Abstract
Purpose
To determine if an oral, tapered methylprednisolone regimen is superior to other commonly used pharmacologic interventions for the treatment of central post-stroke pain (CPSP).
Patients and methods
In this study, the charts of 146 stroke patients admitted to acute inpatient rehabilitation were retrospectively reviewed. Patients diagnosed with CPSP underwent further chart review to assess numerical rating scale for pain scores and as-needed pain medication usage at different time points comparing CPSP patients treated with methylprednisolone to those treated with other pharmacologic interventions.
Results
In the sample, 8.2% were diagnosed with CPSP during acute care or inpatient rehabilitation. Mean numerical rating scale for pain scores day of symptom onset did not differ between those patients treated with methylprednisolone versus those treated with other pharmacologic interventions (mean ± standard deviation; 6.1 ± 2.3 versus 5.7 ± 1.6, P = 0.77). However, mean numerical rating scale for pain scores differed significantly 1-day after treatment initiation (1.7 ± 2.1 versus 5.0 ± 1.9, P = 0.03) and 1-day prior to rehabilitation discharge (0.3 ± 0.9 versus 4.1 ± 3.2, P = 0.01) between the two groups. Compared to day of symptom onset, as-needed pain medication usage within the methylprednisolone group was marginally less 1-day after treatment initiation (Z = −1.73, P = 0.08) and 1-day prior to rehabilitation discharge (Z = −1.89, P = 0.06). No difference in as-needed pain medication usage existed within the non-steroid group at the same time points.
Conclusion
Methylprednisolone is a potential therapeutic option for CPSP. The findings herein warrant study in prospective trials.
Introduction
Central post-stroke pain (CPSP) can be defined as a central neuropathic pain condition occurring after stroke located in the body part(s) corresponding to a cerebrovascular lesion of the somatosensory system characterized by pain and sensory abnormalities where other causes of obvious nociceptive, psychogenic, or peripheral pain have been excluded.Citation1,Citation2 The prevalence and yearly incidence of CPSP has been reported as 7.3% and 8%, respectivelyCitation23 CPSP is considered challenging from a clinical management standpoint and existing treatment options do not result in optimal outcomes.Citation4 One author describes CPSP as an under-recognized complication of stroke despite its potential to impair activities of daily living, deteriorate quality of life, and undermine rehabilitation efforts, and states that CPSP has an overall immense and devastating burden on patients and societyCitation5 Post-stroke complex regional pain syndrome (CRPS) shares similar pain characteristics with CPSP, however, post-stroke CRPS also presents with extremity edema and dystrophic skin changes with temperature and color abnormalities.Citation6 The pathophysiology underlying CPSP and post-stroke CRPS is not understood; however, these two diagnoses share the possible pathophysiologic process of central sensitization resulting in hyperexcitability of central nociceptive neurons.Citation5,Citation6
Given that corticosteroids may regulate the levels of excitatory neuropeptides that contribute to central sensitization and neuronal hyperexcitability,Citation7 Braus et al performed a randomized, non-blinded, placebo-controlled trial assessing the efficacy of an oral, tapered methylprednisolone regimen in the treatment of post-stroke CRPS.Citation8 In this study, 91.2% of patients diagnosed with post-stroke CRPS became symptom free (in an average of 10 days after treatment initiation) and remained symptom free at 6-month follow-up after treatment with the steroid and physical therapy. Similarly, in a randomized, double-blind, active-placebo controlled trial, Kalita et al demonstrated significant improvement in 83.3% of post-stroke CRPS patients treated with an oral, tapered prednisolone regimen versus only 16.7% of patients treated with piroxicam.Citation9 Given the positive results from these two studies, the overlap in pathophysiology between CPSP and post-stroke CRPS, and the difficulties clinicians face in the treatment of CPSP, the retrospective study described herein was initiated in an attempt to assess the efficacy of methylprednisolone versus other pharmacologic interventions for the treatment of CPSP.
