Abstract
Purpose
The spinal nerve ligation (SNL) model is a typical peripheral neuropathic pain model. During its construction, the removal of paraspinal muscles and transverse processes typically occurs, resulting in additional trauma that may potentially affect the pathophysiologic process of neuropathic pain. This study aimed to investigate the feasibility of establishing a more reliable SNL model using an oblique lateral approach.
Methods
36 adult male Sprague-Dawley rats were randomly divided into three groups: the traditional SNL (T-SNL) group, the new SNL (N-SNL) group (where the left L5 spinal nerve was ligated with a titanium clip via an oblique lateral approach), and the sham-operated (Sham) group. The operation time, Intraoperative bleeding, the number of rats that died, gait behavior, mechanical and cold pain threshold were recorded and measured. Stereology technology was used to calculate the number of microglia in spinal dorsal horn, and the Enzyme-linked immunosorbent assay (ELISA) technology was used to detect the expression of TNF-α and IL-1β in spinal cord as well as C-reactive protein (CRP) in serum in order to assess the effect of surgery on animal inflammation.
Results
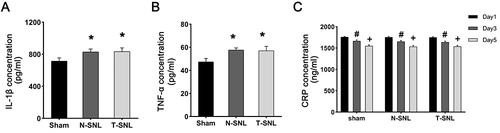
Compared with the T-SNL group, operative time and intraoperative bleeding were significantly decreased in the N-SNL group. Within 14 days postoperation, one rat in the N-SNL group was died, two rats in the T-SNL group were died. Compared with the Sham group, the N-SNL group showed obvious spontaneous pain behavior, decreased the pain thresholds, the number of microglia and the expression of TNF-α and IL-1β were significantly increased, and there was no significant difference in these indexes compared with T-SNL group. There was no significant difference in serum CRP levels among the three groups.
Conclusion
This study suggests that the oblique lateral approach SNL model is a reliable NP model with the advantages of good reproducibility, accessibility, and low trauma.
Introduction
Neuropathic pain (NP) is pain caused by a somatosensory system lesion or illness, in which peripheral nerve injuries are an important cause of induced neuropathic pain.Citation1 NP manifests as spontaneous pain, hyperalgesia, and allodynia. And it is associated with high healthcare costs, decreased quality of life for patients, and increased societal burdens.Citation2 Due to its complex mechanisms, effective therapeutic options for NP are lacking.Citation3
Lumbar disc herniation (LDH) induces NP by compressing and irritating spinal nerve roots, leading to local inflammation and pain. The persistent pain and hyperalgesia symptoms generated by the spinal nerve ligation (SNL) model closely resemble those seen in clinical LDH-induced spinal nerve root irritation or compression. The SNL model, introduced by Kim et al in 1992, and was constructed by firmly ligating of the L5 spinal nerve or the L5 and L6 spinal nerves.Citation4,Citation5 However, this model required animals to be prone position, with surgical exposure achieved by incising the skin along the L4-L6 paraspinal region, followed by removal of paraspinal muscles near the mastoid process and transverse process.Citation4–7 Unfortunately, our previous study and others have encountered difficulties in replicating this SNL model.Citation6,Citation8 Firstly, removal of paraspinal muscles and resection of transverse processes caused additional tissue damage and interfered with pathophysiological mechanisms.Citation9 Secondly, silk ligation of the L5 spinal nerve could easily result in pulling damage to the nerve, and the narrow surgical field made it challenging to achieve consistent ligation strength, thereby affecting post-surgery behavioral test outcomes in animals. Finally, severe postoperative local adhesions, high infection rates, and a high failure rate in modeling further limited the applicability of the model.Citation6,Citation8 Despite improvements in the SNL model, the need for transverse process excision remained, with a high risk of L4/L5 spinal nerve amputation during transversectomy.Citation9,Citation10 We discovered that the current clinical use of oblique lateral lumbar fusion for treating lumbar degenerative diseases is highly effective.Citation11–13
Microglia, the predominant immune cells in the central nervous system (CNS), exhibited a significantly increase in number within the L5 spinal dorsal horn in our previous study of the SNL model.Citation8,Citation14 Activated microglia can release various inflammatory mediators, including IL-1β and TNF-α, through activation of corresponding inflammatory signaling pathways such as mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB). These mediators maintain neuronal hyperexcitability and contribute to the onset and progression of NP.Citation15–17
Therefore, we explored an SNL model that is easier to manipulate, less local injury and higher modeling success rate via an oblique lateral approach. To assess the feasibility of this approach, we introduced titanium clips for spinal nerve ligation following exposure of the surgical field using the oblique lateral approach in the SNL model, and collected operation data, evaluated postoperative pain-related behavior and systemic and local inflammatory responses.
