Abstract
Objective
This study aims to evaluate the effectiveness of Magnetic Resonance Virtual Endoscopy combined with 3D-FIESTA-c and 3D-TOF-MRA in preoperative assessment of MVD for PTN, with a focus on accurately detecting neuromuscular contact.
Methods
We retrospectively analyzed clinical and imaging data from 240 patients with unilateral primary trigeminal neuralgia undergoing MVD surgery between April 2016 and July 2023. Preoperative scans with 3D-FIESTA-c and 3D-TOF-MRA were performed, and MRVE images were obtained to analyze the relationship between the trigeminal nerve and adjacent vessels. Using the findings during microvascular decompression (MVD) surgery as the gold standard, the diagnostic results of 3D-TOF-MRA + 3D-FIESTA-c were considered as group I, while the combined use of MRVE, 3D-TOF-MRA + 3D-FIESTA-c was considered as group II.
Results
In 240 cases, group I had a positive rate of 96.25% and an accuracy rate of 86.25% for identifying responsible blood vessels, while group II had a positive rate of 98.3% and an accuracy rate of 94.17%. There were no statistically significant differences in positive rates between group I and group II, group I and MVD, or group II and MVD (P > 0.05). However, there were statistically significant differences in accuracy rates (P < 0.05). The accuracy for single and multiple arteries with group I was 99.38% and 80.0%, respectively, while with group II, it was 100% and 95.0%. No statistically significant difference was found in accuracy for single or multiple arteries (P>0.05). The accuracy of evaluating responsibility veins with or without other vessels was 52.73% and 80.0%, respectively, with a statistically significant difference (P<0.05).
Conclusion
MRVE combined with 3D-TOF-MRA + 3D-FIESTA-c significantly improves the accuracy of identifying responsibility vessels, especially veins, in preoperative assessment for MVD. This has important clinical implications for preoperative decision-making and surgical planning.
Introduction
Trigeminal neuralgia is a chronic neuropathic pain disorder characterized by brief, recurrent, stabbing pains in one or more branches of the trigeminal nerve on one side of the face, often triggered by simple daily activities such as talking, brushing teeth, and washing the face.Citation1 The global annual incidence ranges from 4.3 to 27 individuals per 100,000, with an onset typically between the ages of 50 and 70. It is more common in females, and the right side is more frequently affected than the left. The incidence increases with age.Citation2,Citation3 Over time, the frequency and duration of pain episodes tend to increase, significantly impacting the patient’s quality of life. Some patients may experience psychological symptoms such as anxiety and depression, and in severe cases, it can lead to suicidal tendencies.Citation4,Citation5
Trigeminal neuralgia is primary classified into two categories based on its etiology: primary and secondary, with primary trigeminal neuralgia (PTN) being more commonly observed in clinical practice.Citation4,Citation6 PTN refers to cases where trigeminal neuralgia occurs without underlying causes such as tumors or tumor-like lesions, inflammatory conditions, or postoperative complications. The pathogenesis of PTN involves multiple theories, with the neurovascular compression (NVC) theory being the most widely accepted currently.Citation4,Citation7,Citation8 Understanding the etiology has driven advancements in PTN treatment, and microvascular decompression (MVD) is considered the most effective method for treating PTN after unsuccessful drug therapy. MVD is currently the only surgical intervention that provides long-term pain relief while preserving normal facial sensation.Citation4,Citation9,Citation10 Challenges during MVD include difficulties in predicting the origin and course of responsible blood vessels and characterizing the responsible vessels. Therefore, a key focus in the field of trigeminal neuralgia treatment is how to preoperatively identify responsible blood vessels and clearly demonstrate their relationship with the trigeminal nerve.