It was hypothesized that methylprednisolone would be superior to other pharmacologic interventions for the treatment of CPSP in the acute rehabilitation inpatient with regard to reduction in numerical rating scale for pain scores (NRS) and as-needed pain medication usage. We have no knowledge of any previously published studies that describe the treatment of CPSP with an oral steroid.
Material and methods
This study was approved by the Wayne State University Institutional Review Board in Detroit, Michigan. All patients admitted to an acute inpatient rehabilitation facility after acute stroke between January 7, 2010 and June 30, 2011 were studied retrospectively. Patients documented as being diagnosed with CPSP during acute care or acute inpatient rehabilitation underwent chart, vital signs, and medical administration record review in order to obtain demographic information (age, gender, race), stroke type, past medical history, symptom onset (days post-stroke), pharmacologic intervention(s), 11-point NRS on day of symptom onset, NRS 1-day after treatment initiation, subjective pain reports 1-day after treatment initiation, NRS 1-day prior to rehabilitation discharge, as-needed pain medication usage on day of symptom onset, as-needed pain medication usage 1-day after treatment initiation, and as-needed pain medication usage 1-day prior to rehabilitation discharge. Additionally, those patients documented as being diagnosed with CPSP during acute care or acute inpatient rehabilitation underwent chart review of the initial outpatient follow-up appointment after rehabilitation discharge in order to obtain data on subjective pain reports.
The primary outcome measure was mean NRS during inpatient care and the secondary outcome measure was mean as-needed pain medication usage during inpatient care. For each inpatient, the mean NRS was calculated from all pain scores documented in the electronic medical record from 6:00 am to 11:59 pm on the specified day (without regard to documentation of painful area). Similarly, for each patient, mean number of as-needed pain medications used was calculated from all documented as-needed pain medication administrations per the medication administration record from 6:00 am to 11:59 pm on the specified day (without regard to type of as-needed pain medication administered).
In addition to the aforementioned analysis of those patients diagnosed with CPSP during acute care and acute inpatient rehabilitation, the remainder of the study population also underwent additional investigations. Patients documented as being diagnosed with post-stroke CRPS during acute care, during acute inpatient rehabilitation, or at the initial outpatient follow-up appointment after rehabilitation discharge and patients documented as being diagnosed with CPSP at the initial outpatient follow-up appointment after rehabilitation discharge were also studied. These patients (combined with those patients diagnosed with CPSP during acute care or acute inpatient rehabilitation) were studied in order to obtain prevalence and symptom onset data for CPSP and post-stroke CRPS. These patients’ demographic information (age, gender, race), stroke type, and past medical history were also recorded.
Statistical methods
Independent t-tests were used for the between group comparisons for NRS at different time points. The Mann–Whitney U test was used for the between group comparisons for as-needed pain medication usage at different time points. Paired t-tests were used for the within group comparisons for NRS at different time points. The Wilcoxon signed rank test was used for the within group comparisons for as-needed pain medication usage at different time points. Criterion for declaring a statistically significant difference was P < 0.05. Criterion for marginal significance was P < 0.10.
Results
During the study period, 146 patients were admitted to an acute inpatient rehabilitation facility after acute stroke. For the entire sample, demographic data, stroke type, and stroke history is presented in . Additionally, for those patients diagnosed with CPSP and post-stroke CRPS during the study period, demographic data, stroke type, stroke history, prevalence, symptom onset (days), timing of symptom onset, and location of patient at diagnosis (inpatient versus outpatient), is presented in . Note that two patients diagnosed with CPSP as an inpatient were later diagnosed with post-stroke CRPS at outpatient follow-up after rehabilitation discharge.
Table 1 Descriptive data
Moving forward, we now focus solely on those patients diagnosed with CPSP during acute care or acute inpatient rehabilitation. Of the sample, 8.2% (N = 12) was diagnosed with CPSP during acute care or acute inpatient rehabilitation. Mean onset of symptoms was 12.3 ± 6.5 days (mean ± standard deviation) post-stroke. Patients presented to acute inpatient rehabilitation 8.8 ± 5.1 days after admission to acute care. Two of the twelve patients developed symptoms during their acute care course. Excluding these two patients, acute rehabilitation inpatients developed CPSP 5.0 ± 4.4 days after admission to rehabilitation.