Materials and Methods
Animals
The Experimental Animal Center of North Sichuan Medical College provided 36 adult clean-grade Sprague-Dawley male rats weighing 180–220 g (Animal Use License No. SYXK (Chuan) 2018–076). Rats were kept at temperatures of 20°C to 25°C under a 12 h/12 h light/dark cycle with add libitum access to food and water. All experimental procedures were approved by the ethic committee of North Sichuan Medical College and followed ethical guidelines for investigating experimental pain in conscious Animals (License No. NSMC-202209).
Since our previous study found that the mortality rate of SNL model rats was much higher than that of the Sham rats, the 36 rats used in this study were divided into three groups: traditional SNL-model group (T-SNL group, n=14) in which the L5 spinal nerve was exposed via the posterior approach, with the nerve being ligated using silk; new SNL-model group (N-SNL group, n=14) in which the L5 spinal nerve was exposed through an oblique lateral approach and ligated with titanium clip; sham-operated group (Sham group, n=8) in which the L5 spinal nerve was exposed via oblique lateral approach without the nerve being ligated. All the operations were on the left sides and the right sides of the rats was untreated. Within 14 days postoperation, 1 and 2 rats died in the N-SNL and T-SNL groups, respectively. Some rats with behavioral tests not meeting the pain behavioral criteria were excluded, resulting in the final number of animals in each group being 6 rats in the Sham group, 10 rats in the N-SNL group, and 10 rats in the T-SNL group.
Surgical Methods
The following surgical operations were carried out in a sterile environment:
Surgical Procedures for the T-SNL Group
For T-SNL group, refer to the method of Kim et al.Citation4,Citation5 In brief, rats were positioned prone under isoflurane anesthesia (3–4% induction, 2% maintenance). Longitudinal incisions of 3–4 cm were made 0.5 cm to the left of the spine at the L4-S2 levels, exposing the skin on the rats’ backs. Paravertebral muscles were separated along the spine, fully exposing the vertebrae. The L6 transverse process was then removed, and the L5 spinal nerve was exposed and ligated using 6–0 sutures.
Surgical Procedures for the N-SNL Group
Preoperative preparation included a U-shaped titanium clip (diameter 6 mm, length 3–4 mm) along with a matching titanium clamp (FQC-1, Linya Medical Instruments Co, Ltd, Nanchang, Jiangxi, China) (), in addition to other commonly used surgical instruments. Under isoflurane anesthesia, experimental animals were positioned in the right lateral decubitus. An oblique incision was made between the upper margin of the L4 spinous process and the upper margin of the S1 spinous process, by starting at the left anterolateral 0.5 cm above the L4 spinous process and extending to the left hip tubercle. The incision was then extended horizontally to the left of the upper margin of the S1 spinous process, with a length of approximately 2.5 cm (). After incising the skin and subcutaneous tissues, the external oblique abdominal muscle, internal oblique abdominal muscle, and transverse abdominal muscle were sequentially incised to expose the peritoneum and lumbar square muscles (). To expose the surgical field, the peritoneum was ventrally pulled while minimizing peritoneal damage using gauze or cotton balls.
Figure 1 Construction of a new L5 spinal nerve ligation model by oblique lateral approach. (A–D) Homemade U-shaped titanium wire clip and matching titanium clamp. (E) Location and size of the surgical opening. (F) Peritoneum and quadratus lumborum. ![]()
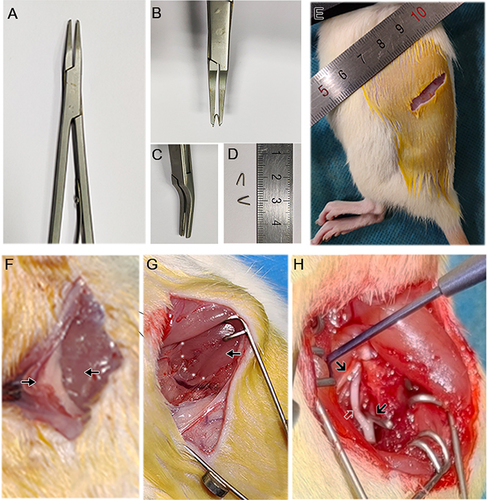
The iliopsoas was located inward along the quadratus lumborum, and the two muscles were separated along the spine by a gap (). A spreader was used to prop open the two muscles, revealing the L3 spinal nerve. On the medial side of the L3 spinal nerve, the L4 spinal nerve was identified, followed by the visualization the L5 spinal nerve along the L4 spinal nerve to the caudal end (). Nerve damage and excessive pulling of the L4 spinal nerve should be avoided throughout the entire procedure.
