Abstract
Purpose
To compare tenderness and pain sensitivity in children (aged 7–17 years) with tension-type headache (TTH) and healthy controls using total tenderness score (TTS), pressure pain threshold (PPT), and pain perceived at suprapressure pain threshold (supraPPT).
Patients and methods
Twenty-three children with frequent episodic TTH, 36 with chronic TTH, and 57 healthy controls were included. TTS was measured bilaterally at seven pericranial myofascial structures. PPT and supraPPT were assessed in the finger, m. temporalis, and m. trapezius by a Somedic® algometer. SupraPPT was defined as the pain perceived at a stimulus calculated as the individual site-specific PPT + 50%.
Statistics
The effect of group, sex, age, headache frequency, intensity, and years on TTS, PPT, and supraPPT was analyzed by general linear models. Confirmatory factor analysis was analyzed for mutual relations between measurements.
Results and conclusion
Tenderness increased uniformly in both frequent episodic TTH (median 14; interquartile range [IQR] 10–18; P < 0.001) and chronic TTH (median 13; IQR 9–20; P < 0.001) compared to controls (median 5, IQR 3–11). However, the children with frequent episodic TTH and chronic TTH did not show significantly increased sensitivity when measured by PPT or supraPPT. Factor analysis confirmed that the site-specific measurements depended on general latent variables. Consequently, the PPT and supraPPT tests can be assumed to measure central pain-processing levels.
Introduction
Tension-type headache (TTH) is a frequent disease among children. The prevalence of TTH in schoolchildren and adolescents is 10%–25%; however, 0.1%–5.9% suffer from chronic tension-type headache (CTTH) (headache ≥ 15 days per month).Citation1,Citation2 This “invisible” and paroxysmal disease deserves attention since quality of life is often impaired in children with primary headaches.Citation3 Chronic pain, which is often associated with mood disorders, lost social relations, lower school attendance, and academic difficulties, might have profound consequences for children suffering from headache in every aspect of their daily lives.Citation4–Citation7 Consequently, there is a need for clarification of the pathophysiological mechanisms of TTH in children in order to identify relevant pharmacological targets and to optimize treatment strategies.
Research in the previous 20 years has provided substantial knowledge on the pathophysiological mechanisms of TTH in adult headache sufferers. Several different test methods have been used, such as: (1) total tenderness score (TTS), (2) pain detection threshold, (3) pain tolerance threshold, (4) electromyographic activity, (5) suprathreshold stimulation, and (6) brain imaging.Citation8 The most prominent finding is that both frequent episodic tension-type headache (FETTH) (headache 1–14 days/month) and CTTH are associated with increased tenderness of the pericranial myofascial tissues.Citation9–Citation11 The majority of studies show that the pressure pain threshold (PPT) is slightly, but significantly, lower in CTTH compared to healthy controls.Citation8,Citation11–Citation16 Two studies indicate that this might also be true in patients with FETTH.Citation16,Citation17 Stimulus-response functions for pressure versus pain have shown altered pain perception in patients with CTTH compared with headache-free controls.Citation18 Various studies have contributed to the hypothesis that altered pain sensitivity in FETTH is established through sensitization of peripheral nociceptorsCitation19 and that prolonged nociceptive input from tender pericranial muscles results in sensitization at the spinal/trigeminal level and in higher-order neurons converting FETTH to CTTH.Citation8,Citation19–Citation27
Significant evidence is being accumulated on adult headache sufferers, but it is still uncertain if these results can be applied directly to children. Since children have typically not suffered from TTH as long as adults, one very important question is whether or not central sensitization exists in children. Until now, very few studies have focused on the pathophysiological mechanisms of TTH in children and adolescents. The studies that do exist primarily focus on TTS in pericranial tissueCitation28–Citation30 and PPTCitation7,Citation28,Citation30,Citation31 in children suffering from FETTH. Results indicate an increased TTS, while the results comparing PPT in children with FETTH and healthy controls are contradictory. Furthermore, a study on trigger points shows an increased number of pericranial trigger points and larger areas of referred pain in children with CTTH.Citation22
To our knowledge, this is the first study to explore pain sensitivity and muscle tenderness in children for the full spectrum of TTH, including FETTH and CTTH. We hypothesized that children with TTH have an increased tenderness of pericranial myofascial tissue and an increased sensitivity for pressure.