After symptom onset, 7 of the 12 patients diagnosed with CPSP were treated with an oral, tapered methylprednisolone regimen (Medrol Dosepak®; Pfizer Inc., New York, NY, USA) while 5 of the 12 patients received other pharmacologic interventions (these included amitriptyline, fluvoxamine, gabapentin, pregabalin, and lamotrigine in varying combinations). Five of the seven patients who received methylprednisolone did not receive any other treatment for CPSP. The other two methylprednisolone patients received pregabalin and amitriptyline with lamotrigine, respectively. Moving forward those patients treated with the methylprednisolone taper will be termed the steroid group and those patients treated with other pharmacologic interventions will be termed the nonsteroid group. details the methylprednisolone taper used. Due to the short duration of the taper, no patients were on methylprednisolone when discharge NRS or discharge as-needed pain medication usage data was collected.
Table 2 Methylprednisolone taper details
Mean NRS day of symptom onset for the steroid group and non-steroid groups did not differ significantly (6.1 ± 2.3 versus 5.7 ± 1.6, t[9] = 0.30, P = 0.77). Mean NRS 1-day after treatment initiation for the steroid group was significantly lower than the mean NRS 1-day after treatment initiation for the non-steroid group (1.7 ± 2.1 versus 5.0 ± 1.9, t[9] = −2.55, P = 0.03). Mean NRS 1-day prior to rehabilitation discharge for the steroid group was also significantly lower than the non-steroid group (0.3 ± 0.9 versus 4.1 ± 3.2, t[9] = − 3.06, P = 0.01). provides a visual representation of these results. details the percent reduction in NRS for the steroid and non-steroid groups 1-day after treatment initiation and 1-day prior to rehabilitation discharge compared to day of symptom onset. Evaluation of subjective pain reports in the daily progress notes 1-day after treatment initiation revealed that five of seven (71.4%) patients in the steroid group reported no pain compared to none (0.0%) of the patients in the non-steroid group. As-needed pain medication usage one day after treatment initiation for the steroid and non-steroid groups did not differ significantly (U = 10.5, P = 0.27). The steroid group demonstrated marginally significantly lower as-needed pain medication usage 1-day prior to rehabilitation discharge compared to the non-steroid group (U = 6.0, P = 0.07).
Figure 1 NRS for pain at different time points.
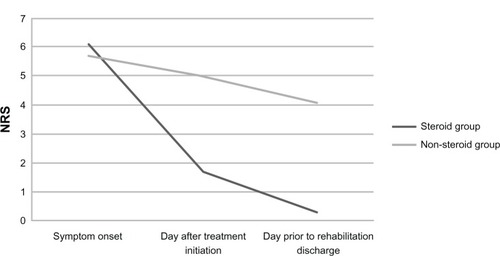
Table 3 Percentage of patients with different percent reductions in NRS at different time points for the steroid and non-steroid groups
For the steroid group, mean NRS day of symptom onset compared to 1-day after treatment initiation differed significantly (6.1 ± 2.3 versus 1.7 ± 2.1, t[6] = 4.98, P = 0.002) and mean NRS day of symptom onset compared to 1-day prior to rehabilitation discharge also differed significantly (6.1 ± 2.3 versus 0.3 ± 0.9, t[6] = 5.88, P = 0.001). For the non-steroid group, mean NRS day of symptom onset compared to 1-day after treatment initiation (5.7 ± 1.6 versus 5.0 ± 1.9, t[3] = 1.20, P = 0.32) and compared to 1-day prior to rehabilitation discharge (5.7 ± 1.6 versus 4.1 ± 3.2, t[3] = 1.85, P = 0.16) did not differ significantly For the steroid group, as-needed pain medication usage day of symptom onset compared to 1-day after treatment initiation (Z = −1.73, P = 0.08) and compared to 1-day prior to rehabilitation discharge (Z = −1.89, P = 0.06) were marginally significantly different. For the non-steroid group, as-needed pain medication usage day of symptom onset compared to 1-day after treatment initiation (Z = −1.13, P = 0.26) and compared to 1-day prior to rehabilitation discharge (Z = −1.07, P = 0.29) did not differ significantly.