Currently, the preoperative assessment of trigeminal neuralgia relies mainly on MRI examinations. In recent years, with the significant advancements in magnetic resonance imaging (MRI) technology, several three-dimensional high-resolution MRI sequences have been developed to evaluate the neurovascular relationships, such as 3D-CISS, 3D-SPACE, 3D-FIESTA, and 3D-TOF-MRA.Citation11–16 However, these imaging methods are often limited to two-dimensional plane images, providing limited assistance in identifying responsible blood vessels and aiding in clinical surgical planning. Magnetic Resonance Virtual Endoscopy (MRVE) is a non-invasive virtual imaging technique that processes three-dimensional MRI datasets to simulate visual images resembling those obtained with standard endoscopy tools.Citation17–19 MRVE offers enhanced visualization of the three-dimensional anatomical relationship between blood vessels and nerves and serves as a powerful complementary tool for preoperative assessment in MVD. It provides crucial imaging evidence for surgeons to accurately assess the relationship between responsible blood vessels and nerves and simulate surgical approaches. This study utilized MRVE technology in conjunction with three-dimensional high-resolution MRI sequences to assess the neurovascular relationships in 240 patients with primary trigeminal neuralgia, aiming to explore the clinical application value of MRVE in preoperative MVD assessments.
Materials and Methods
Study Population
A total of 240 patients with PTN who underwent MVD surgery at our neurosurgery department between April 2016 and April 2023 were retrospectively reviewed. Among these cases, 150 were located on the right side, and 90 on the left side. The cohort comprised 107 male and 133 female patients, with ages ranging from 22 to 88 years (mean age: 58.5±10.0 years). The duration of symptoms ranged from 1 month to 20 years, with an average of (4.7±4.6) years. Prior to surgery, all patients underwent preoperative scanning with 3D-TOF-MRA and 3D-FIESTA-c. Additionally. All patients underwent MVD surgery via a posterior approach through the sigmoid sinus. Inclusion criteria included:①Diagnosed as PTN according to the diagnostic criteria of the third edition of the International Classification of Headache Disorders published by the International Headache Society Classification Committee in 2018;Citation20 ②Unilateral PTN; ③Complete imaging data; ④Normal cognitive function. Exclusion criteria:①Secondary trigeminal neuralgia due to intracranial tumors, inflammation, or vascular malformations; ②History of mental illness; ③Presence of contraindications for MRI examinations. The study was granted approval by the Institutional Ethical Committee for Clinical Research of Nanjing Brain Hospital, and all patients provided written informed consent.
Image Acquisition
Scans were conducted using the GE Discovery 750 3.0T MRI machine with a standard head coil. Patients were positioned in a supine posture, and the scanning range encompassed the entire brainstem. Parameters for the 3D-FIESTA-c scan were as follows: TR=5.05ms, TE=2.26ms, FOV 250 mm × 226 mm, matrix 256 × 256, excitation times 2, and slice thickness 1 mm. Parameters for the 3D-TOF-MRA scan were: TR=12.0ms, TE=2.9ms, FOV 230 mm × 208 mm, matrix 320 × 320, flip angle 18°, excitation times 2, and slice thickness 1mm.
Image Post-Processing
The acquired raw images were transferred to the imaging workstation (GE AW4.6). The Reformat function was utilized for multi-planar reconstruction (MPR) of the 3D-FIESTA-c and maximum intensity projection (MIP) of the 3D-TOF-MRA to observe the relationship between the trigeminal nerve and blood vessels. For MRVE image reconstruction, the 3D-FIESTA-c was selected, and the Reformat Navigator function was used for post-processing. In the parameter interface, including two variables, namely, viewing angle and threshold, the viewing angle ranged from 90° to 160°, and the threshold range was set between 6000 and 10000. Adjustments were made to the viewing angle to ensure the complete display of the target in the image. The threshold was adjusted to maintain the smooth edges of the nerve and blood vessels, avoiding unnecessary structural displays in the surroundings. In the vertical position, the angle between nerves and vessels was observed, while in the tangential position, the distance between nerves and vessels was observed. In the navigation interface, observations were made regarding the distance between nerves and vessels, the presence of surface impressions, and any morphological abnormalities to determine the degree of nerve compression. Finally, three-dimensional images were rotated from multiple angles for further simulation of the surgical path.