The purpose of this study was to use pain measurement tools (TTS, PPT, and pain perceived at individually PPT-adjusted stimuli) to test any differences between children with TTH (FETTH or CTTH) and healthy controls. Secondarily, the aim was to test if there is a relation between the measured variables and headache frequency, headache intensity, or headache years.
Methods
Study design
The study was conducted using a cross-sectional case-control study design.
Participants
Consecutive patients were diagnosed with TTH according to the International Classification of Headache Disorders II (ICHD-II)Citation32 by an experienced pediatrician at the Children’s Headache Clinic (CHC) in Denmark and screened for study enrollment from May 2009 to May 2011. All patients underwent a detailed interview and neurologic examination carried out by a pediatrician during their first visit at the clinic. Seven years of age was assumed to be the limit of cognitive capability to perform the tests correctly. The visual analog scale (VAS) score has previously been shown to be reliable and valid in children over 5–6 years of age.Citation33 The two inclusion criteria were that the patients had: (1) an FETTH or CTTH diagnosis and (2) were between 7–17 years of age, while the exclusion criteria were that the patients: (1) were receiving prophylactic treatment or (2) were suspected of having medication overuse headache or other comorbid headache disorders. Comorbid migraine ≤ 1 day per month was, however, accepted in the included children.
Healthy controls were recruited from two schools in the Copenhagen area. The enrollment and examinations took place from May 2009 to November 2010. The children were informed in person about the study in their classrooms by a pediatrician. Children who were interested in participating received a letter with written information to take home to their parents, who were asked to respond by email. The same pediatrician subsequently contacted the parents who had responded and informed oral consent was given. Written informed consent was provided later.
The exclusion criteria were that the healthy controls: (1) had more than 12 headache days per year or (2) suffered from some kind of chronic illness. The exclusion criteria were based on the assumption that infrequent TTH often occurs among children, thus making it difficult to recruit children with no headache at all.Citation2,Citation34 Furthermore, the assumption was made that the presence of infrequent headaches (<1 day per month) would not affect pain sensitivity measured by the tests being applied.
Setting
Patients were tested at the CHC within the space of 3 weeks after their first and before any interventions were initiated. Healthy controls were tested in their own schools during the school day. The participating children were not permitted to take pain medication 12 hours before the tests in order to prevent interference with pain sensitivity in the myofascial tissues. During the tests, all children were examined by the same physiotherapist with pediatric experience and each child was assisted by the same pediatrician. The physiotherapist was blinded for the child’s answers, but not for disease/control status. She had no information about the patient’s headache history and the children were unknown to her. The tests lasted 20 minutes and were conducted in a quiet, comfortable room. The FETTH and CTTH children were accompanied by a parent. Parents of the healthy school children were invited to participate, but refrained from attending in the belief that their children felt secure. All procedures were carefully explained before each test. The child was placed in a vertical position in an adjustable chair with head and armrests. The chair was individually adjusted to obtain relaxed muscles during the test. The child was examined fully clothed, but was asked to uncover the shoulder and neck area.
Self-reported measurements during the first visit
(1) Headache frequency was recorded as headache days per month, (2) Headache intensity was the most dominating VAS score during each headache attack, and (3) Headache years was the total number of years with headache. Data were obtained by interviewing the parents and child, and from a headache diary comprising the 1-month period prior to the interview.
Outcome variables
(1) TTS, (2) PPT, and (3) the VAS score at the suprapressure pain threshold (supraPPT).
Test 1: total tenderness score
Tenderness in pericranial structures was assessed by the TTS system,Citation9 which previously has proved to be reliable in adults.Citation35 The investigator was positioned in front of the child. Seven myofascial structures were palpated bilaterally: m. masseter, m. temporalis, m. frontalis, processus mastoideus, the neck muscle insertions on basis cranii, m. trapezius, and m. sternocleidomastoideus. Each structure was palpated with the pulpa of the second and third finger in a rotating manner, point to point, for 4–5 seconds. If a difference between the two sides was detected, each side was palpated separately to obtain a score. The palpation pressure was moderate, and in order to be able to compare our results with previous studies in adult patients, it was standardized at 120 arbitrary units on a palpometer kindly on loan from the Danish Headache Centre, Glostrup University Hospital, Denmark. The palpometer has previously been described and validated as a useful tool to obtain a standardized pressure.Citation36 Tenderness was evaluated on a four-point scale: 0 = no visible reaction and denial of tenderness; 1 = no visible reaction but verbal report of discomfort or mild pain; 2 = verbal report of painful tenderness, facial expression of discomfort or no reaction; and 3 = marked grimacing or withdrawal, verbal report of marked painful tenderness and pain. Values from both sides were added together for a TTS between 0–42.