The self-made sterile U-shaped titanium clip was then installed onto the matching titanium clamp and tightly ligated the L5 spinal nerve (). Upon confirmation of nerve ligation, the muscle layer and skin were sutured, the skin was sterilized again, and the modeling procedure was completed. Rats were provided unrestricted access to food and water post-surgery.
Considerations while using this modeling method include: i. Accurate identification of the gap between the lumbar square muscle and the iliopsoas muscle; ii. Precise determination of the anatomical positions of the L3, L4, and L5 spinal nerves to avoid misidentification and misligation; and iii. Minimization of excessive stretching of L4 during the search for and ligation of L5 nerves.
Surgical Procedures of the Sham Group
The surgical procedures were identical to those outlined for the N-SNL group, except that the L5 spinal nerves were not ligated.
Observation Indicators
Detailed records of operation duration were kept for all three groups of rats during modeling, with the total length of time calculated as the duration from the beginning of anesthesia to the end of anesthesia. Intraoperative bleeding was estimated using gelatin sponges, and the difference in weight of the sponges before and after the operation indicated intraoperative bleeding in the rats. At 14 days postoperatively, following completion of behavioral tests, the number of deaths in each group of rats was recorded.
Observation of General Behaviors
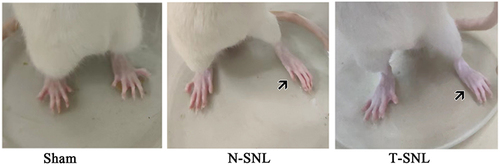
The mental health, hair luster, and weight changes of the rats were documented before and after surgery. Wounds were examined for damage, infection, or suppuration post-surgery, among other factors. Postural responses were observed and documented, such as everting of the left rear paws, spontaneous foot lifting/shaking/licking, and other behaviors.
Behavioral Testing
Behavioral tests were tested in a double-blind manner by two researchers with the same training and testing criteria. The researchers did not know how the rats were grouped before the test. The average of the two researchers’ results was recorded as the pain threshold. Behavioral tests were performed at consistent times to minimize the influence of the rats’ biological clock phenomenon on the experimental results. The positive reaction was paw withdrawal and foot lifting in rats.
50% Paw Withdrawal Mechanical Threshold (PWT)
Rats were acclimated to the test area for at least three days before measurements. Von-Frey filaments of varying (0.6 g, 1.0 g, 1.4 g, 2.0 g, 4.0 g, 6.0 g, 8.0 g, 10.0 g, and 15.0 g) were selected for vertical stimulation of the lateral plantar aspect. Measurements were taken one day prior to surgery and at 4, 7, 10, and 14 days post-surgery. First, 4.0 g of fibrils were selected, and then the 50% PWT threshold was calculated for rats according to the “up-down” method.Citation18
Percentage of Paw Withdrawal After 6.0 g Mechanical Stimulation
Using only the 6.0 g von Frey filaments, the fraction of paw withdrawal in response to increased mechanical stimulation was measured. Three groups of rats were evaluated in turn, with five measurements (5-minute intervals) on each side. Lastly, the five-fold paw withdrawal reaction rate was calculated, which is the proportion of paw withdrawal produced by rats in response to 6.0 g of mechanical stimulation.
Cold Allodynia Threshold
To assess foot sensitivity to cold, foot responses to acetone were measured. Pre-cooled acetone (100 μL) was applied to the plantar surface of the foot while the rat was at quiet. The time of positive response (lifting/shaking/licking, etc) was recorded for 30 seconds. Each paw was tested three times (5-minute intervals), and the average of the three measurements was recorded as the response time on that side.
Iba-1 Immunohistochemical Staining
Retrieval and Embedding: Rat intumescentia lumbalis were retrieved under anesthesia on the 15th postoperative day. The intumescentia lumbalis was divided into two parts along the L5 spinal nerve root: the head end and the tail end. Either the head end or the tail end was randomly selected for subsequent experiments. The selected tissue blocks were fixed in 4% paraformaldehyde for 48 hours, dehydrated in 70% ethanol, and stored in fresh 70% ethanol solution for 24 hours. Standard paraffin dehydration and embedding were performed.
Sections of 10 μm thickness were cut using a paraffin microtome (YGQ-3126D, Yaguang Medical Electronic Technology Co, Ltd, China).
