Abstract
Purpose
Percutaneous transforaminal endoscopic discectomy (PTED) was used as a minimally invasive treatment option for lumbar disc herniation (LDH). However, studies focusing on the clinical outcomes of PTED for elderly patients with adjacent segment disease (ASD) were limited. This study aims to compare the clinical outcomes of PTED between ASD and LDH in elderly patients.
Patients and Methods
This retrospective study enrolled 39 patients with ASD and 39 patients with LDH. Both groups had undergone PTED in Beijing Chaoyang Hospital from July 4, 2016 to July 30, 2021. Visual analog scale for back pain (VAS-BP) and leg pain (VAS-LP) and Oswestry disability index (ODI) were used to value the clinical outcomes of patients preoperatively, immediately postoperatively, 12, and 24 months postoperatively, and at final follow-up. Patients’ satisfaction was evaluated based on the MacNab criteria.
Results
All operations were completed. The excellent or good clinical outcomes at final follow-up was demonstrated by 87.15% (34/39) and 89.74% (35/39) in ASD and non-ASD patients, respectively. Clinical improvement was observed immediately postoperatively in both groups and sustained stability during the postoperative follow-up. The ASD group demonstrated significantly longer hospital stays (p = 0.02) and operative time (p < 0.01) than the non-ASD group.
Conclusion
PTED is an effective and minimally invasive treatment option for revision surgery of ASD, especially for elderly patients. However, the long-term prognosis of PTED treating ASD still needs further exploration.
Introduction
Posterior lumbar decompression and interbody fusion with instrumentation has been the standard treatment for degenerative lumbar diseases.Citation1 This surgery markedly offers immediate segment stability and notable clinical outcomes. However, adjacent segment degeneration (ASD) is not a rare complication because spinal fusion surgery has been more widely performed. The incidence rate of asymptomatic ASD was reported to range from 5.2% to 18.5%.Citation2 Revision surgery might have been a priority strategy after the failed conservative treatment.
Open revision surgery, in terms of surgical management of patients with ASD, has been the main therapy method so far, including extended decompression and further lengthening of the fusion segment.Citation3 However, open revision surgery may bring patients a second painful experience and substantial trauma to the muscle tissue.Citation4,Citation5 Furthermore, the majority of patients with ASD are elderly people with comorbidities, such as cardiopulmonary disorders and diabetes, which may cause a high incidence of complications related to open revision surgery.Citation6,Citation7 In recent years, percutaneous transforaminal endoscopic discectomy (PTED) was used as a minimally invasive treatment option for ASD because of the advantages of local anesthesia, avoidance of repetitive damage to surrounding muscle tissue, and fast recovery.Citation8,Citation9
Previous studies have been focusing on the clinical outcomes of PTED for elderly patients,Citation10 but studies concerning patients with ASD were limited. Therefore, this study aims to explore the efficacy and safety of PTED in elderly patients with ASD. Patients with LDH were enrolled for comparison and further illustration.
Material and Methods
Patient Population
This study consecutively enrolled 78 patients July 4, 2016 to July 30, 2021 in Beijing Chaoyang Hospital, Capital Medical University. All study participants were considered suitable for PTED based on current indications. All patients agreed to participate in this study after explaining its detailed aims and scope. The hospital’s institutional review board and ethics committee approved this study. Furthermore, all aspects of this study conformed to the principles outlined in the Helsinki Declaration.
Inclusion criteria of ASD include 1) previous posterior fusion surgery for lumbar degenerative disease; 2) low-back pain or radicular pain in the unilateral lower extremities; 3) ASDs confirmed by radiologic images; 4) symptoms that cannot be relieved after 6 months of strict conservative treatment. Inclusion criteria of LDH include 1) positive symptoms of nerve root compression; 2) positive clinical examination findings for sensory or motor neurological deficits; 3) clear visualization of lumbar disc herniation on preoperative computed tomography and magnetic resonance imaging; 4) persistence of symptoms or recurrent episodes after strict conservative treatment for at least 6 months.
Exclusion criteria include 1) disc sequestration, segmental instability, vertebral slippage, or other abnormalities; 2) history of surgery at the affected segment or recurrent disc herniation at the same level; 3) spinal tumors, infections, or vertebral fractures; 4) patients with psychiatric disorders that affect accurate assessment.