Image Analysis
Based on the original transverse images of 3D-FIESTA-c, 3D-TOF-MRA, and MRVE reconstructed images, a comprehensive assessment was made to determine the type and source of nerve-vessel compression on the affected side, and annotations were added to the three-dimensional images. All images were evaluated by two experienced physicians with extensive neuroimaging diagnostic experience, using a double-blind approach. They were unaware of the patients’ symptom locations and surgical outcomes. A consensus judgment was reached on whether the trigeminal nerve in the brainstem area experienced vascular compression and the degree of compression. The source of the responsible vessel was also analyzed. According to the criteria of Arbab et alCitation21 the degree of contact between nerves and vessels was classified into three types. Type I: No contact, with no vascular display around the nerve or a clear distance between vessels and nerves. Type II: Contact, with no gap between vessels and nerves, and the nerve contact point shows no compressed deformation. Type III: Compression, with noticeable deformation and displacement at the nerve-vessel contact point. Type I was considered negative, while Type II + Type III was considered positive. Using the results of MVD surgery as the gold standard, the diagnostic results of 3D-TOF-MRA + 3D-FIESTA-c were designated as group I, while the combined use of MRVE, 3D-TOF-MRA + 3D-FIESTA-c was designated as group II. A comparative analysis was conducted to assess the accuracy of different MRI methods in evaluating responsible vessels.
Statistical Analysis
Statistical analysis of the data was performed using SPSS 21.0 software. A comparative analysis was conducted to compare the diagnostic results between group I and group II, group I and MVD, and group II and MVD. The count data were expressed as number of cases (%). The comparison between the two groups was performed using the chi-square (χ2) test. A significance level of P < 0.05 was considered statistically significant.
Results
MVD Surgical results
Among the 240 confirmed cases of PTN undergoing MVD surgery, the positive rate was 98.75% (237/240). In 3 cases (1.25%), no responsible vessel compression or contact was observed during surgery, attributed to the restricted space in the cerebellopontine angle (CPA) with arachnoid adhesions causing thickening and compression on the trigeminal nerve. Responsible vessels included Superior Cerebellar Artery (SCA) in 148 cases (61.67%), Anterior Inferior Cerebellar Artery (AICA) in 9 cases (3.75%), Petrosal Vein (PV) in 14 cases (5.83%), Vertebral Artery (VA) in 5 cases (2.08%), SCA + AICA in 13 cases (5.42%), SCA + PV in 39 cases (16.25%), SCA + VA in 5 cases (2.08%), AICA + PV in 2 cases (0.83%), and AICA + VA in 2 cases (0.83%). Combining responsibility vessel types, they were categorized as follows: Arterial group in 182 cases (75.83%), comprising Single Artery group in 162 cases (67.50%) and Multiple Arteries group in 20 cases (8.33%); Venous group in 14 cases (5.83%); Arteriovenous group in 41 cases (17.08%) ().
Table 1 MRI Findings and MVD Results of NVC
Comparison of Preoperative Imaging Examination Results Between Two Groups
The diagnostic positive rate of responsibility vessels for group I was 96.25% (231/240), with an accuracy of 86.25% (207/240). For group II the positive rate was 98.3% (236/240), with an accuracy of 94.17% (226/240). There were no statistically significant differences in positive rates between group I and group II, group I and MVD, or group II and MVD (χ2=1.977, χ2=3.077, χ2=0.145; P > 0.05). However, there were statistically significant differences in accuracy rates (χ2=8.515, χ2=35.436, χ2=14.421; P < 0.05).
Comparison of Preoperative Imaging Evaluation of Responsibility Vessels Between Two Groups
The accuracy of evaluating single and multiple arteries with 3D-TOF-MRA + 3D-FIESTA-c was 99.38% (161/162) and 80.0% (16/20), respectively. With MRVE combined with 3D-TOF-MRA + 3D-FIESTA-c, the accuracy was 100% (162/162) and 95.0% (19/20) for single and multiple arteries, respectively. There was no statistically significant difference in accuracy between the two imaging methods when evaluating responsibility vessels as single or multiple arteries (χ2=1.003, P>0.05; χ2=2.057, P>0.05). The accuracy of 3D-TOF-MRA + 3D-FIESTA-c and MRVE combined sequence in evaluating responsibility veins with or without other vessels was 52.73% (29/55) and 80.0% (44/55), respectively, with a statistically significant difference (χ2=9.162, P<0.05)().