Six of the 12 patients diagnosed with CPSP during acute care or acute inpatient rehabilitation followed-up as outpatients after discharge from acute inpatient rehabilitation. Of these six, three patients were in the steroid group and three patients were in the non-steroid group. Two of the three steroid group patients reported no pain at outpatient follow-up. The other steroid group patient’s pain was deemed musculoskeletal. All three patients in the non-steroid group reported pain at outpatient follow-up. One of the non-steroid group patient’s pain was deemed musculoskeletal. The other two non-steroid group patients were diagnosed with post-stroke CRPS at outpatient follow-up.
Discussion
The prevalence and yearly incidence of CPSP has been reported as 7.3% and 8%, respectivelyCitation2,Citation3 and the majority of patients are diagnosed with CPSP within 3 months of stroke with immediate and delayed onset (>1 year) being possible but atypical.Citation2,Citation3,Citation10–Citation12 Fitting with existing literature, the study described here reports a 9.6% prevalence of CPSP with 92.9% of CPSP patients developing symptoms 30 days or less post-stroke (). The occurrence and timing of post-stroke CRPS is less clearly defined. For example, McLean studied stroke rehabilitation inpatients admitted over a 1-year period and diagnosed post-stroke CRPS in only 1.5% of patients.Citation13 However, in this study, diagnosis required a positive bone scan which may have resulted in under-diagnosis. Similarly, in another study by Davis et al, 12.6% of ischemic stroke rehabilitation inpatients were diagnosed with CRPS with most patients developing signs and symptoms between the second and fourth months post-stroke.Citation14 Conversely, Kocabas et al reported a higher incidence of post-stroke CRPS (48.8%) in patients followed for 28 weeks with 70% of cases developing 6 weeks post-stroke or later.Citation15 Also, Gokkaya et al reported that 30.5% of stroke rehabilitation inpatients developed post-stroke CRPS.Citation16 However, it should be noted that these patients were admitted to rehabilitation 67.8 ± 38.9 days after stroke due to considerable patient load at the study site. In the study described here, we report 5.5% prevalence for post-stroke CRPS with 87.5% of patients developing symptoms 31 days or more post-stroke (). The variability in the reported occurrence of post-stroke CRPS is typically considered to be the result of differences in diagnostic criteria coupled with overlapping signs and symptoms in the stroke patient both with and without a CRPS diagnosis.Citation6 However, we feel the timing of patient assessment should also be considered as a contributor to this variability as well. For example, Kocabas et alCitation15 and Gokkaya et alCitation16 demonstrated higher percentages of post-stroke CRPS; however, they followed their patients longer and first assessed their patients later (respectively) than the studies that demonstrated lower percentages of post-stroke CRPS. Further, in the study described here, patients were diagnosed with post-stroke CRPS 55.9 ± 28.7 days after stroke and 87.5% were diagnosed as outpatients. These figures are in stark contrast to the CPSP patients who were diagnosed 20.9 ± 27.2 days after stroke with 85.7% diagnosed as inpatients (). As a result, it seems that post-stroke CRPS is a complication after stroke that may develop later than CPSP and studies who fail to follow patients for an adequate amount of time may underestimate the occurrence of post-stroke CRPS.