Surgical Procedures
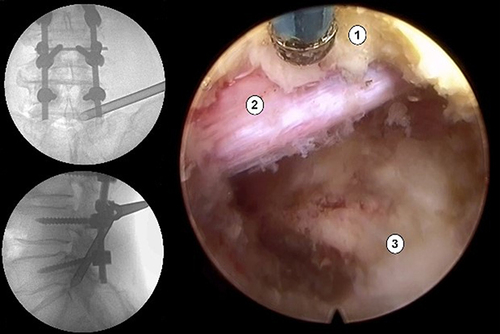
All operations were performed using PTED under local anesthesia and prone position. Surgical segments and needle placements were guided under anteroposterior and lateral C-arm fluoroscopy. The entry point was established at a lateral distance of 12–14 cm from the spinal midline, targeting the intervertebral level of intent. A puncture needle was then inserted into the superior articular process (SAP) of the designated segment under the guidance of C-arm fluoroscopic after infiltration of the entry point with 3–5 mL of lidocaine. Serial cannula and dilator were percutaneously introduced into the SAP under the guidance of the puncture needle. Subsequently, a trepan was used to excise the ligamentum flavum and ventral elements of the SAP. Burrs were employed to further expand the foramen when necessary. The endoscope was inserted, with the working channel and irrigation channels placed eccentrically, after placing the working cannula. Removal of herniated disc material was performed under direct vision. The traversing nerve root and dural sac were exposed with adequate mobility and good pulse to ensure complete decompression. Appropriate irrigation and hemostasis management were used to minimize the risk of postoperative infection and hematoma formation.
Clinical Evaluation
This study recorded the demographic and perioperative parameters. The clinical outcome was evaluated by the visual analog scale (VAS) and Oswestry disability index (ODI) scores preoperatively, immediately postoperatively, 12 and 24 months postoperatively, and at the final follow-up. Patients’ satisfaction was evaluated via MacNab criteria at the final follow-up.
Statistical Analysis
Statistical Package for the Social Sciences (version 27.00, Chicago, Illinois, USA) and MedCalc (version 22.016) were used for data analyses. Continuous variables were expressed as mean ± standard deviation and analyzed by t-tests if assuming a normal distribution or Yuen-Welch tests if not, in which the trimming was set as 10%. Statistical analysis for categorical variables was performed using the chi-square test. A p-value of <0.05 indicated statistical significance.
Results
Demographic Characteristics
This study ultimately enrolled 78 patients who were categorized into the ASD and the non-ASD groups (LDH group). 3 patients in the ASD group and 2 patients in the non-ASD group lost follow-up, and the follow-up rates of the two groups were respectively 92.86% and 95.12%. Of the participants, 56.41% were women (n = 44). The average age was 68.49 ± 9.74 years old, ranging from 38 to 87 years. shows similar demographic features between the two groups. Significant statistical differences were observed in operation (p < 0.001) and hospitalization times (p = 0.02). Level of significance was determined at p-value=0.05.
Table 1 Demographic Characteristics of Enrolled Patients
Clinical Outcomes
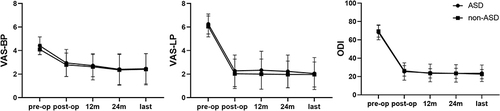
presents significantly improved postoperative symptoms in both groups (p < 0.001). shows the details of other information on postoperative parameters. The follow-up time was at least 2 years (range: 25–74 months). The VAS-back pain (VAS-BP) scores of the ASD and non-ASD groups significantly improved from 4.41 ± 0.75 and 4.1 ± 0.45 preoperatively to 2.95 ± 1.15 and 2.79 ± 1.00 immediately postoperatively, respectively. So did the VAS-leg pain (VAS-LP) scores of the ASD and non-ASD group, respectively from 6.26 ± 0.85 and 6.05 ± 0.89 preoperatively to 2.28 ± 1.34 and 2.03 ± 1.25 immediately postoperatively. The ODI scores in both groups also demonstrated significant improvement from preoperative scores of 68.23 ± 8.11 (ASD) and 68.38 ± 6.36 (non-ASD) to immediate postoperative scores of 25.46 ± 9.46 (ASD) and 26.21 ± 5.76 (non-ASD). These improvements remained stable during the subsequent follow-up period. Preoperative VAS-BP scores indicated statistically significant differences in the severity of lumbar pain between the two groups (p < 0.05). However, all valued scores demonstrated no statistical differences between the two groups during the postoperative follow-up time. The excellent and good rate was 87.15% in the ASD group and 89.74% in the non-ASD group, with no significant statistical difference.