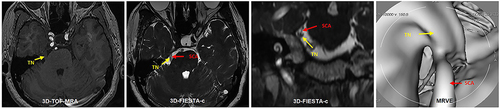
Figure 1 A 50-year-old female with left TN for 3 years. 3D-FIESTA-c axial (A) and 3D-TOF-MRA axial (B) showed vascular contact (red arrow) at the left trigeminal nerve (yellow arrow). MRVE image (C) showed the left Superior Cerebellar Artery (red arrow) compressing the trigeminal nerve from above (yellow arrow).
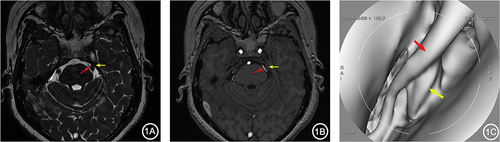
Figure 2 A 66-year-old female with right TN for over 5 years. 3D-FIESTA-c axial (A) and 3D-TOF-MRA axial (B) showed two branches of the right Superior Cerebellar Artery (red arrows) individually compressing the trigeminal nerve (yellow arrows). MRVE image (C) showed the two branches of the right Superior Cerebellar Artery (red arrows) separately compressing the trigeminal nerve from above (yellow arrows).
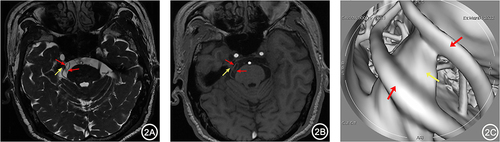
Figure 3 A 65-year-old male with right TN for 4 years. 3D-FIESTA-c axial (A) and coronal (B) showed close contact between the right Petrosal Vein (blue arrow) and the trigeminal nerve (yellow arrow) below. 3D-TOF-MRA axial (B) showed no apparent responsible vessels around the right trigeminal nerve (yellow arrow). MRVE image (D) showed the intimate relationship between the right Petrosal Vein (blue arrow) and the trigeminal nerve (yellow arrow).
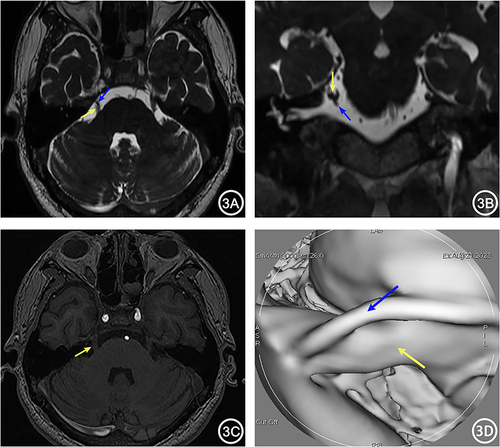
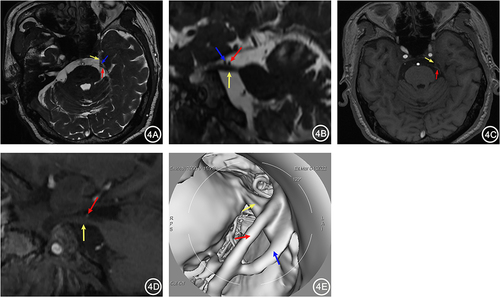
Figure 4 A 67-year-old female with left TN for 1 year. 3D-FIESTA-c axial (A) and sagittal (B) showed compression on the left trigeminal nerve (yellow arrow) by the Petrosal Vein (blue arrow) and Superior Cerebellar Artery (red arrow) from above. 3D-TOF-MRA axial (C) and sagittal (D) showed only the compression of the left trigeminal nerve by the Superior Cerebellar Artery (red arrow), with no other visible vascular. MRVE image (E) showed compression on the left trigeminal nerve by the Petrosal Vein (blue arrow) and Superior Cerebellar Artery (red arrow).
