Abstract
Purpose
This study compared the efficacy, tolerability, and safety of rimegepant 75 mg oral tablet – a small molecule calcitonin-gene receptor peptide (CGRP) receptor antagonist – with placebo in the acute treatment of migraine.
Methods
This double-blind, randomized, placebo-controlled trial enrolled adults aged ≥18 years with at least a 1-year history of migraine. Participants randomized to rimegepant 75 mg oral tablet or placebo treated a single migraine attack of moderate or severe pain intensity. The coprimary endpoints, pain freedom and freedom from the most bothersome symptom ([MBS] nausea, photophobia, or phonophobia) at 2 hours postdose, were evaluated using Mantel–Haenszel risk estimation.
Results
Of the 1485 participants enrolled, 1162 (78.2%) were randomized to rimegepant (n = 582) or placebo (n = 580). Most participants (85.5%) were female; the population had a mean (SD) age of 41.6 (12.2) years and a history of 4.7 (1.8) migraine attacks per month. At 2 hours postdose, rimegepant-treated participants had higher pain freedom rates (19.2% [104/543] vs 14.2% [77/541]; risk difference 4.9; 95% confidence interval [CI] 0.5 to 9.3; P=0.0298) and MBS freedom rates (36.6% [199/543] vs 27.7% [150/541]; risk difference 8.9; 95% CI 3.4 to 14.4; P=0.0016) than placebo-treated participants. Rimegepant-treated participants also had higher rates of pain relief (56.0% [304/543] vs 45.7% [247/541]; risk difference 10.3; 95% CI 4.4 to 16.2, P=0.0006) at 2 hours postdose. The most common adverse events were nausea (0.9% [5/546] vs 1.1% [6/549]) and dizziness (0.7% [4/546] vs 0.4% [2/549]). No signal of drug-induced liver injury due to rimegepant was identified.
Conclusion
Rimegepant 75 mg oral tablet was effective in the acute treatment of migraine. Tolerability and safety were similar to placebo, with no evidence of hepatotoxicity.
Trial Registration
Clinicaltrials.gov Identifier: NCT03235479.
Plain Language Summary
Researchers wanted to know if rimegepant 75 mg is effective and safe for the acute treatment of migraine. They gave half the participants rimegepant and half placebo and waited 2 hours. Then, they measured the percentages of participants whose headache and most bothersome migraine symptom besides pain (nausea, sensitivity to light or sound) were gone. They also measured side effects to make sure rimegepant is safe.
The study included 1084 adults with migraine, 927 (86%) of whom were women. Two hours after taking the medicine: pain freedom was 19% with rimegepant and 14% with placebo. Freedom from the most bothersome symptom was 37% with rimegepant and 28% with placebo. Pain relief rates, defined as the transition from moderate or severe pain to pain that was mild or absent, occurred in 56% with rimegepant and 46% with placebo. The most common side effects were nausea and dizziness, which affected fewer than 1% of rimegepant patients. Rimegepant 75 mg was more effective than placebo for migraine, with similar tolerability and safety.
Introduction
Migraine is a chronic neurologic disorder characterized by episodic attacks of headache, nausea and/or vomiting, and sensitivity to light and/or sound.Citation1 Migraine affects more than 1 billion people worldwide, with risk highest among working-age women.Citation2 As the leading cause of disability in people younger than age 50,Citation3 migraine is increasingly recognized as an important public health problem that places a substantial burden on individuals and families and presents an ongoing challenge to medical professionals, employers, and health systems.
In the acute treatment of adults with migraine, the utility of the most widely prescribed guidelines- and consensus-recommended treatments, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and serotonin 5-HT1B/1D receptor agonists (triptans),Citation4–7 is limited by insufficient response, cardiovascular and gastrointestinal safety issues, and the risk of medication-overuse headache.Citation8–11 These limitations, well-known sources of dissatisfaction with acute treatment,Citation12–15 have created a longstanding need for alternatives. Acute treatments that target the calcitonin gene-related peptide (CGRP) receptor have emerged as an important approach to addressing these needs.Citation7
Rimegepant is an orally administered small-molecule CGRP receptor antagonist indicated for the acute treatment of migraine and the preventive treatment of episodic migraine.Citation16 This study was part of the Phase 3 development program that demonstrated the efficacy and safety of rimegepant 75 mg for the acute treatment of migraine.Citation17–20 Its rationale was the observation that rimegepant 75 mg was more effective than placebo on the 2-hour pain freedom endpoint (31.4% vs 15.3%, P=0.002), with good safety and tolerability in a Phase 2b dose-ranging trial.Citation21 Based on those results, it was hypothesized that a single dose of rimegepant 75 mg oral tablet would be more effective than placebo in the acute treatment of migraine, and that rimegepant and placebo would have similar safety and tolerability profiles. The objective of this study was to compare the efficacy, tolerability, and safety of rimegepant 75 mg tablet with placebo in the acute treatment of migraine.