Table 2 Comparison of Scores Between Two Groups
Figure 1 Postoperative scores of VAS-BP, VAS-LP and ODI significantly improved in both groups. p-value<0.001 in comparison between preoperative and all postoperative valued scores.
Complications and Typical Case
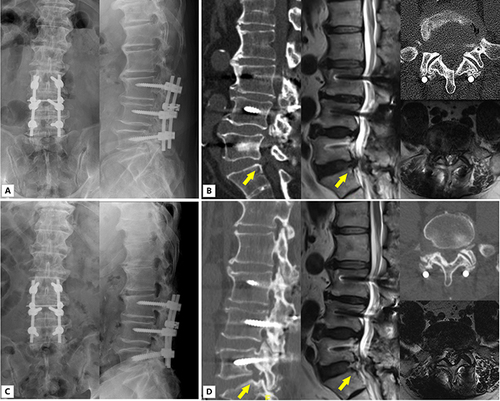
The ASD group showed two cases of postoperative complications. One case involved an intraoperative dural tear but showed a favorable recovery following postoperative treatment. Another case exhibited radiating pain 2 days postoperatively, indicating recurrent disc herniation. Symptoms improved after subsequent PTED. The non-ASD group showed one patient who suffered a postoperative complication, characterized by 4-grade strength and residual pain in the right iliopsoas muscle. Neuraxial steroids demonstrated satisfactory results. Typical case is shown in , .
Figure 2 A 71-year-old man with ASD accepted PTED for L5-S1 disc herniation. (A) Preoperative anterior and lateral X-rays showing the L3-L5 having undergone PLIF surgery previously (B) Preoperative CT and MRI showing herniation of the intervertebral disc in the right of adjacent segment L5-S1 (yellow arrow) (C) Postoperative anterior and lateral X-rays showing the L5-S1 having undergone PTED (D) Postoperative CT and MRI showing the herniated intervertebral disc in the right side of L5-S1 having been removed (yellow arrow).
Discussion
Several studies have supported the efficacy and reliability of lumbar fusion, but considering the fusion-related complications is crucial.Citation11 ASD is the symptomatic deterioration of spinal levels neighboring the fused vertebrae.Citation12 Compared to the natural history, fusion surgery accelerates degenerative changes in adjacent segments,Citation13 with an increasing occurrence rate with the extended follow-up period.Citation14,Citation15 Hence, the need for treatment grows more urgent with the accumulation of patients undergoing lumbar fusion surgery. Surgical intervention becomes a viable option once conservative treatment is ineffective. Traditional approaches extended internal fixation to the adjacent vertebral level. However, PTED has been a recently attractive potential alternative, considering the comparatively little trauma, fast recovery, and low complication incidence.
Our study included 39 patients with ASD accepting follow-up for at least 2 years. Most treatments resulted in satisfactory therapeutic outcomes. Postoperative VAS-BP, VAS-LP, and ODI scores, which remained stable during the later follow-up period, demonstrated significant improvement in comparison with preoperative scores. Of the patients with ASD, 87.15% (34/39) showed excellent and good clinical outcomes at final follow-up, which is akin to the rate of 90.63% (29/32) in the study of Feng and others.Citation16 All measured indexes exhibited statistically significant improvements in all follow-up sections compared to preoperative scores. Previous research has explored the application of variable treatment for ASD following lumbar fusion.Citation17 The comparison conformed to a study conducted by Li and others, which compared the outcomes of PELD and posterior lumbar interbody fusion in managing disc herniation of adjacent segments.Citation18 The study concluded that both procedures showed similar clinical outcomes, but PELD demonstrated advantages in terms of reduced trauma, decreased blood loss, and faster recovery. These findings indicate that PTED, as well as minimally invasive, significantly improves symptoms and reduces functional impairment in patients with ASD.