Discussion
The etiology and pathogenesis of primary trigeminal neuralgia remain contentious, with the predominant consensus among scholars favoring the NVC theory.Citation4,Citation7,Citation8 According to this theory, compression or conflict between neurovascular structures at the root entry zone (REZ) leads to demyelination of the compressed nerve root. The exposed axons then come into contact with adjacent non-myelinated fibers, causing a “short circuit” and altering pain mechanisms, thereby resulting in neuropathic pain.Citation1,Citation4 Among the common responsible vessels for trigeminal neuralgia, the superior cerebellar artery is the most frequently implicated, followed by the anterior inferior cerebellar artery, the basilar artery, the petrosal veins, and arterial-venous compression. Wei et alCitation14 reported that among 146 patients undergoing MVD surgery for TN, responsible vessels were found in 143 cases, with arteries accounting for 75.34%, veins for 7.53%, and arterial-venous mixed for 15.07%. Anwar et alCitation15 found that the occurrence rate of NVC caused by the SCA was 77%, while the AICA was 23%, with no cases attributed to venous compression. In our study, arterial compression accounted for 75.83% of responsible vessels, with SCA was 61.67%, AICA was 3.75%, and VA was 2.08%,venous was 5.83%, and arterial-venous mixed was 17.08%.
MVD is regarded as the most effective treatment strategy for trigeminal neuralgia caused by NVC.Citation4,Citation9,Citation10 Compared to other ablative surgical methods, MVD achieves immediate pain relief rates ranging from 87% to 98%, with 1-year pain-free rates reaching 80% and 8–10 year pain-free rates ranging from 58% to 68%.Citation22,Citation23 Even in elderly patients, MVD is equally safe and effective.Citation24 However, MVD surgery involves further exploration of the cranial cavity, thereby carrying certain surgical risks and complications. Enhancing treatment efficacy and reducing complication rates have become significant challenges for clinical neurosurgeons. Various factors influence the outcomes of MVD surgery, with preoperative identification of responsible vessels and determination of the relationship between nerves and vessels crucial for surgical success.Citation25–27
In recent years, magnetic resonance imaging (MRI) has been employed for preoperative detection of NVC in trigeminal neuralgia patients. Conventional MRI, due to its low resolution, has been disappointing in assessing the relationship between the trigeminal nerve and adjacent vessels. With rapid advancements in MR technology, high-resolution imaging sequences, particularly three-dimensional high-resolution T2-weighted imaging, play a crucial role in excluding secondary etiologies such as tumors, inflammation, and demyelination-induced spasms, and in identifying and evaluating NVC.Citation11–13,Citation28 In this study, we used 3D-FIESTA-c and 3D-TOF-MRA imaging for preoperative detection of NVC in trigeminal neuralgia patients. The 3D-FIESTA-c utilizes the effects of heavy T2-weighted imaging to highlight the signal of cerebrospinal fluid (CSF), achieving a similar effect to “ventriculography”. In 3D-FIESTA-c imaging, arteries, veins, and nerves all appear as low signals, while surrounding CSF appears as high signal. With the use of refocused gradient echo pulses, the 3D-FIESTA-c demonstrates high contrast and good spatial resolution in depicting small structures around CSF, providing detailed visualization of the boundaries between CSF and nerves, vessels, and dura mater. However, a major limitation of this sequence is the low signal of both vessels and nerves, leading to unclear signal contrast between them. This restriction hampers the detection and differentiation of NVC, particularly when responsible vessels overlap closely with the trigeminal nerve, and when there is minimal or no CSF between them, resulting in some false-positive or false-negative results.Citation14 Additionally, a significant drawback of 3D-FIESTA-c is its inability to differentiate between arteries and veins.3D-TOF-MRA is a flow-enhanced imaging technique that selectively images rapidly flowing vessels. It serves as an important complement to 3D-FIESTA-c, as it demonstrates fast-flowing small vessels as high signals, with CSF showing low signal, and is able to display high signal small vessels coursing within the low signal CSF background, thus facilitating clear identification of nerves and vessels. However, the 3D-TOF-MRA has limitations in imaging slow-flowing vessels such as veins and tortuous small arteries, especially the commonly implicated responsible vessel, the petrosal vein, in trigeminal neuralgia.Citation11,Citation14,Citation28,Citation29 In such cases, combining both sequences compensates for the limitations of each, enabling better tracking of the relationship between nerves and vessels.Citation14–16,Citation28 Muller et alCitation25 reported that using 3D-FIESTA combined with TOF-MRA allows visualization of the trigeminal