Methods
Ethics
This study was conducted in accordance with the principles of the Guidelines for Good Clinical Practice, the Declaration of Helsinki, and all applicable local regulations. The protocol was approved by independent ethics committees and/or institutional review boards at each study center. Participants provided written informed consent when they were screened. The study was prospectively registered at clinicaltrials.gov (Identifier: NCT03235479).
Design
This was a double-blind, randomized, multicenter, outpatient Phase 3 evaluation of the safety and efficacy of a single 75 mg dose of rimegepant versus placebo in the acute treatment of moderate-to-severe migraine. The study lasted approximately 11 weeks, including a screening period of 3 to 28 days, an acute treatment phase of up to 45 days, and an end-of-treatment visit 7 days after the administration of the study medication.
All screened participants were entered into an interactive web response system. After undergoing all screening procedures, participants returned to study centers within 3 to 28 days of signing informed consent. They were randomized to blinded study treatment and given an electronic diary (eDiary). Study personnel instructed participants on the proper use of the eDiary and ensured proper understanding and use of the tool before participants left the office.
Participants were given a single dose of study medication – rimegepant 75 mg oral tablet or matching placebo – and instructed to treat a migraine attack of moderate or severe pain intensity with study medication immediately after answering eDiary questions about their current pain and symptoms and identifying their currently most bothersome symptom (MBS) from among the migraine-associated symptoms of phonophobia, photophobia, and nausea. Pain intensity level, the presence or absence of migraine-associated symptoms, and functional disability level were assessed immediately before dosing and at 15, 30, 45, 60, and 90 minutes and at 2, 3, 4, 6, 8, 24, and 48 hours postdose. Pain intensity level was assessed on a 4-point rating scale (none, mild, moderate, or severe), and functional disability level was also assessed on a 4-point rating scale (normal, mildly impaired, severely impaired, or requires bedrest). Participants were instructed to not take other acute migraine medication before taking study medication and to not take rescue medication at or before 2 hours postdose. Participants also completed questionnaires about migraine-specific quality-of-lifeCitation22 and preference of medication 24 hours postdose.
Participants who treated a migraine attack of moderate or severe pain intensity within 45 days of randomization were instructed to return to the study site within 7 days (plus 2 days if necessary) of the treated migraine attack for review of the eDiary, assessment of medication compliance, and monitoring of tolerability and safety. Participants who had not experienced a migraine attack of moderate or severe pain intensity within 45 days of randomization were terminated and instructed to return unused study medication and the eDiary to the study center.
Participants
The trial population included males and females aged 18 years and older who had migraine with or without aura according to the criteria of the International Classification of Headache Disorders, 3rd Edition, beta version;Citation23 a 1-year history of attacks lasting about 4 to 72 hours if untreated, with the age of onset prior to 50 years of age; at least 2 and not more than 8 migraine attacks of moderate or severe intensity per month, as well as fewer than 15 days with migraine or nonmigraine headache within the last 3 months. Participants had to be able to distinguish migraine attacks from attacks of tension-type and cluster headache, and those taking preventive migraine medication had to be on a stable dose for at least 3 months before study entry. If they met all other criteria for inclusion, participants with contraindications to triptans could be included. Females of childbearing potential and nonsterile males could participate if they were using 2 acceptable methods of contraception to avoid pregnancy throughout the study; the 2 methods had to include 1 barrier method (eg, condom with spermicidal gel, intrauterine devices, cervical cap, etc.) and 1 other method that could include oral contraceptives or another barrier method. Females also had to have a negative serum or urine pregnancy test (minimum sensitivity 25 IU/L or equivalent units of human chorionic gonadotropin) at baseline and could not be breastfeeding.