In comparison to the non-ASD group, the ASD group achieved similar clinical improvement after PTED. Cho and others indicated that postoperative lumbar pain symptoms may be more common postoperatively in patients with ASD,Citation19 but we found no significant difference between the two groups throughout the subsequent follow-up period. Multiple surgical techniques reporting the efficiency of PTED on spinal degeneration diseases demonstrated analogous outcomes. Gadjradj and others considered PTED as an effective alternative to open microdiscectomy in treating sciatica.Citation20 Li and others revealed PTED as an effective supplement in elderly patients with degenerative lumbar scoliosis combined with spinal stenosis, with excellent rates of 90.0%.Citation21 Additionally, Cheng and others explored the application of PETD on central spinal stenosis and degenerative lumbar spondylolisthesis and revealed a satisfactory outcome at a good-to-excellent rate of 93.3%.Citation22 However, our groups demonstrated differences. First, we found higher preoperative VAS-BP scores in the ASD group than in the non-ASD group, which is consistent with the results of Kapetanakis and others.Citation23 One possible reason is a previous intervertebral fusion internal fixation.Citation24 Further, we observed significant statistical disparities between the two groups in operation and hospitalization times, which can be attributed to the complex nature of conditions prevalent among patients with ASD, including previous fusion approach, resultant mechanical alterations, and enduring scars. Different distribution of affected segments may also play a role, considering factors, such as puncture angle and anatomical characteristics.Citation25,Citation26 Thus, extensive expertise and experience should be necessary for perioperative preparation. Benefits to patients with ASD could still be observed through PTED in our study despite these complexities.
Elderly patients, often with multiple comorbidities, make up a vastly considerable proportion of the ASD group. Our current investigation revealed that the mean age of patients undergoing revision surgery was 70.2 years. Other research reported the mean age of patients with ASD as 57.2, 66.2, and 64.8 years, respectively.Citation23,Citation27,Citation28 This indicates a more cautious approach when selecting surgical strategies. Revision surgeries present significant challenges, particularly for the elderly in the context of previous fusion procedures, for prior internal fixation disrupting the natural anatomy of paraspinal muscles, spinal bone structures, and ligaments.Citation24,Citation29 Moreover, the overlap of surgical incisions with the postoperative scar tissue of the initial procedure makes open revision surgery difficult. However, PTED accesses the intervertebral foramen via a reduced incision, eliminating the need to disturb pre-existing scar tissue when dealing with recurrent disc herniation.Citation30 Small surgical incision also reduces the amount of intraoperative blood loss and shorten recovery time. Additionally, PTED merely decompresses the adjacent segment nerve without internal fixation and fusion. This simplifies the surgical procedure, thereby allowing PTED as a tolerable option for the elderly requiring spinal revision surgery.
Complications pose a significant concern in ASD treatment. The incidence of complications in our ASD group was 5.13% (2/39), which was comparable to the non-ASD group and notably lower than what has been reported in traditional lumbar fusion surgery.Citation31,Citation32 Importantly, our study revealed no severe complications in patients, such as cardiopulmonary dysfunction, thrombosis, nerve root injuries, major bleeding, or postoperative infections. We also observed no recurrence during the whole follow-up (25–74 months). Performing fusion surgery under general anesthesia exposes the elderly to an increased risk of serious cardiopulmonary complications.Citation33 However, PTED offers the advantage of using local anesthesia, making it a safer option for the elderly. Additionally, PTED allows patients to remain awake during the procedure, providing real-time feedback on sensory and motor functions, thereby helping the surgeon avoid potential nerve root damage and ensure sufficient decompression. Moreover, recent studies indicated that patients undergoing open revision spinal fusion surgery have a higher risk of surgical site infection (SSI) than those who underwent primary fusion surgery.Citation34 However, PTED has the advantages of a low SSI rate due to the small incision and working environment. Previous studies have explored the recurrence following multiple fusion revision techniques, respectively revealing that 5.1% and 11.1% of patients required further surgery due to recurring ASD.Citation17,Citation35 Clinical data indicates that post-revision spinal fusions cause a greater segmental rigidity, which in turn triggers compensatory motion in adjacent segments, eventually causing additional ASD and fixation-related complications such as screw loosening or rod fractures.Citation36,Citation37 These results indicate that additional fusion surgeries may offer only temporary relief and fail to effectively prevent recurring ASD. In terms of the aforementioned problem, PTED retains a larger portion of the facet joint structure, preserving the motion and minimizing the occurrence of subsequent adjacent segment diseases postoperatively.Citation38,Citation39
PTED shows promise in addressing ASD after lumbar fusion, but it does have limitations. Not all patients with ASD after lumbar fusion are suitable candidates for PTED, because cases of lumbar instability may be better suited for open surgery. Furthermore, the current study has limitations, such as a small sample size and potential selection bias due to its retrospective nature. Currently, large-scale, long-term follow-up data to facilitate the sustained effectiveness of PTED in treating ASD are lacking.
Conclusion
PTED, as a minimally invasive surgical technique, has shown promising efficacy and safety in treating ASD after lumbar fusion surgery especially for elderly patients. However, postoperative symptoms may worsen due to progressive degeneration and further intervention may subsequently be needed.