nerve, optimizing the identification of NVC and potentially providing more reliable differentiation between veins and arteries. Wei et alCitation14 reported that the accuracy of diagnosing neurovascular compression using a combination of 3D-TOF-MRA and 3D-FIESTA significantly improves compared to using either sequence alone. Although the combination of these two sequences can compensate for some of their respective shortcomings, both display two-dimensional images, making it difficult to dynamically visualize the spatial anatomy of the cerebellopontine angle region and to display the spatial relationship between nerves and vessels from multiple angles and perspectives, especially with lower accuracy in displaying venous vessels or unknown small vessels,Citation14,Citation16 posing challenges for preoperative anatomical localization in neurosurgical MVD. In this study, the accuracy of diagnosing responsible vessels using a combination of 3D-TOF-MRA and 3D-FIESTA-c was 86.25%, with accuracies of 99.38% for evaluating single arterial branches and 80.0% for multiple arterial branches, but only 52.73% accuracy for evaluating responsible veins with or without other vessels. It is evident that while 3D-FIESTA-c combined with 3D-TOF-MRA effectively displays responsible arterial vessels, more advanced imaging techniques are required for cases where responsible vessels are veins.
MRVE is a three-dimensional visualization technique based on magnetic resonance imaging data, which utilizes computer software to post-process MR thin-slice three-dimensional image data to construct anatomical morphologies of cavity organ cavities. It provides three-dimensional visual feedback similar to or equivalent to standard fiber endoscopic techniques. Currently, this technology has been successfully applied in the examination of cavity organs (airways, gastrointestinal tracts, bladders, etc), as well as the fine structures of blood vessels, internal ear canals, and joint cavities.Citation28,Citation30–33 In neurosurgery, the main applications of MRVE include endoscopic third ventriculostomy (ETV), endoscopic nasal surgery, and assessment of cerebral vascular lesions.Citation34 However, there is currently limited literature on the application of MRVE in preoperative evaluation of neurovascular relationships in trigeminal neuralgia, both domestically and internationally. MRVE possesses imaging advantages of safety, non-invasiveness, and repeatability, allowing for multi-angle, comprehensive, three-dimensional observation of the complex spatial relationships between nerves and vessels.Citation35 It overcomes some of the shortcomings of MR two-dimensional images, allowing for better tracking of vessels and nerves, increasing the detection rate of responsible vessels, and enabling better differentiation between small arteries and veins. This provides clinical surgeons with a very intuitive understanding of the relationship between nerves and vessels, serving as important imaging evidence for preoperative simulation of MVD surgery and reducing the occurrence of intraoperative and postoperative complications. Shen et alCitation36 analyzed imaging data from 98 trigeminal neuralgia patients and found that the accuracy of diagnosing responsible vessels using MRVE combined with 3D-FIESTA and 3D-TOF-MRA was 98.0%, superior to that of combined diagnosis with 3D-FIESTA and 3D-TOF-MRA, and the difference was statistically significant. Chai et alCitation37 reported that MRVE combined with MR three-dimensional high-resolution imaging had a higher detection rate (95%) for responsible vessels compared to single MR three-dimensional high-resolution imaging (85%), and the detection rate (71.4%) for venous and arterial-venous mixed compression was higher than that of single MR three-dimensional high-resolution imaging (14.3%). In this study, it was found that the accuracy of diagnosing responsible vessels using MRVE combined with 3D-TOF-MRA+3D-FIESTA-c was 94.17%, higher than that of detection accuracy using 3D-TOF-MRA+3D-FIESTA-c (86.25%), and the difference was statistically significant (P < 0.05). The accuracy of diagnosing responsible veins with or without other vessels using MRVE combined with 3D-TOF-MRA+3D-FIESTA-c was 80.0%, significantly higher than the detection accuracy using 3D-TOF-MRA+3D-FIESTA-c for veins (52.73%), and the difference was statistically significant (P < 0.05). These results indicate that MRVE technology, by utilizing three-dimensional simulated images for observation and combining them with images from 3D-TOF-MRA and 3D-FIESTA-c, can significantly improve the detection rate of responsible vessels (especially small veins). Additionally, MRVE imaging technology can simulate the surgical path through three-dimensional images, providing appropriate perspectives for neurosurgeons to observe the spatial relationships between vessels and nerves, helping to optimize surgical plans to avoid missing vessels, and further reducing unnecessary exploration and traction during surgery.