Participants were excluded if they had any medical condition that might interfere with study assessments of efficacy and safety or expose them to undue risk of a significant adverse event (AE). Participants were also excluded if they had been treated for or showed evidence of alcohol or drug abuse within the past 12 months; had a history of drug or other allergy that made them unsuitable for participation; or had electrocardiogram or laboratory test findings that raised safety or tolerability concerns. Complete criteria for exclusion from the study are available in the study protocol (https://classic.clinicaltrials.gov/ProvidedDocs/79/NCT03235479/Prot_000.pdf).
Randomization and Blinding
Participants were randomized in a 1:1 ratio via an interactive web-response system to rimegepant 75 mg oral tablet or placebo for treatment of a single migraine attack of moderate-to-severe pain intensity. Randomization was stratified by the use of preventive migraine medication at randomization (yes or no). All participants, investigators, and study personnel were blinded to treatment assignments.
Outcomes
The coprimary efficacy endpoints were pain freedom and freedom from the MBS associated with migraine (ie, phonophobia, photophobia, or nausea) at 2 hours postdose. Pain freedom was assessed using the percentage of evaluable participants who reported pain intensity of none at 2 hours postdose. Freedom from the MBS was assessed using the percentage of evaluable participants who reported MBS before dosing and the absence of the MBS at 2 hours postdose.
Secondary efficacy endpoints were specified in the protocol to be tested hierarchically in the following order: photophobia freedom at 2 hours postdose; phonophobia freedom at 2 hours postdose; pain relief at 2 hours postdose; nausea freedom at 2 hours postdose; rescue medication use within 24 hours postdose; sustained pain freedom and sustained pain relief from 2 to 24 hours postdose; sustained pain freedom and sustained pain relief from 2 to 48 hours postdose; pain relapse from 2 to 48 hours postdose; and ability to function normally at 2 hours postdose.
Freedom from photophobia, phonophobia, and nausea was assessed using the percentage of participants reporting the absence of the symptom at 2 hours postdose in the subset of participants who experienced the symptom at the time of dosing. Pain relief at 2 hours postdose was assessed using the percentage of participants reporting pain intensity of none or mild at 2 hours postdose. Rescue medication use within 24 hours postdose was assessed using the percentage of participants who took rescue medication within 24 hours of the administration of study medication. Sustained pain freedom from 2 to 24 hours and from 2 to 48 hours postdose was assessed using the percentage of participants reporting pain intensities of none at all time points in the time periods of interest. Sustained pain relief from 2 to 24 hours and from 2 to 48 hours postdose was assessed using the percentage of participants reporting pain intensities of none or mild at all time points in the time periods of interest. Pain relapse was assessed using the percentage of participants reporting pain intensity of mild, moderate, or severe at any time point after 2 hours through 48 hours postdose in the subset of participants with pain freedom at 2 hours postdose. Ability to function normally at 2 hours postdose was assessed using the percentage of participants reporting functional disability level of normal at 2 hours postdose.
Safety and tolerability assessments included AEs, serious AEs (SAEs), electrocardiography, vital signs, physical measurements, routine laboratory tests, the Sheehan Suicidality Tracking Scale,Citation24 preference of medication, and the Migraine-Specific Quality of Life Questionnaire.Citation22
Statistical Analysis
Sample Size
Sample size requirements were calculated based on the assumption that approximately 90% of the 600 participants randomized to each treatment group would treat a migraine attack in the allotted time period, resulting in approximately 550 participants per group. The results from a previous Phase 2b studyCitation21 suggested that 550 participants would provide more than 95% power to detect a difference between rimegepant and placebo on each of the coprimary efficacy endpoints, assuming a 2-sided alpha level of 0.05, pain freedom at 2 hours postdose response rates of 29.7% and 15.2%, respectively, for rimegepant 75 mg and placebo, and MBS freedom at 2 hours postdose response rates of 34.6% and 19.6%, respectively, for rimegepant 75 mg and placebo. Having at least 95% power on each coprimary endpoint provides at least 90% power to detect a difference on both endpoints jointly. The protocol was amended before database lock and unblinding to increase the sample size from 425 to 600 participants randomized to each treatment group, and to revise the hierarchical testing order of the secondary endpoints.
