pain Anxiety Symptoms Scale (CPASS) in a clinical sample of children and adolescents with acute postsurgical painPain201115291958196521489692
- SilvermanWKFleisigWRabianBPetersonRAChildhood Anxiety Sensitivity ScaleJ Clin Child Psychol1991202162168
- CrombezGBijttebierPEcclestonCThe child version of the pain catastrophization scale (PCS-C): a preliminary validationPain2003104363964612927636
- SullivanMJLBishopSRPivikJThe pain catastrophizing scale: development and validationPsychol Assess199574524532
- CrombezGEcclestonCBaeyensFEelenPWhen somatic information threatens, pain catastrophizing enhances attentional interferencePain1998752–31871989583754
- WalkerLSGreeneJWThe functional disability inventory: measuring a neglected dimension of child health statusJ Pediatr Psychol199116139581826329
- Kashikar-ZuckSVaughtMHGoldschneiderKRGrahamTBMillerJCDepression, coping, and functional disability in juvenile fibromyalgia syndromeJ Pain20023541241914622745
- LynchAMKashikar-ZuckSGoldschneiderKRJonesBAPsychosocial risks for disability in children with chronic back painJ Pain20067424425116618468
- ReidGJMcGrathPJLangBAParent-child interactions among children with juvenile fibromyalgia, arthritis and healthy controlsPain20051131–220121015621381
- GidronYMcGrathPJGooddayRThe physical and psychosocial predictors of adolescents’ recovery from oral surgeryJ Behav Med19951843853997500329
- Von BaeyerCLNumerical rating scale for self-report of pain intensity in children and adolescents: recent progress and further questionsEur J Pain200913101005100719766028
- von BaeyerCLSpagrudLJMcCormickJCChooENevilleKConnellyMAThree new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensityPain2009143322322719359097
- FaulstichMECareyMPRuggieroLEnyartPGreshamFAssessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC)Am J Psychiatry19861438102410273728717
- MarchJSParkerJDASullivanKStallingsPConnersCKThe Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability and validityJ Am Acad Child Adolesc Psychiatry19973645545659100431
- FaulFErdfelderEBuchnerALangAGStatistical power analyses using G*Power 3.1: tests for correlation and regression analysesBehav Res Methods20094141149116019897823
- GelmanAHillJSample size and power calculationsGelmanAHillJData Analysis Using Regression and Multilevel/Hierarchical ModelsNew YorkCambridge University Press2007437455
- HsiehFYBlochDALarsenMDA simple method of sample size calculation for linear and logistic regressionStat Med19981714162316349699234
- KatzJSeltzerZTransition from acute to chronic postsurgical pain: risk factors and protective factorsExpert Rev Neurother20099572374419402781
- von BaeyerCLInterpreting the high prevalence of pediatric chronic pain revealed in community surveysPain2011152122683268421920669
- HuguetAMiroJThe severity of chronic pediatric pain: an epidemiological studyJ Pain20089322623618088558
- EcclestonCFisherEAVervoortTCrombezGWorry and catastrophizing about pain in youth: a reappraisalPain201215381560156222516589
- OcanezKLMcHughRKOttoMWA meta-analytic review of the association between anxiety sensitivity and painDepress Anxiety201027876076720336798
- MartinALHalketEAsmundsonGJFloraDBKatzJPosttraumatic stress symptoms and the diathesis-stress model of chronic pain and disabilityClin J Pain201026651852720551727
- AsmundsonGJNortonPJVlaeyenJWSFear-avoidance models of chronic pain: an overviewAsmundsonGJGVlaeyenJWSCrombezGUnderstanding and Treating Fear of PainNew YorkOxford University Press2004324
- AsmundsonGJNoelMPetterMParkersonHPediatric fear-avoidance model of chronic pain: Foundation, application and future directionsPain Res Manag201217639740523248813
- KatzJSeltzerZTransition from acute to chronic postsurgical pain: risk factors and protective factorsExpert Rev Neurother20099572374419402781