Test 2: pressure pain threshold
Algometry has previously been shown to be reliable (intra- and interrater) in measuring PPT in children.Citation37 The PPT was assessed using a Somedic® Algometer II (SBMEDIC Electronics, Solna, Sweden).Citation38 The algometer comprises a gauge attached to a hard rubber tip and the gauge is connected to a finger button. Pressure was applied though the rubber surface area of 1 cm2 at a rate of 10 kPa per second. The instrument was placed perpendicular to the skin’s surface. Only one side of the body was tested since we assumed that the duration of the applied tests was the upper limit of concentration in the participating children. Consequently, the non-dominant side was tested in all children to avoid any differences in tissue composition and pain sensitivity according to hand dominance. We tested PPT at three different sites: P1, dorsum of the second finger’s interphalanx; P2, the anterior temporal region where palpation revealed the belly of the muscle during contraction; and P3, m. trapezius, the point halfway between C7 and acromion. The method was demonstrated once at each site before testing to ensure that the participants were familiar with the test. The participants were asked to indicate when the pressure became painful based on the following definition, “When you feel the sensation changes from pressure to the slightest pain, press the button immediately”. The electronic display was then read. This procedure was in accordance with the International Association for the Study of Pain’s definition of pain threshold as “the minimum intensity of a stimulus that is perceived as painful”.Citation39 Each measure site was tested three times with 2 minutes between each test, but the site was different for each measure. The mean of the three consecutive trials for each site was calculated (PPTP1, PPTP2, and PPTP3) and used in further analysis to reduce intraindividual error.
Test 3: visual analog score at suprapressure pain threshold
The supraPPT in each individual child and test site was defined as the mean of the child’s three consecutive PPT measures tested in Test 2 + 50%. The algometer was placed perpendicular to the skin. The pressure of the supraPPT calculated was applied to the child for a total of 3–4 seconds at each site. The child was asked to mark the pain level with a pencil on a printed VAS showing a happy face on one end and a crying face on the other. The child was told that the happy face represented no pain and the crying face the worst pain they could imagine. The VAS score was later measured at 0.0–10.0 cm.
Study size
Our goal was to include 60 patients and 60 healthy controls. On the basis of published studies before 2008, we did not have relevant information to make power calculations a priori.
Approval
Written informed consent was obtained in all patients and controls. The study was approved by the local biomedical research ethics committee H-D-2009-019 and the Danish Data Protection Agency 2009-41-3172.
Statistics
SPSS software (version 20; IBM, Armonk, NY, USA) and Mplus (Muthén and Muthén 2007) were used for data analysis. Data that do not follow a parametric distribution (self-measured variables, TTS, PPT, and VAS at supraPPT) are characterized by median and interquartile range (IQR). The Mann–Whitney U test was used to analyze for differences between groups at the seven test sites in the TTS test. Given that it was a non-parametric analysis, multiplicity was not taken into account; however, the results were assessed in that light. Simple difference between test sites (P1, P2, P3) in Test 2 and Test 3 were determined by a Wilcoxon signed-rank test.
Factor analysis is a statistical method that confirms if a number of observed variables mainly reflect fewer unobserved general latent variables, known as factors. The information gained about the inter-dependencies between observed variables can be used later to reduce the set of variables for the main analysis and to confirm hypothesis about the mutual relations between the observed variables. Let PPTP1, PPTP2, and PPTP3 be the three measures of Test 2 and let supraPPTP1, supraPPTP2, and supraPPTP3 be the measures of Test 3. During the statistical analysis, we regarded the two sets of variables as indicators of unobserved general levels of PPT and supraPPT, respectively. We therefore focused on the association with the general PPT and general supraPPT level and the TTS on the one hand and the explanatory variables on the other. To support this point of view, we conducted a confirmatory factor analysis by structural equation models (Bollen, 1989 and Muthén and Muthén, 2007), where PPT and supraPPT were regarded as unobserved general latent variables and where PPTP1, PPTP2, and PPTP3 were assumed to load on the general PPT level, while supraPPTP1, supraPPTP2, and supraPPTP3 were assumed to load on the general supraPPT level of pain. Finally, the structural equation model included the TTS, assuming that TTS depended on the latent PPT and supraPPT variables but not on the manifest PPTP1, PPTP2, PPTP3, and supraPPTP1, supraPPTP2, and supraPPTP3 variables.