Efficacy
Efficacy analyses were performed using the analysis set of modified intent-to-treat (mITT) participants, consisting of randomized participants who were treated with study medication, had a migraine attack of moderate or severe pain intensity at the time of dosing, and provided at least 1 postdose efficacy measurement. Rimegepant was tested for superiority to placebo at the 2-sided alpha level of 0.05 on pain freedom and MBS freedom at 2 hours postdose. Because these were coprimary endpoints, and significance on both endpoints was required for success, no alpha-level adjustment was required. Both endpoints were evaluated using Mantel–Haenszel risk estimation with stratification by the use of preventive migraine medication at randomization (yes or no). Secondary efficacy endpoints were assessed using the same method.
To control the type I statistical error rate at 0.05, a hierarchical gate-keeping procedure was employed.Citation25 First, the coprimary endpoints were tested at a 2-sided alpha level of 0.05. If the tests for both coprimary endpoints were significant, then the secondary endpoints were tested sequentially, each at a 2-sided alpha level of 0.05, in the order specified in the protocol (see Outcomes section). Thus, a secondary efficacy endpoint was tested only if the preceding secondary endpoint in the hierarchy was determined to be significant (ie, p-value ≤0.05). If a test in the hierarchy was not significant, then any further tests on secondary endpoints in the sequence had nominal p-values presented only for descriptive purposes. Results are presented in the sequence in which they were evaluated.
For coprimary and secondary endpoints evaluated at 2 hours postdose, participants with missing data at 2 hours postdose were imputed as failures. For the secondary endpoints of sustained pain relief, sustained pain freedom, and pain relapse, participants with missing pain intensity at 2, 24, or 48 hours postdose, or more than 1 time point from 2 to 48 hours postdose were imputed as failures. For all endpoints except the secondary endpoint of rescue medication use within 24 hours postdose, participants who used rescue medication at or before the time point(s) of interest defining the endpoint were considered failures.
Safety
Safety analyses were performed using the analysis set of enrolled participants who received at least 1 dose of study medication. Safety assessment included evaluation of AEs, laboratory values, vital signs, and electrocardiography. AEs were coded using the Medical Dictionary for Regulatory Activities, version 20.0, and on-study AEs were defined as those with onset date on or after the randomization date.
Results
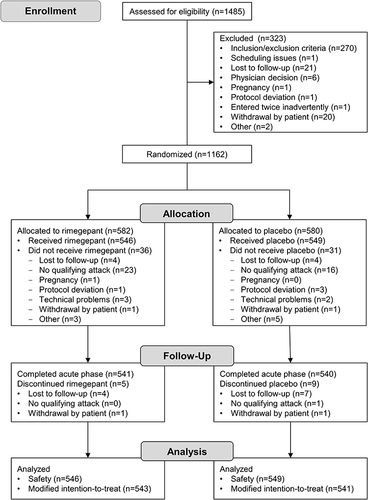
Participants
Fifty study centers in the United States participated. The duration of the study was 193 days; the first participant began the study on 18 July 2017, and the last participant completed the study on 26 January 2018. In total, 1162 participants were randomized to rimegepant (n = 582) or placebo (n = 580), 1095 were treated with rimegepant (n = 546) or placebo (n = 549), and 1084 were evaluated for efficacy (rimegepant n = 543, placebo n = 541) (). Baseline demographic and clinical characteristics were consistent between the rimegepant and placebo groups (). Most participants (85.5%) were female, and the population reported a mean (standard deviation [SD]) age of 41.6 (12.2) years, a history of 4.7 (1.8) attacks per month, and an average duration of untreated attacks of 29.8 (21.7) hours. Historically, the MBS was photophobia for 55.7% (604/1084) of participants, nausea for 26.8% (290/1084) of participants, and phonophobia for 17.5% (190/1084) of participants. The MBS immediately before dosing was photophobia for 54.2% (588/1084) participants, nausea for 29.6% (321/1084) participants, and phonophobia for 13.1% (142/1084) participants.