It has been suggested that that amitriptyline, lamotrigine, and pregabalin are all reasonable first-line treatment options for CPSP.Citation1,Citation4,Citation17,Citation18 The seminal works that helped to establish these assertions were performed by Leijon et al,Citation19 Vestergaard et al,Citation20 and Kim et al.Citation21 For amitriptyline, a randomized, blinded, crossover, placebo-controlled trial demonstrated that 75 mg daily of the tricyclic antidepressant yielded significantly lower mean daily pain ratings on a 10-step verbal scale compared to placebo (4.2 ± 1.6 versus 5.3 ± 2.0 by week 4 of the treatment period); and, perhaps more importantly, 67% of the amitriptyline patients reported improvement in pain on global assessment compared to only 7% of the placebo group.Citation19 For lamotrigine, a randomized, blinded, crossover, placebo-controlled trial demonstrated that a 200 mg daily dose of the anticonvulsant resulted in significantly lower median daily pain ratings on an 11-point Likert scale compared to placebo (5 versus 7) after 8 weeks of medication titration; however, only 44% of patients were deemed clinical responders (defined by a lamotrigine pain score ≥2 points lower than the corresponding placebo value).Citation20 For pregabalin, a randomized, blinded, parallel group, placebo-controlled trial assessed the efficacy of 150 mg to 600 mg per day of the anticonvulsant in 219 patients with CPSP.Citation21 Mean pain score on the Daily Pain Rating Scale decreased in both groups; however, there was no significant difference between the two groups at baseline or endpoint (6.5 to 4.9 in the pregabalin group; 6.3 to 5.0 in the placebo group). Additionally, the majority of patients treated with pregabalin did not achieve a 30% or 50% reduction in mean pain score compared to baseline. However, the pregabalin group did improve significantly over the placebo group in some of the secondary outcome measures including those regarding sleep and anxiety, and on the Clinician Global Impression of Change rating scale. In comparison to these studies, a short, oral, tapered methylprednisolone regimen appears to be far superior for the treatment of CPSP with regard to absolute reduction and rate of reduction in a pain scale score. Further, the percentage of methylprednisolone-treated patients with ≥30%, ≥50%, and 100% reductions in NRS at different time points is remarkable compared to: (1) the non-steroid patient treated patients in this study, (2) the aforementioned pregabalin study,Citation21 and (3) existing literature commenting on clinically meaningful percent reduction in pain scores.Citation22–Citation26 Additionally, the NRS scores 1-day prior to rehabilitation discharge and the subjective pain reports noted at outpatient follow-up suggests long-term benefit for CPSP patients treated with methylprednisolone. The authors are unaware of any existing literature investigating changes in as-needed pain medication usage in response to treatment in patients with CPSP. Here, within the steroid group (but not within the non-steroid group) at different time points, and between the steroid and non-steroid group at discharge, there was less as-needed pain medication usage noted.
Currently, CPSP and post-stroke CRPS are considered distinct diagnoses. Perhaps CPSP and post-stroke CRPS could be considered one diagnosis that exist on a continuum with CPSP representing a more limited and post-stroke CRPS representing a more florid manifestation of the same syndrome. Given that two patients initially diagnosed with CPSP were later diagnosed with post-stroke CRPS in this study may speak to this possibility. Relatedly, the differences in mean symptom onset and timing of symptom onset for CPSP and post-stroke CRPS described in this study () may not demonstrate different onset times for two different post-stroke complications. Instead, these differences may represent the time required for a patient to transition from the more limited to the overt presentation of the same diagnosis. The consideration that CRPS possesses a variety of phenotypes is not without precedent and is applicable to this discussion. Specifically, Bruehl et al postulated CRPS as existing in subtypes; namely, a limited neuropathic pain/sensory syndrome, a limited vasomotor syndrome, and a florid syndrome.Citation27 While their study did not focus on stroke exclusively, it is apparent that their limited neuropathic pain/sensory syndrome might exist on a spectrum that closely approximates CPSP. Given the overlap in proposed pathophysiology underlying these two diagnoses,Citation5,Citation6,Citation17,Citation28 considering that they may be one diagnosis existing on a continuum is not unreasonable. Additionally, since autonomic dysfunction post-stroke can occur in the absence of CRPSCitation29 this further blurs the line between CPSP and post-stroke CRPS.
Conclusion
This study introduces methylprednisolone as a potential therapeutic option for patients suffering from CPSP. The findings herein warrant study in large prospective clinical trials. Additionally, this study proposes that CPSP and post-stroke CRPS might exist on a continuum given the two diagnoses overlap in pathophysiology and treatment, and since two patients first diagnosed with CPSP later developed post-stroke CRPS. Importantly, it should be noted that both CPSP patients who later developed post-stroke CRPS were not treated with methylprednisolone. This, coupled with the abrupt improvement in NRS noted in the steroid group, might suggest that methylprednisolone has abortive properties in the treatment and prevention of progression of central pain phenomena after stroke.