The confirmatory factor analysis accepted Muthén and Muthén’s model (2007) (Chi squared test of model fit = 7.9, df = 12; P = 0.79) (). From this model, it follows that the separate PPT and supraPPT indicators can be summarized into overall measures of PPT and supraPPT that can be used during the analysis of the association between the PPT and supraPPT levels and other variables. Consequently, the mean of the PPT measurements for the three sites (T-PPT) and the mean of the VAS measurements at the three sites (T-supraPPT) were calculated and used as measures of the general latent levels of PPT and supraPPT. shows the estimates of the factor loading (the effects of the underlying latent variables on the manifest indicators).
Figure 1 Diagram of the confirmatory factor analysis.
Abbreviations: PPT, pressure pain threshold; P1, second finger; P2, m. temporalis; P3, m. trapezius; TTS, total tenderness score; supraPPT, visual analog scale at suprapressure pain threshold; VAS, visual analog scale.
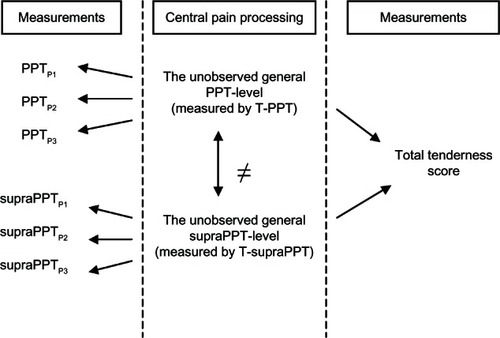
Table 1 Estimates of loadings of PPT and supraPPT indicators on general levels of PPT and supraPPT, respectively
According to the results of the factor analysis, the effect of group (FETTH/CTTH/control), sex, and age on TTS, T-PPT, and T-supraPPT were analyzed separately by general linear models (GLM) taking frequency, intensity, headache years, and interactions into account. As the assumption of normal distribution was not fulfilled, dependent variables were transformed with the square root (TTS) and the logarithmic function (T-PPT). T-supraPPT remained untransformed in the analysis. A P-value less than 5% was considered statistically significant.
Results
Descriptive data
Patients
Around 400 children with either primary or secondary headache disorders attended the clinic in the study period. The pediatrician screened all patients for the inclusion and exclusion criteria during their first visit. Sixty-four children were confirmed eligible and present on days when the research team was also present and could provide oral information in person. Five children declined to participate, but 59 agreed to participate and performed the tests. No records of the five children who declined were registered. We included 23 children with FETTH, mean age 10.5 (standard deviation [SD] 2.5), 13 females/10 males and 36 children with CTTH, mean age 12.6 (SD 2.2) and 28 females/8 males.
Controls
Twenty-eight classes with around 20 pupils (aged 7–15 years) each were told about the study in person in the classroom by a pediatrician. Sixty healthy pupils were included. Fifty-seven children performed the tests. Three children were absent on the day of examination. The control group had a mean age of 10.7 (SD 2.3) and 36 were females, 21 males.
Missing values
(1) one child suffering from FETTH only completed the TTS test; (2) the PPT test was stopped in all three sites for fear of tissue contusion in one healthy control; (3) the calculated supraPPT in the finger was not applied since there was the risk of tissue contusion in one healthy control; (4) in one healthy control and in one FETTH patient, a calculation error was performed, which resulted in the wrong supraPPT stimuli in the m. temporalis, the results of which were later erased from the database ().
Table 2 Diagram of participating children
Self-reported measurements
In the FETTH group, the median headache frequency was seven days per month (IQR 4.0–8.5), the headache intensity was 5.0 cm (IQR 4.0–5.5) on a VAS and the children had suffered from headache for a median duration of two years (IQR 1.0–5.0) prior to the examination. The CTTH group had a median headache frequency of 30 days per month (IQR 21.5–30.0), a VAS score of 5 cm (IQR 4.0–6.0) and had suffered from headache for a median duration of 2 years (IQR 1.0–3.8) prior to the examination. Twenty-six percent of the FETTH children and 86% of the CTTH children had headache at the time of examination.
Test measurements
presents the median and IQR among patients and controls.