Table 1 Demographics and Baseline Characteristics for mITT Participants
Efficacy
As shown in , rimegepant was superior to placebo for the coprimary efficacy endpoints of pain freedom at 2 hours postdose (19.2% [104/543] vs 14.2% [77/541]; risk difference 4.9; 95% confidence interval [CI] 0.5 to 9.3; P=0.0298) and MBS freedom at 2 hours postdose (36.6% [199/543] vs 27.7% [150/541]; risk difference 8.9; 95% CI 3.4 to 14.4; P=0.0016).
Figure 2 Forest plot of coprimary and secondary efficacy endpoints for mITT participants.
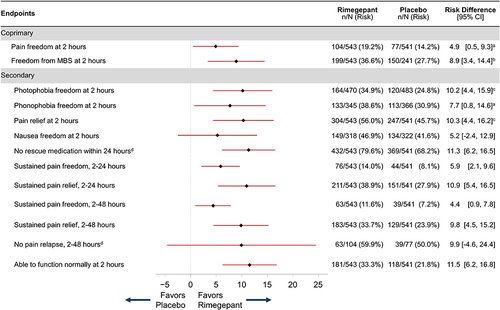
Rimegepant was also superior to placebo at 2 hours postdose for freedom from photophobia (34.9% [164/470] vs 24.8% [120/483], P=0.0005) and phonophobia (38.6% [133/345] vs 30.9% [113/366], P=0.0299), as shown in . At 2 hours postdose, participants who received rimegepant had higher rates of pain relief than participants who received placebo (56.0% [304/543] vs 45.7% [247/541], P=0.0006). Because nausea freedom at 2 hours postdose did not significantly differ between treatment groups (rimegepant 46.9% [149/318] vs placebo 41.6% [134/322], P=0.1815), no formal testing was conducted on the remaining secondary efficacy endpoints in the testing hierarchy ().
Safety
A total of 12.6% (69/546) and 10.7% (59/549) of participants in the rimegepant and placebo groups, respectively, experienced at least 1 AE (). The most common AEs, occurring in ≥0.5% of treated participants in either treatment group, are presented in . Nausea (0.9% [5/546] vs 1.1% [6/549]) was the only AE that occurred in at least 1% of treated participants in either treatment group.
Table 2 Adverse Events and Liver Function Tests in Treated Participants
Table 3 Adverse Eventsa Occurring in ≥0.5% of Treated Participants in Either Treatment Group
Serum alanine transaminase or aspartate transaminase levels greater than the upper limit of normal (ULN) were seen in 2.0% (11/546) of participants treated with rimegepant and 3.6% (20/549) of participants treated with placebo. One participant (0.2%) in each treatment group had a transaminase level greater than 3x ULN, and no participant in either group had a level greater than 5x ULN. No bilirubin elevations greater than 2x ULN were observed. Serious AEs on study were observed in 0.4% (2/546) of participants in the rimegepant group and 0.2% (1/549) in the placebo group (), but they were considered unrelated to study treatment. Both participants with SAEs in the rimegepant group had not been dosed before onset of the SAEs.
Discussion
This study was conducted to assess the efficacy, tolerability, and safety of a single dose of rimegepant 75 mg oral tablet in the acute treatment of adults with migraine. Results showed that rimegepant 75 mg oral tablet was significantly more effective than placebo on the coprimary endpoints of pain freedom and MBS freedom at 2 hours postdose and the hierarchically tested secondary endpoints of freedom from photophobia and phonophobia as well as pain relief, all 2 hours postdose. Safety and tolerability with rimegepant were excellent, and liver function testing showed no evidence of hepatotoxicity.
The current results are consistent with prior reports on rimegepant for the acute treatment of migraine.Citation17–19,Citation21 In a Phase 2b dose-ranging study, rimegepant 75 mg tablet was more effective than placebo at 2 hours postdose for freedom from pain, nausea, photophobia, and phonophobia, and it remained significantly superior to placebo on these endpoints at 24 hours postdose.Citation21 At the time of the Phase 2b study, MBS had not yet emerged as an important endpoint, and it was not assessed. Subsequently, 3 already-published Phase 3 studies found that rimegepant 75 mg was more effective than placebo on the coprimary endpoints of freedom from pain and the MBS, as well as on multiple secondary endpoints.Citation17–19 One of these studies was conducted using the tablet formulationCitation17 and 2 were conducted using the now marketed orally disintegrating tablet (ODT) formulation. The 2 formulations are bioequivalent,Citation26 making these results relevant to the marketed ODT product.