Iba-1 immunohistochemical staining: After conventional paraffin dehydration, immunostaining was conducted using a commercial kit (E-IR-R217, Elabscience, Wuhan, Hubei, China). Briefly, sections were washed with PBS, treated with 0.4% Triton X-100 for 25 minutes, and subjected to antigen retrieval in citrate antigen repair solution (pH = 6.4) in a microwave oven over medium heat for 15 minutes. Subsequently, 3% H2O2 was incubated for 10 minutes. The sections were then blocked with 10% goat serum for 30 minutes, followed by incubation with rabbit anti-mouse Iba-1 (1:300, Proteintech, Wuhan, Hubei, China) overnight at 4°C. After washing with PBS, the sections were incubated with the secondary antibody for 30 minutes at 37°C. DAB was used for color development for 30–90 seconds. The sections were rinsed with tap water, counterstained with hematoxylin for 2.5 minutes, dehydrated, cleared, and sealed with neutral resin. PBS was used as the primary antibody in the negative control.
Stereological Measurement
Sections were observed and measured in a blinded manner. The sections were renumbered prior to measurement to ensure that the group and side of the section were unknown to the observer at the time of measurement. A professional stereology set (NewCAST, Visiopharm, Denmark) equipped with an Olympus BX51 (Olympus, Japan) light microscope was used for examination and photography. Sections stained with Iba-1 (3 sections in each group) were delineated under the × 4 objective lens, followed by examination under the × 20 lens. Each field of view was overlaid with 4 rectangular test frames (area: 14,400.00 μm2) and 9 measuring points (area: 40,673.66 μm2). Test fields were systematically sampled (spaced 0.5 mm apart) using a motorized stage, and the number of microglia within the frames was counted. The numerical density of microglia (NA, number per acreages) in the spinal dorsal horn thus could be calculated with the total microglia number counted and the total acreages of the test frames used.Citation19
Enzyme-Linked Immunosorbent Assay (ELISA) Assay
The testers were blinded to the specific grouping of specimens in the experiment.
IL-1β and TNF-α protein: The L5 left side of the spinal cord was dissected and stored at −80°C. Tissues were homogenized in 200 μL ice-cold RIPA buffer and centrifuged at 5000 g at 4°C for 5 minutes. Supernatants were collected and stored at −20°C or −80°C for future assay. IL-1β and TNF-α levels were determined using ELISA kits (ER1094 and ER1393, FineTest, Wuhan, Hubei, China) according to the manufacturer’s instructions.
C-reactive protein (CRP): Blood was drawn from the tail veins of the three groups of rats 1 day, 3 days, and 5 days after surgery, and centrifuged at 2000 rpm for 10 minutes. The supernatants were collected and CRP levels were determined using an ELISA kit (JM-01488M2, Jingmei, Jiangsu, China) according to the manufacturer’s instructions.
Statistical Analysis
Data are presented as mean ± SEM and were analyzed using SPSS 25.0 software. Normality was assessed using the Shapiro–Wilk test. Paired t-tests or Wilcoxon signed rank tests were used to compare the surgical side and non-surgical side within the same group. One-way analysis of variance or Kruskal–Wallis tests were used for within-group comparisons of CRP levels on different days and among multiple groups, followed by SNK or pairwise test methods for pairwise comparisons. Significance was set at p < 0.05.
Results
Operation Data
Compared with the Sham group, the operative time respectively increased by 33% and 92% in the N-SNL and T-SNL groups (p < 0.05), and increased by 44% in the T-SNL group compared with the N-SNL group (p < 0.05). Intraoperative bleeding significantly increased by 152% and 578% in the N-SNL and T-SNL groups compared with the Sham group (p < 0.05), and increased by 169% in the T-SNL group compared with the N-SNL group (p < 0.05). Within 14 days postoperatively, one rat in the N-SNL group was died, two rats in the T-SNL group were died, and no rats in the Sham group was died ().
Table 1 General Observational Indicators ()
General Behavioral Changes of Rats
There were no significant differences in weight among the groups. There were no differences before and after surgery postures of rats in the Sham group, and there was no spontaneous discomfort in the left hind paw. However, in the N-SNL and T-SNL groups, post-surgery, the toes of left hind paws were held together and left hind paws were everted. Additionally, spontaneous lifting/shaking/licking of the left hind paws were observed, with the rats avoiding weight-bearing on that side by maintaining an abducted forward position (). During exploration, the rats exhibited a skewed center of gravity to the right.