Table 3 Measurements
Differences between test sites P1, P2, and P3
The PPT did not significantly differ between the three test sites in the individual child. However, in the supraPPT test, the children were significantly more sensitive to pressure in the m. temporalis (P < 0.001) and in the m. trapezius (P < 0.001) compared to the finger.
Associations between outcome variables and explanatory variables
TTS
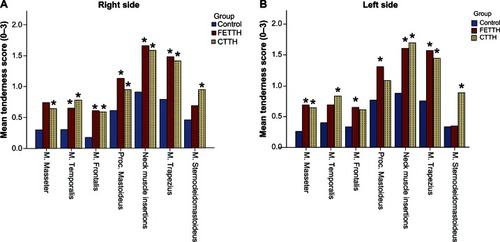
Both the FETTH group (P < 0.001) and the CTTH group (P < 0.001) had a significantly higher TTS than the control group (). The median TTS was almost equal between the two headache groups (P = 0.914). Sex, age, headache frequency, intensity, and headache years were all insignificant and had no association with TTS.
The tenderness was almost uniformly raised in all of the seven examination sites bilaterally indicating general increased pericranial tenderness in muscles and tendons in those children suffering from both FETTH and CTTH (). The most tender tissues were m. trapezius and the neck muscle insertions in all three groups.
Figure 2 Tenderness score (0–3) of pericranial muscles and tendons – (A) right side; (B) left side.
Abbreviation: TTH, tension-type headache; CTTH, chronic tension-type headache; FETTH, frequent episodic tension-type headache.
PPT and supraPPT
No differences in T-PPT and T-supraPPT were detected in the FETTH and the CTTH groups compared with healthy controls. Sex, age, headache frequency, intensity, and headache years did not influence T-PPT or T-supraPPT.
Factor analysis
Factor analysis confirmed that PPT measurements in the finger, m. temporalis, and m. trapezius depended on one general latent level of pain threshold in the individual child. Likewise, VAS measurements in the finger, m. temporalis, and m. trapezius depended on one general latent level of experienced pain adjusted for the pain thresholds in the individual child.
When we tested our hypothesis of mutual relations between the three tests (TTS, PPT, and supraPPT), factor analysis confirmed the following, which are also illustrated in : the estimate of the correlation between the general latent PPT and the general latent supraPPT was, according to the structural equation model, r = 0.095 (standard error [SE] = 0.110). This corresponds to the correlation between the measured T-PPT and T-supraPPT, which was equal to 0.091. Since the general PPT level and the general supraPPT level did not correlate, we must conclude that the two measurements of pain were independent and measured two different aspects of pain sensitivity in the individual child.
Furthermore, according to the structural equation model, the dependence of TTS on the general latent PPT level and the general latent supraPPT level can be described by a linear regression model with the two latent variables as independent variables (TTS = α + β1PPT + β2SupraPPT + ε). The estimates of the effects of PPT and VAS at SupraPPT were both significantly different from zero (β1 = 1.464, SE = 0.390) and (β2 = −0.046, SE = 0.012) in the confimatory factor analysis. This confirmed that TTS depended on both the general latent level of PPT and the general latent level of supraPPT (). In other words, the individual child’s tenderness in the pericranial area was associated to the child’s general level of pain processing, which in part can be measured by the PPT test and in part by the supraPPT test.
Discussion
TTS, PPT, and supraPPT
The present study has detected an almost equally increased and uniformly distributed tenderness of pericranial tissue in children with either FETTH or CTTH. However, headache days per month did not affect the tenderness. This could indicate that the increased tenderness is a key factor in TTH, but other processes must determine whether the pain is only frequent or chronic. Increased tenderness has been a consistent finding in studies of both adultsCitation19 and children with TTH.Citation29,Citation30
Our study did not detect any differences in PPT or supraPPT in either the FETTH group or the CTTH group. PPT studies in adults with CTTH have demonstrated a widespread and non-specific nature of decreased PPT.Citation8,Citation11–Citation16 Studies of PPT in patients with episodic TTH are contradictory.Citation8 Furthermore, Bendtsen et al also demonstrated altered pain perception in adult CTTH sufferers when he conducted stimulus-response functions in a range of suprathreshold stimuli.Citation18 Only four studies of PPT have been conducted in children with TTH and they all examined children with FETTH. Fernandez-de-las-Peñas et al documented significantly decreased PPT in both the muscles and above nerves of children with FETTH (mean 12.7 and 14 days/month).Citation30,Citation31 Two other studies could not detect any difference in PPT between the children with FETTH and the healthy controls. However, in both studies, the headache frequency was very low (mean 3 days/month).Citation7,Citation28
The present study could not detect any effect of sex on pain sensitivity. This finding is not consistent with previous studies of PPT in adults, which have shown a higher sensitivity in females compared with males.Citation16,Citation40,Citation41 Previous studies of PPT in children, however, are contradictory. Buskila et alCitation42 found that healthy females were more sensitive than males, but Hogeweg et al found no sex difference in healthy children.Citation43 These differences may be attributed to thresholds being measured at different sites and with different algometers.