Ethics Statement
This study was approved by the ethics committee of Beijing Chaoyang Hospital, Capital Medical University (Registration number: 2021-KE-478) and the research was performed in accordance with the guidelines of the Declaration of Helsinki. Informed consent for this study was obtained from all patients by both written and verbal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Fenton-White HA. Trailblazing: the historical development of the posterior lumbar interbody fusion (PLIF). Spine J. 2021;21(9):1528–1541. doi:10.1016/j.spinee.2021.03.016
- Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29(17):1938–1944. doi:10.1097/01.brs.0000137069.88904.03
- Aiki H, Ohwada O, Kobayashi H, et al. Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci. 2005;10(5):490–495. doi:10.1007/s00776-005-0919-3
- Lee JC, Kim Y, Soh JW, Shin BJ. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine (Phila Pa. 2014;39(5):E339–45. doi:10.1097/brs.0000000000000164
- Yamamoto S, Malakoutian M, Theret M, et al. The effect of posterior lumbar spinal surgery on biomechanical properties of rat paraspinal muscles 13 weeks after surgery. Spine. 2021;46(21):E1125–e1135. doi:10.1097/brs.0000000000004036
- Wang N, Xie Y, Liu X, et al. Safety and clinical efficacy of endoscopic procedures for the treatment of adjacent segmental disease after lumbar fusion: a systematic review and meta-analysis. PLoS One. 2023;18(2):e0280135. doi:10.1371/journal.pone.0280135
- Kuan V, Denaxas S, Patalay P, et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit Health. 2023;5(1):e16–e27. doi:10.1016/s2589-7500(22)00187-x
- Gibson JNA, Subramanian AS, Scott CEH. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J Mar. 2017;26(3):847–856. doi:10.1007/s00586-016-4885-6
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa. 2008;33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Zhu Y, Zhang X, Gu G, et al. Clinical outcomes of percutaneous transforaminal endoscopic discectomy assisted with selective nerve root block for treating radicular pain with diagnostic uncertainty in the elderly. J Pain Res. 2024;17:753–759. doi:10.2147/JPR.S402033
- Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;2016(1):Cd010264. doi:10.1002/14651858.CD010264.pub2
- Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339–1349. doi:10.1016/j.spinee.2013.03.020
- Ekman P, Möller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18(8):1175–1186. doi:10.1007/s00586-009-0947-3
- Sears WR, Sergides IG, Kazemi N, Smith M, White GJ, Osburg B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11(1):11–20. doi:10.1016/j.spinee.2010.09.026
- Okuda S, Nagamoto Y, Matsumoto T, Sugiura T, Takahashi Y, Iwasaki M. Adjacent segment disease after single segment posterior lumbar interbody fusion for degenerative spondylolisthesis: minimum 10 years follow-up. Spine. 2018;43(23):E1384–e1388. doi:10.1097/BRS.0000000000002710
- Feng P, Kong Q, Zhang B, Liu J, Ma J, Hu Y. Percutaneous full endoscopic lumbar discectomy for symptomatic adjacent segment disease after lumbar fusion in elderly patients. Orthop Surg. 2023;15(7):1749–1755. doi:10.1111/os.13725
- Miwa T, Sakaura H, Yamashita T, Suzuki S, Ohwada T. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level posterior lumbar interbody fusion. Eur Spine J. 2013;22(12):2864–2868. doi:10.1007/s00586-013-2863-9
- Li T, Zhu B, Liu X. Revision strategy of symptomatic lumbar adjacent segment degeneration: full endoscopic decompression versus extended posterior interbody fusion. World Neurosurg. 2020;142:e215–e222. doi:10.1016/j.wneu.2020.06.168
- Cho KS, Kang SG, Yoo DS, Huh PW, Kim DS, Lee SB. Risk factors and surgical treatment for symptomatic adjacent segment degeneration after lumbar spine fusion. J Korean Neurosurg Soc. 2009;46(5):425–430. doi:10.3340/jkns.2009.46.5.425
- Gadjradj PS, Rubinstein SM, Peul WC, et al. Full endoscopic versus open discectomy for sciatica: randomised controlled non-inferiority trial. BMJ. 2022;376:065846.