Postoperative PWT Values of Rats
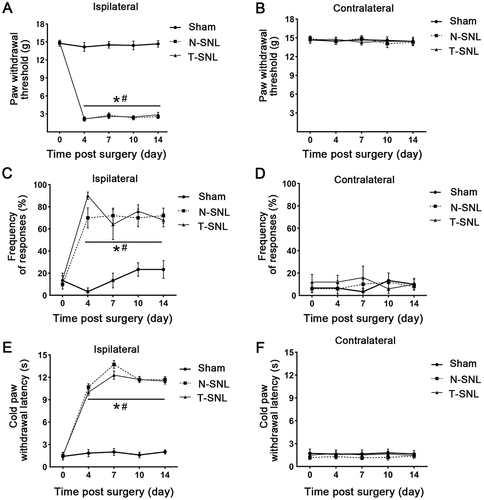
On the operated side, the PWT values in the N-SNL and T-SNL groups was significantly decreased than in the Sham group (p < 0.05), with no significant differences between the N-SNL and T-SNL groups (p > 0.05, ). The PWT values on the non-surgical side did not differ significantly among the three groups (p > 0.05, ). Compared with the non-operated side, there was no significant change in the PWT values on the operated side in the sham-operated group (p > 0.05); however, the PWT values were significantly decreased on the operated side in the N-SNL and T-SNL groups (p < 0.05, and ).
Figure 3 The mechanical pain threshold and cold allodynia threshold responses of the three groups of rats were evaluated. (A and B) Comparison of paw withdrawal threshold between the operative and non-operative sides in the three groups. (C and D) Comparison of the percentage of 6.0 g paw withdrawal between the operative and non-operative sides in the three groups. (E and F) Comparison of cold paw withdrawal latency between the operative and non-operative sides in the three groups. *p < 0.05 vs contralateral to the injury of the same group; #p <0.05 vs ipsilateral to the injury of Sham group.
Percentage of Paw Withdrawal After 6.0 g Mechanical Stimulation
On the operated side, the percentages of postoperative paw withdrawal in the N-SNL and T-SNL groups were significantly increased than in the Sham group (p < 0.05), with no significant differences between the N-SNL and T-SNL groups (p > 0.05, ). Differences in non-surgical side ratios among the three groups were not significant (p > 0.05, ). However, the percentages were significantly increased on the operated side in the N-SNL and T-SNL groups, compared with the non-operated side (p < 0.05, and ).
Cold Allodynia Threshold
On the operated side, Cold allodynia thresholds in the N-SNL and T-SNL groups were significantly decreased than in the Sham group (p < 0.05), with no significant differences between the N-SNL and T-SNL groups (p > 0.05, ). Cold allodynia threshold on the non-surgical side did not differ significantly among the three groups (p > 0.05, ). Compared with the non-operated side, there were no significant change in cold allodynia threshold on the operated side in the sham-operated group (p > 0.05); however, cold allodynia thresholds were significantly decreased on the operated side in the N-SNL and T-SNL (p < 0.05, and ).
The Number of Microglia in L5 Spinal Dorsal Horns
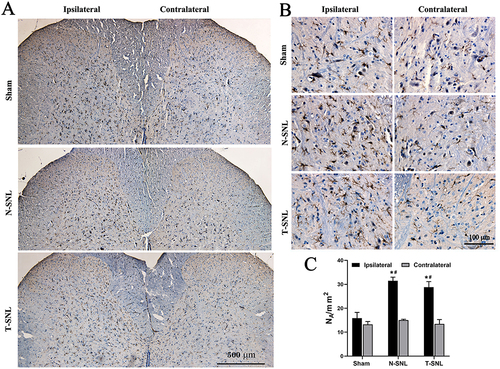
By immunohistochemical staining ( and ), the number of microglia increased by 98% on the operated side in the N-SNL group and 82% in the T-SNL group compared to the Sham group (p < 0.05). The difference in microglial counts between the N-SNL and T-SNL groups was not statistically significant (p > 0.05). Similarly, no significant differences were observed between the groups on the non-surgical side (p > 0.05). Compared with the nonsurgical side, the number of microglia on the surgical side in the Sham group increased by up to 20%, but the difference was not statistically significant (p > 0.05), whereas the number of microglia on the surgical side in the N-SNL and T-SNL groups increased by 109% and 114%, respectively (p < 0.05, ).
Figure 4 Changes in microglia numbers in the spinal dorsal horn. Immunostaining was conducted using the microglial marker Iba-1 antibody in the entire spinal dorsal horn ((A) magnification ×100, scale bar: 500 μm) and a portion of the dorsal horn ((B) magnification ×400, scale bar: 100 μm). (C) Bar graph of positive number changes of Iba-1 in the spinal dorsal horn among the three groups. *p < 0.05 vs contralateral to the injury of the same group; #p <0.05 vs ipsilateral to the injury of Sham group.
Postoperative Serum IL-1β and TNF-α Levels
The IL-1β and TNF-α levels in the N-SNL and T-SNL groups were significantly increased than in the Sham group (p < 0.05). However, there were no significant differences between the N-SNL and T-SNL groups (p > 0.05, and ).