Measures of peripheral or central pain processing
It has been proposed that increased TTS in TTH patients reflects the peripheral sensitization of nociceptors and their neurons, whereas altered PPT primarily, or in part, reflects central sensitization at the level of second-order neurons in the spinal dorsal horn/trigeminal nucleus or supraspinal neurons.Citation18,Citation19 The factor analysis confirmed this hypothesis.
The PPT measurements and VAS measurements in the finger, m. temporalis, and m. trapezius were dependent on latent general variables and must consequently mainly measure central level of pain pathways. In other words, the same child might have different PPT and VAS measures in different sites, but latent general levels of these measures exist, which determines the individual’s “over-all” sensitivity level compared to others’ sensitivity levels. The differences in sensitivity between test sites might be explained by differences in peripheral nociception at various sites in the individual child. Given that PPT and supraPPT tests mainly reflect the central level of pain processing, we must conclude that we could not detect differences between groups indicating the presence of central sensitization in children with TTH. The present negative findings may be caused by the high interindividual variability of these measurements in children (). A very large sample size is necessary to detect changes between groups.
In contrast, we found, in an earlier study by the present authors, indications of altered pain sensitivity and possible central sensitization in the same children when examined with stimulus-response functions.Citation44 In our earlier study, pressures of five increasing intensities were applied to m. trapezius and m. temporalis with the algometer and VAS was rated at each pressure. Area under the curve was calculated and significantly increased in the children with CTTH, indicating altered pain sensitivity. The method and results will be published elsewhere. Measuring pain sensitivity by stimulus-response functions might amplify the differences between groups and compensate for the problem of high interindividual variability, because it is a sum measure in a broad range of stimuli intensities.
Another interesting finding was the results from the factor analysis that concerned the mutual relation between the three tests and the three test sites in the individual child. The analysis confirmed that we succeeded in producing an individual adjusted suprathreshold-stimuli test, which measured something different than the PPT test. Furthermore, we found that TTS was affected by both the general PPT level and the general supraPPT level in the individual child.
Limitations
The nonblinding of the physiotherapist is the major limitation of the present study. Blinding would have been the optimal study design; however, it was not possible to get the healthy controls to participate at the headache clinic in a blinded setting. Bias would create differences between headache groups as our physiotherapist would expect the patients to be more sensitized. Furthermore, the patients were examined in unknown surroundings. Anxiety might induce increased sensitivity compared to the healthy controls. However, we did not detect any differences in the PPT or supraPPT tests. Only the patients were accompanied by a parent at examination. If the healthy controls were more anxious due to participating alone, it could induce bias in terms of higher sensitivity in the control group, thus counteracting the real differences seen between patients and healthy controls. This bias could contribute to the negative findings in the PPT and supraPPT tests. The lack of differences between patients and healthy controls might also be related to a small sample size.
Another limitation is the fact that we did not distinguish between children with or without headache on the day of examination in our study design. We did register it. One could argue that the actual headache status could interfere with pain sensitivity and tenderness. This was not the case in a study of PPT in adults.Citation16 Pericranial tenderness assessed by TTS seems to be influenced by the actual headache status, although increased TTS is found in TTH both on days with and without headache.Citation45 However GLM analysis using headache status on the day of examination as an additional factor did not change the results in our study. The factor was insignificant in all analyses. We did not examine for psychiatric comorbidity such as anxiety or depression in a structured way, but our impression was that none of the children suffered from serious comorbidities.
Our results add to the evidence of increased peripheral myofascial tenderness in both FETTH and CTTH, but contradict the previous findings of alterations in PPT in children and adults suffering from TTH. This leaves us with the following open questions yet to be answered: (1) Are the tests not sensitive enough to reveal differences in pain sensitivity between healthy controls and children with TTH? (2) Is this because of the high interindividual variability in children? (3) Are the mechanisms of TTH in children different from those in adults? Further research in pain sensitivity in children with TTH is urgently needed.