In the present study, a single 75 mg rimegepant oral tablet provided favorable results on a range of clinically important endpoints from 2 hours postdose through 48 hours postdose, with a very low rate of AEs – qualities patients value in medications used for acute treatment.Citation27 These results are also comparable to findings with other trials with orally administered CGRP receptor antagonists for the acute treatment of migraineCitation18,Citation28,Citation29 and suggest that rimegepant and other gepants may join lasmiditan as new therapeutic options for the acute treatment of migraine.
The tolerability and safety of rimegepant were comparable to placebo. The only AE reported by more than 1% of participants in either treatment group was nausea, and assessment of the 2 rimegepant-treated participants who experienced SAEs found that the events occurred prior to study treatment. Liver function test elevations for rimegepant and placebo were comparable; no evidence of hepatoxicity was observed. Because it is common for patients with migraine to require some form of medication for acute treatment over a period of decades, often with the use of multiple concomitant medications, the finding that rimegepant showed no evidence of hepatotoxicity is reassuring and corroborates the large body of evidence that supports the safety of rimegepant.Citation17–20,Citation30 While additional studies are needed to confirm the long-term safety and tolerability of rimegepant, in clinical practice the absence of cardiovascular effects and the excellent liver safety record, coupled with robust efficacy, indicate that rimegepant may represent an important new option for the acute treatment of migraine. Awareness and educational initiatives targeting patients and medical professionals may help to expand access to the full range of medications for the treatment of individuals with migraine.
Despite the large cohort, the use of standardized diagnostic criteria for migraine, and utilization of efficacy endpoints recommended by the International Headache Society,Citation31 this study has some limitations. Because all agents in the CGRP class were investigational at the time of the study, participants who had recently received an anti-CGRP antibody were excluded. Due to the single-attack study design, no information about consistency of response across multiple attacks or the long-term safety of rimegepant could be collected. In addition, treatment during mild pain was not assessed.
Conclusions
A single 75 mg dose of rimegepant oral tablet was effective in the acute treatment of migraine. The tolerability and safety of rimegepant were comparable to placebo, and there was no signal of hepatotoxicity.
Abbreviations
AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; CGRP, calcitonin gene-related peptide; CI, confidence interval; eDiary, electronic diary; mITT, modified intent-to-treat; MBS, most bothersome symptom; NSAID, nonsteroidal anti-inflammatory drug; ODT, orally disintegrating tablet; SAE, serious adverse event; SD, standard deviation; ULN, upper limit of normal.
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of the Guidelines for Good Clinical Practice, the Declaration of Helsinki, and all applicable local regulations. The protocol was approved by Schulman Associates Institutional Review Board, Inc. (Cincinnati, OH, USA). Participants provided written informed consent when they were screened.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Richard B. Lipton serves on the editorial board of Neurology and as senior advisor to Headache but is not paid for his roles on these journals. He has received research support from the NIH. He also receives support from the National Headache Foundation. He receives research grants from Allergan/AbbVie, Amgen, Dr. Reddy’s Laboratories, and Novartis. He has reviewed for the NIA and NINDS and serves as consultant, advisory board member, or has received honoraria from Allergan/AbbVie, Amgen, Avanir, Axsome, Biohaven, Biovision, BDSI, Boston Scientific, Cool Tech, Cult Health, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, Genentech, Grifols, GlaxoSmithKline, LinPharma, Lundbeck, Manistee, Merck, Novartis, Pfizer, Satsuma, Teva, and Vedanta. He receives royalties from Wolff’s Headache (8th Edition, Oxford Press University, 2009) and Informa. He holds stock in Biohaven Pharmaceuticals and holds options in Axon, CoolTech, and Manistee. Robert Croop was an employee of Biohaven Pharmaceuticals, owns stock in Biohaven Ltd, was an employee of Pfizer, has received research payments from Pfizer, and provides services to Collima LLC which has had consulting agreements with Pfizer, Actio Biosciences, Inc., Aptose Biosciences Inc., Biohaven Pharmaceuticals, Inc., Manistee Therapeutics, and Vida Ventures Management Co., L.L.C. In addition, Dr Robert Croop reports a patent regarding CGRP antagonists, originally assigned to Biohaven Pharmaceuticals and now owned by Pfizer Inc. Alexandra Thiry is employed by Pfizer and owns stock/stock options in Biohaven Pharmaceuticals and Pfizer. Beth A Morris is employed by and owns stock/stock options in Biohaven Pharmaceuticals. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors are grateful to the study participants and site staff. Medical writing services were provided by Christopher Caiazza at Polymedia Corporation and funded by Pfizer.