Figure 5 Changes in the protein expression of IL-1β and TNF-α in the left L5 spinal cord and the C-reactive protein in the serum. (A) Changes in IL-1β protein expression. (B) Changes in TNF-α protein expression. *p < 0.05 vs ipsilateral to the injury of Sham group. (C) Changes in C-reactive protein expression. #p < 0.05 Postoperative Day 3 vs Postoperative Day 1. +p < 0.05 Postoperative Day 5 vs Postoperative Day 3.
Postoperative Serum CRP Levels
Serum CRP levels did not show substantial differences among the three groups of rats on postoperative days 1, 3, and 5 (p > 0.05). However, on postoperative day 5, serum CRP levels in the three groups of rats were significantly decreased than on postoperative days 1 and 3 (p < 0.05, ).
Discussion
The treatment of neuropathic pain is a common clinical challenge. In this study, we established a new minimally invasive SNL model by oblique lateral approach followed by titanium clip ligation. Our Results showed that both the new oblique lateral approach and the traditional posterior approach SNL model induced pain-related behavioral changes in rats, such as the production of spontaneous pain, the decrease of mechanical pain threshold and cold allodynia threshold; and the increase in the number of microglia in the spinal dorsal horn and the expression of inflammatory factors in the spinal cord, which suggests that the construction of the rat model of N-SNL-induced NP was successful. Meanwhile, we demonstrated that the new minimally invasive SNL model has the advantages of shortening surgical time, reducing intraoperative bleeding and decreasing postoperative animal mortality. Therefore, based on the above results, we believe that we have successfully constructed a minimally invasive SNL model that is easier to operate and has less local damage, which can be used for NP mechanism research and drug screening.
The traditional SNL model, developed by Kim et al in 1992, has been valuable in studying neuropathic pain (NP) formation and development.Citation4,Citation20 However, its posterior approach involves complex operations and causes significant tissue damage, limiting its widespread application.Citation5,Citation6 In this study, the researchers explored an oblique lateral approach, commonly used in clinical lumbar fusion.Citation11–13 The oblique lumbar interbody fusion avoids the richly innervated paraspinous region, avoids damaging spinal paraspinal muscles, interspinous ligaments, small joints, better restoration of intervertebral height compared with the traditional posterior lumbar interbody, thereby reducing surgical trauma and accelerating postoperative recovery.Citation21–23 The maximal incision is only 2.5 cm, reducing vascular and nerve injury. To address concerns about additional tissue damage and inconsistent ligation strength associated with silk ligation, titanium clips were used as a novel ligation material. Titanium is biocompatible and less likely to elicit adverse tissue reactions, making it suitable for biomedical applications. The use of titanium clips simplifies the ligation procedure and allows for adjustable clamping sites.Citation24,Citation25 In summary, our results suggest that the oblique approach followed by titanium clamp ligation can effectively reduce surgical complexity and tissue damage, and significantly reduce operative time, intraoperative blood loss, and the number of death in rats. It is worth emphasizing that the oblique lateral approach can also be used to construct other NP models, such as the chronic compression dorsal root ganglion (CCD) model.Citation26 Compared with the posterior approach, the oblique lateral approach is more favorable for exposing the intervertebral foramen and inserting the L-shaped steel rod into the intervertebral foramen to compress the dorsal root ganglion.
Spontaneous pain, hyperpathia and allodynia were the primary features of neuropathic pain (NP), with pain-related behavioral tests serving as indirect evidence for assessing pain severity and progression.Citation27,Citation28 Studies have found that rats in NP models such as (CCI), SNL and spared nerve injury (SNI) show significant spontaneous pain behaviors such as spontaneous foot licking and lifting as well as significant decreases in the thresholds of mechanical pain and cold allodynia.Citation6,Citation29,Citation30 In this study, these changes in spontaneous pain behaviors as well as decreased mechanical and cold pain sensitivity thresholds in the hind foot of the ligated side of the rats were also observed in the new SNL rat model, and there were no significant differences in the results when compared with those of the T-SNL model. These results indicate the successful establishment of a new SNL model in rats. They also suggest that the core part of SNL models is exposure and ligation of spinal nerves, and that tissue damage during surgical access is not the main cause of NP.