Conclusion
Children suffering from either episodic or chronic TTH have increased tenderness in myofascial tissues of the head and neck area compared with healthy controls. The tenderness is equally higher in both groups and universally distributed in the head and neck area. The increased tenderness is not associated with headache frequency, headache intensity, or years suffering from headache.
PPT measurements and pain sensitivity at individually PPT-adjusted stimuli are not changed in children suffering from FETTH or CTTH compared with healthy controls. The present negative findings may be caused by the high interindividual variability of these measurements in children and do not exclude the presence of hypersensitivity in children with TTH.
PPT measures and perceived pain at individually PPT-adjusted stimuli appear to measure central levels of pain processing. Further studies in the full spectrum of TTH are needed to confirm these findings.
Acknowledgments
We would like to thank the healthy children from Sengeløse Skole and Søndervangskolen as well as the CHC patients who agreed to participate. We would also like to thank the Danish Headache Centre, University Hospital of Copenhagen in Glostrup for providing the palpometer.
Disclosure
The authors report no conflicts of interest in this work. This project was supported by the Lundbeck Foundation (grant numbers R19-A2040 and R34-A3618); the Dagmar Marshalls Foundation; the Master Carpenter Jørgen Holm and Wife Elisa F Hansen’s Memorial Trust; the Capital Region of Denmark (grant number R120-A3256); the Danish Headache Society; and the Torben Iversen’s Travelling Foundation.
References
- AnttilaPTension-type headache in childhood and adolescenceLancet Neurol2006526827416488382
- SeshiaSSAbu-ArafehIHersheyADTension-type headache in children: the Cinderella of headache disorders!Can J Neurol Sci20093668769519960746
- NodariEBattistellaPANaccarellaCVidiMQuality of life in young Italian patients with primary headacheHeadache20024226827412010383
- AndrasikFSchwartzMSBehavioral assessment and treatment of pediatric headacheBehav Modif2006309311316330521
- AnttilaPMetsahonkalaLAromaaMDeterminants of tensiontype headache in childrenCephalalgia20022240140812110116
- EspositoMPascottoAGallaiBCan headache impair intellectual abilities in children? An observational studyNeuropsychiatr Dis Treat2012850951323139628
- MetsahonkalaLAnttilaPLaimiKExtracephalic tenderness and pressure pain threshold in children with headacheEur J Pain20061058158516203164
- BezovDAshinaSJensenRBendtsenLPain perception studies in tension-type headacheHeadache20115126227121029081
- LangemarkMOlesenJPericranial tenderness in tension headache. A blind, controlled studyCephalalgia198772492553427625
- JensenRRasmussenBKPedersenBOlesenJMuscle tenderness and pressure pain thresholds in headache. A population studyPain1993521931998455967
- JensenRBendtsenLOlesenJMuscular factors are of importance in tension-type headacheHeadache19983810179504997
- AshinaSBabenkoLJensenRAshinaMMagerlWBendtsenLIncreased muscular and cutaneous pain sensitivity in cephalic region in patients with chronic tension-type headacheEur J Neurol20051254354915958095
- BendtsenLJensenROlesenJDecreased pain detection and tolerance thresholds in chronic tension-type headacheArch Neurol1996533733768929161
- JensenROlesenJInitiating mechanisms of experimentally induced tension-type headacheCephalalgia1996161751828734769
- LangemarkMJensenKJensenTSOlesenJPressure pain thresholds and thermal nociceptive thresholds in chronic tension-type headachePain1989382032102780074
- Schmidt-HansenPTSvenssonPBendtsenLGraven-NielsenTBachFWIncreased muscle pain sensitivity in patients with tension-type headachePain200712911312117161538
- MorkHAshinaMBendtsenLOlesenJJensenRInduction of prolonged tenderness in patients with tension-type headache by means of a new experimental model of myofascial painEur J Neurol20031024925612752398
- BendtsenLJensenROlesenJQualitatively altered nociception in chronic myofascial painPain1996652592648826515
- BendtsenLCentral sensitization in tension-type headache–possible pathophysiological mechanismsCephalalgia20002048650811037746