- Li P, Tong Y, Chen Y, Zhang Z, Song Y. Comparison of percutaneous transforaminal endoscopic decompression and short-segment fusion in the treatment of elderly degenerative lumbar scoliosis with spinal stenosis. BMC Musculoskelet Disord. 2021;22(1):906. doi:10.1186/s12891-021-04804-6
- Cheng XK, Chen B. Percutaneous transforaminal endoscopic decompression for geriatric patients with central spinal stenosis and degenerative lumbar spondylolisthesis: a novel surgical technique and clinical outcomes. Clin Interv Aging. 2020;15:1213–1219. doi:10.2147/CIA.S258702
- Kapetanakis S, Gkantsinikoudis N, Gkasdaris G, Charitoudis G. Treatment of adjacent segment disease with percutaneous transforaminal endoscopic discectomy: Early experience and results. J Orthop Surg (Hong Kong). Sep-Dec. 2020;28(3):2309499020960560.
- Anandjiwala J, Seo JY, Ha KY, Oh IS, Shin DC. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: A prospective cohort study with a minimum five-year follow-up. Eur Spine J. 2011;20(11):1951–1960. doi:10.1007/s00586-011-1917-0
- Chow DH, Luk KD, Evans JH, Leong JC. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996;21(5):549–555. doi:10.1097/00007632-199603010-00004
- Aono H, Takenaka S, Tobimatsu H, et al. Adjacent-segment disease after L3-4 posterior lumbar interbody fusion: does L3-4 fusion have cranial adjacent-segment degeneration similar to that after L4-5 fusion? J Neurosurg Spine. 2020;33:1–6.
- Telfeian AE. Transforaminal endoscopic surgery for adjacent segment disease after lumbar fusion. World Neurosurg. 2017;97:231–235. doi:10.1016/j.wneu.2016.09.099
- Iwai H, Oshima Y, Kitagawa T, et al. A less invasive treatment by a full-endoscopic spine surgery for adjacent segment disease after lumbar interbody fusion. J Spine Surg. 2020;6(2):472–482. doi:10.21037/jss.2019.08.04
- Yuan C, Wang J, Zhou Y, Pan Y. Endoscopic lumbar discectomy and minimally invasive lumbar interbody fusion: a contrastive review. Wideochir Inne Tech Maloinwazyjne. 2018;13(4):429–434. doi:10.5114/wiitm.2018.77744
- Pan M, Li Q, Li S, et al. Percutaneous Endoscopic Lumbar Discectomy: indications and Complications. Pain Physician. 2020;23(1):49–56.
- Aichmair A, Alimi M, Hughes AP, et al. Single-level lateral lumbar interbody fusion for the treatment of adjacent segment disease: A retrospective two-center study. Spine. 2017;42(9):E515–e522. doi:10.1097/BRS.0000000000001871
- Phillips FM, Carlson GD, Bohlman HH, Hughes SS. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord. 2000;13(5):432–437. doi:10.1097/00002517-200010000-00011
- Devereaux PJ, Sessler DI, Longo DL. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258–2269. doi:10.1056/NEJMra1502824
- Rajaee SS, Kanim LE, Bae HW. National trends in revision spinal fusion in the USA: patient characteristics and complications. Bone Joint J. 2014;96-B-b(6):807–816. doi:10.1302/0301-620X.96B6.31149
- Chen WJ, Lai PL, Niu CC, Chen LH, Fu TS, Wong CB. Surgical treatment of adjacent instability after lumbar spine fusion. Spine. 2001;26(22):E519–24. doi:10.1097/00007632-200111150-00024
- Lee CH, Hyun SJ, Kim KJ, Jahng TA, Yoon SH, Kim HJ. The efficacy of lumbar hybrid stabilization using the DIAM to delay adjacent segment degeneration: an intervention comparison study with a minimum 2-year follow-up. Neurosurgery. 2013;73(2):224–231. doi:10.1227/01.neu.0000430310.63702.3e
- Mageswaran P, Techy F, Colbrunn RW, Bonner TF, McLain RF. Hybrid dynamic stabilization: a biomechanical assessment of adjacent and supraadjacent levels of the lumbar spine. J Neurosurg Spine. 2012;17(3):232–242. doi:10.3171/2012.6.SPINE111054
- Pan A, Hai Y, Yang J, Zhou L, Chen X, Guo H. Adjacent segment degeneration after lumbar spinal fusion compared with motion-preservation procedures: a meta-analysis. Eur Spine J. 2016;25(5):1522–1532. doi:10.1007/s00586-016-4415-6
- Ren C, Song Y, Liu L, Xue Y. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol. 2014;24(1):S245–53. doi:10.1007/s00590-014-1445-9