Limitations and Conclusions
MRVE has some limitations that have hindered its widespread application in clinical practice. Firstly, MRVE is a time-consuming post-processing technique that requires specialized operators and experienced neuroradiologists for image interpretation. Therefore, mastering this technique requires professional training and practical experience. Secondly, obtaining clear MRVE images requires a certain amount of cerebrospinal fluid in the cerebellopontine angle cistern. Patients with small cerebrospinal fluid spaces may have insufficient fluid, resulting in lower resolution and difficulties in MRVE reconstruction. Conversely, patients with excessive cerebrospinal fluid may experience motion artifacts, which can affect diagnostic accuracy.
In conclusion, the combined application of MRVE with three-dimensional high-resolution MRI sequences (3D-TOF-MRA, 3D-FIESTA-c) provides a powerful tool for assessing the intricate adjacent relationships between the trigeminal nerve and surrounding vessels. This combination technique enables multi-angle, comprehensive observation, significantly enhancing the accuracy of detecting potential responsible vessels. In neurosurgical procedures, it offers surgeons detailed three-dimensional anatomical maps, allowing for more precise surgical planning and greatly reducing the risk of damaging critical nerves or vessels during the operation, thereby ensuring surgical safety.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethical Committee for clinical research of Nanjing Brain Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. We will always comply with relevant laws, regulations, and international ethical guidelines, particularly the “Declaration of Helsinki”.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors are grateful to everyone who offered their time, insight, and assistance to this article.
Data Sharing Statement
The data are available from the corresponding author on reasonable request.
References
- Cruccu G, Di Stefano G, Truini A, Ropper AH. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–762. doi:10.1056/NEJMra1914484
- Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Therap Clin Risk Manag. 2015;289–299. doi:10.2147/TCRM.S37592
- De toledo IP, Réus JC, Fernandes M, et al. Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc. 2016;147(7):570–576.e2. doi:10.1016/j.adaj.2016.02.014
- Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784–796. doi:10.1016/S1474-4422(20)30233-7
- Melek LN, Smith JG, Karamat A, Ranton T. Comparison of the neuropathic pain symptoms and psychosocial impacts of trigeminal neuralgia and painful posttraumatic trigeminal neuropathy. J Oral Facial Pain Headache. 2019;33(1):77–88. doi:10.11607/ofph.2157
- Brinzeu A, Dumot C, Sindou M. Role of the petrous ridge and angulation of the trigeminal nerve in the pathogenesis of trigeminal neuralgia, with implications for microvascular decompression. Acta neurochirurgica. 2018;160:971–976. doi:10.1007/s00701-018-3468-1
- Harsha KJ, Kesavadas C, Chinchure S, Thomas B, Jagtap S. Imaging of vascular causes of trigeminal neuralgia. J Neuroradio. 2012;39(5):281–289. doi:10.1016/j.neurad.2012.08.006
- Gajski D, Dennis AR, Arnautović KI. Microsurgical decompression of trigeminal neuralgia caused by simultaneous double arterial (SCA and AICA) and petrosal vein complex compression. J Neurol Surg B Skull Base. 2018;79:05):S428–S430.