- SchoenenJBottinDHardyFGerardPCephalic and extracephalic pressure pain thresholds in chronic tension-type headachePain1991471451491762808
- Fernandez-de-las-PeñasCCuadradoMLArendt-NielsenLSimonsDGParejaJAMyofascial trigger points and sensitization: an updated pain model for tension-type headacheCephalalgia20072738339317359516
- Fernandez-de-las-PeñasCFernandez-MayoralasDMOrtega-SantiagoRAmbite-QuesadaSPalacios-CenaDParejaJAReferred pain from myofascial trigger points in head and neck-shoulder muscles reproduces head pain features in children with chronic tension type headacheJ Headache Pain201112354321359873
- BuchgreitzLLyngbergACBendtsenLJensenRFrequency of headache is related to sensitization: a population studyPain2006123192716630694
- BuchgreitzLLyngbergACBendtsenLJensenRIncreased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up studyPain200813762363018061350
- BendtsenLJensenRAmitriptyline reduces myofascial tenderness in patients with chronic tension-type headacheCephalalgia20002060361011075846
- BendtsenLFernandez-de-la-PeñasCThe role of muscles in tensiontype headacheCurr Pain Headache Rep20111545145821735049
- AshinaSBendtsenLAshinaMMagerlWJensenRGeneralized hyperalgesia in patients with chronic tension-type headacheCephalalgia20062694094816886930
- AnttilaPMetsahonkalaLMikkelssonMMuscle tenderness in pericranial and neck-shoulder region in children with headache. A controlled studyCephalalgia20022234034412110109
- CarlssonJTenderness of pericranial muscles in schoolchildren with headacheThe Pain Linic199694956
- Fernandez-MayoralasDMFernandez-de-las-PeñasCOrtega-SantiagoRAmbite-QuesadaSJimenez-GarciaRFernandez-JaenAGeneralized mechanical nerve pain hypersensitivity in children with episodic tension-type headachePediatrics2010126e187e19420530075
- Fernandez-de-las-PeñasCFernandez-MayoralasDMOrtega-SantiagoRAmbite-QuesadaSGil-CrujeraAFernandez-JaenABilateral, widespread, mechanical pain sensitivity in children with frequent episodic tension-type headache suggesting impairment in central nociceptive processingCephalalgia2010301049105520713555
- The International Classification of Headache Disorders2nd edCephalalgia200424Suppl 1916014979299
- MelzackWallTextbook of PainFifth Edition edNew York, NYElsevier2006
- LewisDWGozzoYFAvnerMTThe “other” primary headaches in children and adolescentsPediatr Neurol20053330331316243216
- BendtsenLJensenRJensenNKOlesenJPressure-controlled palpation: a new technique which increases the reliability of manual palpationCephalalgia1995152052107553810
- BendtsenLJensenRJensenNKOlesenJMuscle palpation with controlled finger pressure: new equipment for the study of tender myofascial tissuesPain1994592352397892021
- ChavesTCNagamineHMde SousaLMde OliveiraASGrossiDBIntra- and interrater agreement of pressure pain threshold for masticatory structures in children reporting orofacial pain related to temporomandibular disorders and symptom-free childrenJ Orofac Pain20072113314217547125
- Somedic ElectronicsWeb site2012 Available from: http://www.sbmedic.seAccessed on. August 15, 2012
- International Association for the Study of PainTaxonomy2012 Available from: http://www.iasp-pain.orgAccessed on May 22, 2012
- NeziriAYScaramozzinoPAndersenOKDickensonAHArendt-NielsenLCuratoloMReference values of mechanical and thermal pain tests in a pain-free populationEur J Pain20111537638320932788
- Kroner-HerwigBGassmannJTromsdorfMZahrendEThe effects of sex and gender role on responses to pressure painPsychosoc Med20129Doc0122400065
- BuskilaDPressJGedaliaAAssessment of nonarticular tenderness and prevalence of fibromyalgia in childrenJ Rheumatol1993203683708474077
- HogewegJAKuisWOostendorpRAHeldersPJThe influence of site of stimulation, age, and gender on pain threshold in healthy childrenPhys Ther199676133113398960002
- SoeeABThomsenLLKreinerSTornoeBSkovLAltered pain perception in children with chronic tension-type headache: Is this a sign of central sensitisationCephalalgia2252013 [Epub ahead of print.]
- JensenRMechanisms of spontaneous tension-type headaches: an analysis of tenderness, pain thresholds and EMGPain1996642512568740601