Furthermore, we assessed changes in the number of microglia in the spinal dorsal horn, the expression of IL-1β and TNF-α inflammatory factors in the spinal cord, and the level of CRP expression in the serum of the three groups of rats after surgery. Microglia, intrinsic immune cells of the central nervous system, are activated in the spinal dorsal horn following peripheral nerve injury.Citation14,Citation31 Activated microglia can release a variety of injurious inflammatory mediators such as IL-1β and TNF-α. IL-1β and TNF-α are pro-inflammatory cytokines that play an important role in NP regulation, and the amounts of both IL-1β and TNF-α in the spinal cord were significantly increased in peripheral NP models such as SNL, CCI and Spinal nerve transection (SNT).Citation15,Citation16,Citation32 These two proinflammatory factors may be involved in NP formation through different mechanisms, such as IL-1β possibly by enhancing NMDA receptor expression in spinal dorsal horn neurons, and TNF-α possibly by activating the intracellular p38 MAPK signaling pathway; or through similar mechanisms such as facilitating further release of other inflammatory mediators.Citation33 Our results showed a significant increase in the number of microglia and expression of IL-1β and TNF-α pro-inflammatory factors in the spinal cord of rats in both the N-SNL and T-SNL groups on the operated side, with no statistically significant differences between the two groups. This indicates that spinal nerve ligation-induced local inflammatory responses significantly contribute to NP. Systemic CRP levels are an important criterion for determining whether a systemic inflammatory response has occurred.Citation34 Our results suggested that systemic inflammatory responses may not be involved in NP development. There are also some shortcomings in this study: i. Because the traditional SNL model can induce depression, and we did not perform depression-related behavioral tests such as the water maze et al; ii. Our study only lasted until postoperative day 14, and the extent of ectopic pain in the new model over a longer modeling time and the associated inflammatory mechanisms still need to be further investigated.
Since their development, animal models have undergone improvements to ensure alignment with clinical symptoms. For instance, to improve CCI models, various materials such as silicone tubing, PE tubing, and silk thread have been utilized, alongside adjustments in ligature strength, such as ligature 4 passes and ligature 3 passes.Citation35 To mitigate significant muscular injury induced by SNL model creation, paravertebral muscle retraction has been employed to laterally retract the muscles.Citation9,Citation10 Different models also elicit varying levels of NP, thereby opening up new avenues for research into NP-related biological pathways. The selection of appropriate animal models based on research objectives, coupled with ongoing exploration of simpler and more user-friendly animal models, is imperative for investigating NP pathophysiology and identifying novel treatment targets.
Conclusion
In Conclusion, we have introduced a novel rat model of spinal nerve ligation constructed through an oblique lateral approach following by titanium clip ligation. This new SNL model effectively induced significant pain-related behaviors and spinal cord inflammatory responses, while also demonstrating short operative times, minimal intraoperative bleeding, and low postoperative animal mortality. We anticipate that this model can be used to study the mechanisms underlying the pathological processes associated with the development of neuropathic pain. The study also has clinical implications as the model can be used as a drug screening tool to facilitate the development of therapeutic measures for NP.
Ethics Approval
All experimental procedures were approved by the ethic committee of North Sichuan Medical College and followed the ethical guidelines for the investigation of experimental pain in conscious animals. The studies were conducted in accordance with the local legislation and institutional requirements (License No. NSMC-202209).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Weiting Qin, Yidan Kang, Chengfei Yu, Zhaoyang Li, Jian He, Jiao Meng, Jiebin Dong, Jiayang Wang, Cunyue Gong, and Weilin Jiang (North Sichuan Medical College undergraduates), the Institute of Medical Imaging, and the Innovation platform of Basic Medical School for their assist in the lab and with animal care. We are very grateful to Professor Zhengwei Yang for guiding the revision of the paper. The authors would like to thank MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Additional information
Funding
References
- Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi:10.1016/j.pain.2010.07.031
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. doi:10.1152/physrev.00045.2019
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Ho Kim S, Mo Chung J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi:10.1016/0304-3959(92)90041-9
- Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med. 2004;99:35–45. doi:10.1385/1-59259-770-X:035
- Abboud C, Duveau A, Bouali-Benazzouz R, et al. Animal models of pain: diversity and benefits. J Neurosci Methods. 2021;348:108997. doi:10.1016/j.jneumeth.2020.108997