Data Sharing Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Additional information
Funding
References
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496–505. doi:10.1111/head.13281
- Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17. doi:10.1186/s10194-018-0846-2
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754–762. doi:10.1212/WNL.55.6.754
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20. doi:10.1111/head.12499
- National Institute for Health and Care Excellence: Guidelines. Headaches in Over 12s: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2021.
- Ailani J, Burch RC, Robbins MS. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi:10.1111/head.14153
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60(6):1259–1287. doi:10.2165/00003495-200060060-00003
- Bigal ME, Rapoport AM, Sheftell FD, Tepper SJ, Lipton RB. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes. Cephalalgia. 2004;24(6):483–490. doi:10.1111/j.1468-2982.2004.00691.x
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database Syst Rev. 2012;2(2):CD008615–CD. doi:10.1002/14651858.CD008615.pub2
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–1675. doi:10.1016/s0140-6736(01)06711-3
- Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. 2007;47(4):475–479. doi:10.1111/j.1526-4610.2007.00752.x
- Malik SN, Hopkins M, Young WB, Silberstein SD. Acute migraine treatment: patterns of use and satisfaction in a clinical population. Headache. 2006;46(5):773–780. doi:10.1111/j.1526-4610.2006.00437.x
- Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache. 2002;42(Suppl 1):3–9. doi:10.1046/j.1526-4610.2002.0420s1003.x
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache. 1999;39(s2):S20–S6. doi:10.1111/j.1526-4610.1999.00006.x
- Chiang CC, Schwedt TJ. Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine - The monoclonal antibodies and gepants. Prog Brain Res. 2020;255:143–170. doi:10.1016/bs.pbr.2020.06.019
- Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142–149. doi:10.1056/NEJMoa1811090
- Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–745. doi:10.1016/s0140-6736(19)31606-x
- Yu S, Kim BK, Guo A, et al. Safety and efficacy of rimegepant orally disintegrating tablet for the acute treatment of migraine in China and South Korea: a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2023;22(6):476–484. doi:10.1016/s1474-4422(23)00126-6
- Croop R, Berman G, Kudrow D, et al. A multicenter, open-label long-term safety study of rimegepant for the acute treatment of migraine. Cephalalgia. 2024;44(4):03331024241232944.
- Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34(2):114–125. doi:10.1177/0333102413500727
- Bagley CL, Rendas-Baum R, Maglinte GA, et al. Validating Migraine-Specific Quality of Life Questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52(3):409–421. doi:10.1111/j.1526-4610.2011.01997.x
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
- Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan suicidality tracking scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry. 2009;6(1):26–31.
- Dmitrienko A, Tamhane AC. Gatekeeping procedures with clinical trial applications. Pharm Stat. 2007;6(3):171–180. doi:10.1002/pst.291
- Croop R, Bhardwaj R, Anderson M, et al. Bioequivalence of rimegepant, a small molecule CGRP receptor antagonist, administered as an oral tablet, a sublingual orally disintegrating tablet, and a supralingual orally disintegrating tablet: two Phase 1 randomized studies in healthy adults. Cephalalgia. 2024;44(2):03331024231219505.
- Xu X, Ji Q, Shen M. Patient preferences and values in decision making for migraines: a systematic literature review. Pain Res Manag. 2021;2021:9919773. doi:10.1155/2021/9919773
- Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372(9656):2115–2123. doi:10.1016/s0140-6736(08)61626-8
- Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230–2241. doi:10.1056/NEJMoa1813049
- Johnston K, Popoff E, Deighton A, et al. Comparative efficacy and safety of rimegepant, ubrogepant, and lasmiditan for acute treatment of migraine: a network meta-analysis. Expert Rev Pharmacoecon Outcomes Res. 2022;22(1):155–166. doi:10.1080/14737167.2021.1945444
- Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39(6):687–710. doi:10.1177/0333102419828967