Disclosure
The authors report no conflicts of interest in this work.
References
- KlitHFinnerupNBJensenTSCentral post-stroke pain: clinical characteristics, pathophysiology, and managementLancet Neurol20098985786819679277
- KlitHFinnerupNBAndersenGJensenTSCentral poststroke pain: a population-based studyPain2011152481882421272999
- AndersenGVestergaardKIngeman-NielsenMJensenTSIncidence of central post-stroke painPain19956121871937659428
- HarveyRLCentral poststroke pain syndromeTop Stroke Rehabil201017316317220797959
- KumarGSoniCRCentral post-stroke pain: current evidenceJ Neurol Sci20092841–2101719419737
- PertoldiSDi BenedettoPShoulder-hand syndrome after stroke. A complex regional pain syndromeEura Medicophys200541428329216474282
- SmithGDSecklJRShewardWJEffect of adrenalectomy and dexamethasone on neuropeptide content of dorsal root ganglia in the ratBrain Res1991564127301723340
- BrausDFKraussJKStrobelJThe shoulder-hand syndrome after stroke: a prospective clinical trialAnn Neurol19943657287337526774
- KalitaJVajpayeeAMisraUKComparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: a randomized controlled trialQJM2006992899516428335
- LeijonGBoivieJJohanssonICentral post-stroke pain – neurological symptoms and pain characteristicsPain198936113252919091
- NasreddineZSSaverJLPain after thalamic stroke: right diencephalic predominance and clinical features in 180 patientsNeurology1997485119611999153442
- BowsherDCentral pain: clinical and physiological characteristicsJ Neurol Neurosurg Psychiatry199661162698676164
- McLeanDEMedical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitationArch Phys Med Rehabil200485346646915031834
- DavisSWPetrilloCREichbergRDChuDSShoulder-hand syndrome in a hemiplegic population: a 5-year retrospective studyArch Phys Med Rehabil197758835335669426
- KocabasHLevendogluFOzerbilOMYurutenBComplex regional pain syndrome in stroke patientsInt J Rehabil Res2007301333817293718
- GokkayaNKArasMYesiltepeEKoseogluFReflex sympathetic dystrophy in hemiplegiaInt J Rehabil Res200629427527917106342
- KumarBKalitaJKumarGMisraUKCentral poststroke pain: a review of pathophysiology and treatmentAnesth Analg200910851645165719372350
- FreseAHusstedtIWRingelsteinEBEversSPharmacologic treatment of central post-stroke painClin J Pain200622325226016514325
- LeijonGBoivieJCentral post-stroke pain – a controlled trial of amitriptyline and carbamazepinePain198936127362465530
- VestergaardKAndersenGGottrupHKristensenBTJensenTSLamotrigine for central poststroke pain: a randomized controlled trialNeurology200156218419011160953
- KimJSBashfordGMurphyTKMartinADrorVCheungRSafety and efficacy of pregabalin in patients with central post-stroke painPain201115251018102321316855
- RowbothamMCWhat is a “clinically meaningful” reduction in pain?Pain200194213113211690725
- CepedaMSAfricanoJMPoloRAlcalaRCarrDBWhat decline in pain intensity is meaningful to patients with acute pain?Pain20031051–215115714499431
- FarrarJTYoungJPJrLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain200194214915811690728
- FelsonDTAndersonJJBoersMAmerican College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritisArthritis Rheum19953867277357779114
- ForouzanfarTWeberWEKemlerMvan KleefMWhat is a meaningful pain reduction in patients with complex regional pain syndrome type 1?Clin J Pain200319528128512966253
- BruehlSHardenRNGalerBSSaltzSBackonjaMStanton-HicksMComplex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome?Pain2002951–211912411790474
- ChaeJPoststroke complex regional pain syndromeTop Stroke Rehabil201017315116220797958
- KorpelainenJTSotaniemiKAMyllylaVVAutonomic nervous system disorders in strokeClin Auton Res19999632533310638806