- Challa SR. Surgical animal models of neuropathic pain: pros and Cons. Int J Neurosci. 2015;125(3):170–174. doi:10.3109/00207454.2014.922559
- Huang XL, Tan C, Lin JY, et al. Stereological study on the dynamic changes in the number of glial cells in the spinal dorsal horn of rats with spinal nerve ligation. Chin J Neuroanat. 2016;32(05):629–634. doi:10.16557/j.cnki.1000-7547.2016.05.0014
- Huang YG, Zhang Q, Wu H, Zhang CQ. A Comparison of Surgical Invasions for Spinal Nerve Ligation with or without Paraspinal Muscle Removal in a Rat Neuropathic Pain Model. Biomed Res Int. 2016;2016:6741295. doi:10.1155/2016/6741295
- Koh WU, Choi SS, Lee JH, et al. Perineural pretreatment of bee venom attenuated the development of allodynia in the spinal nerve ligation injured neuropathic pain model; an experimental study. BMC Complement Altern Med. 2014;14(1):431. doi:10.1186/1472-6882-14-431
- Li R, Li X, Zhou H, Jiang W. Development and application of oblique lumbar interbody fusion. Orthop Surg. 2020;12(2):355–365. doi:10.1111/os.12625
- Quillo-Olvera J, Lin GX, Jo HJ, Kim JS. Complications on minimally invasive oblique lumbar interbody fusion at L2-L5 levels: a review of the literature and surgical strategies. Ann Translat Med. 2018;6(6):101. doi:10.21037/atm.2018.01.22
- Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6(2):89–97. doi:10.4184/asj.2012.6.2.89
- Karavis MY, Siafaka I, Vadalouca A, Georgoudis G. Role of microglia in neuropathic pain. Cureus. 2023;15(8):e43555. doi:10.7759/cureus.43555
- Zhao L, Tao X, Wang Q, Yu X, Dong D. Diosmetin alleviates neuropathic pain by regulating the Keap1/Nrf2/NF-κB signaling pathway. Biomed Pharmacother. 2024;170:116067. doi:10.1016/j.biopha.2023.116067
- Ji A, Xu J. Neuropathic pain: biomolecular intervention and imaging via targeting microglia activation. Biomolecules. 2021;11(9):1343. doi:10.3390/biom11091343
- Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1–2):210–226. doi:10.1016/j.pharmthera.2005.07.001
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
- Lin JY, Zhu N, He YN, Xu BL, Peng B. Stereological study on the numerical plasticity of myelinated fibers and oligodendrocytes in the rat spinal cord with painful diabetic neuropathy. Neuroreport. 2020;31(4):319–324. doi:10.1097/WNR.0000000000001407
- Martin PY, Doly S, Hamieh AM, et al. mTOR activation by constitutively active serotonin6 receptors as new paradigm in neuropathic pain and its treatment. Prog Neurobiol. 2020;193:101846. doi:10.1016/j.pneurobio.2020.101846
- Li X, Chen X, Wang Y, Diwan AD, Lu S. Early outcomes of oblique lateral interbody fusion with posterior fixation versus posterior interbody fusion with fixation for treating adult degenerative scoliosis. J Orthop Surg Res. 2023;18(1):873. doi:10.1186/s13018-023-04363-7
- An B, Ren B, Han Z, Mao K, Liu J. Comparison between oblique lumbar interbody fusion and posterior lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18(1):856. doi:10.1186/s13018-023-04312-4
- Wu M, Li J, Zhang M, et al. Efficacy and radiographic analysis of oblique lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Orthop Surg Res. 2019;14(1):399. doi:10.1186/s13018-019-1416-2
- Kaur M, Singh K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater Sci Eng C Mater Biol Appl. 2019;102:844–862. doi:10.1016/j.msec.2019.04.064
- Mantsopoulos K, Thimsen V, Taha L, et al. Comparative analysis of titanium clip prostheses for partial ossiculoplasty. Am J Otolaryngol. 2021;42(5):103062. doi:10.1016/j.amjoto.2021.103062
- Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77(1):15–23. doi:10.1016/S0304-3959(98)00067-0
- Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. doi:10.3389/fnmol.2017.00284
- Seto T, Suzuki H, Okazaki T, et al. Three-dimensional analysis of the characteristics of joint motion and gait pattern in a rodent model following spinal nerve ligation. Biomed Eng Online. 2021;20(1):55. doi:10.1186/s12938-021-00892-6
- Sun J, Zhou YQ, Xu BY, et al. STING/NF-κB/IL-6-mediated inflammation in microglia contributes to spared nerve injury (SNI)-induced pain initiation. J Neuroimmune Pharmacol. 2022;17(3–4):453–469. doi:10.1007/s11481-021-10031-6
- Gosnell ME, Staikopoulos V, Anwer AG, et al. Autofluorescent imprint of chronic constriction nerve injury identified by deep learning. Neurobiol Dis. 2021;160:105528. doi:10.1016/j.nbd.2021.105528
- Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol. 2023;19(4):199–220. doi:10.1038/s41582-023-00777-3
- Yi MH, Liu YU, Liu K, et al. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav Immun. 2021;92:78–89. doi:10.1016/j.bbi.2020.11.030
- Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7(1):27. doi:10.1186/1742-2094-7-27
- Xiong HY, Zhang ZJ, Wang XQ. Bibliometric analysis of research on the comorbidity of pain and inflammation. Pain Res Manag. 2021;2021:6655211. doi:10.1155/2021/6655211
- Wang C, Chen P, Lin DF, Chen Y, Wu ZB, Lin XD. Effects of different materials for partial sciatic nerve ligation on glial cell activation in rat models of chronic constriction injury. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(08):1207–1212. doi:10.12122/j.issn.1673-4254.2020.08.20