- Holste K, Chan AY, Rolston JD, Englot DJ. Pain outcomes following microvascular decompression for drug-resistant trigeminal neuralgia: a systematic review and meta-analysis. Neurosurgery. 2020;86(2):182. doi:10.1093/neuros/nyz075
- Liu MX, Zhong J, Xia L, Dou NN, Shi J. Treatment of Trigeminal Neuralgia with “Microvascular Decompression Plus” Technique. J Neurol Surg B Skull Base. 2020;82:e295–e299. doi:10.1055/s-0040-1710520
- Gamaleldin OA, Donia MM, Elsebaie NA, Abdelrazek AA, Rayan T, Khalifa MH. Role of fused three-dimensional time-of-flight magnetic resonance angiography and 3-dimensional T2-weighted imaging sequences in neurovascular compression. World Neurosurg. 2020;133:e180–e186. doi:10.1016/j.wneu.2019.08.190
- Busse S, Taylor J, Field M. Correlation of Preoperative High-Resolution Neurovascular Imaging and Surgical Success in Neurovascular Compression Syndromes. World Neurosurg. 2023;172:e593–e598. doi:10.1016/j.wneu.2023.01.094
- Pham HD, Dang TH, Duong TK, et al. Predictability of fused 3D-T2-SPACE and 3D-TOF-MRA images in identifying conflict in trigeminal neuralgia. J Pain Re;2021. 3421–3428. doi:10.2147/JPR.S331054
- Wei SC, Yu R, Meng Q, Qu C. Efficacy of microvascular decompression in patients with trigeminal neuralgia with negative neurovascular relationship shown by magnetic resonance tomography. Clini Neuro Neurosur. 2020;197:106063. doi:10.1016/j.clineuro.2020.106063
- Anwar HA, Ramya Krishna M, Sadiq S, Ramesh Kumar R, Venkatarathnam V, Saikiran G. A study to evaluate neurovascular conflict of trigeminal nerve in trigeminal neuralgia patients with the help of 1.5 T MR imaging. Egypt J Radiol Nucl Med. 2022;53(1):66. doi:10.1186/s43055-022-00746-8
- Hu M, Zhou W, Shen W, Zhang H, Shen J. A Combination of 3D TOF MRA and FIESTA Predicts Surgery-Needed Primary Trigeminal Neuralgia and Specific Offending Vessels. J Integr Neuroscie. 2022;21(6):169. doi:10.31083/j.jin2106169
- Nowé V, Michiels JL, Salgado R, et al. High-resolution virtual MR endoscopy of the cerebellopontine angle. Am J Roentgenol. 2004;182(2):379–384. doi:10.2214/ajr.182.2.1820379
- Werner H, Lopes J, Ribeiro G, et al. Three-dimensional virtual cystoscopy: noninvasive approach for the assessment of urinary tract in fetuses with lower urinary tract obstruction. Prenat Diagn. 2017;37(13):1350–1352. doi:10.1002/pd.5188
- Bao Y, Wan W, Li Q, et al. MR Virtual Endoscopy of the Fetal Limb Anomalies Using Three-Dimensional Fast Imaging Employing Steady-State Acquisition Sequence. Fetal Diagn Thera. 2021;48(5):333–341. doi:10.1159/000514327
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
- Arbab AS, Nishiyama Y, Aoki S, et al. Simultaneous display of MRA and MPR in detecting vascular compression for trigeminal neuralgia or hemifacial spasm: comparison with oblique sagittal views of MRI. Eur Radiol. 2000;10:1056–1060. doi:10.1007/s003309900291
- Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F. Micro-vascular decompression for primary Trigeminal Neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta neurochirurgica. 2006;148:1235–1245. doi:10.1007/s00701-006-0809-2
- Oesman C, Mooij JJA. Long-term follow-up of microvascular decompression for trigeminal neuralgia. Skull Base. 2011;313–322. doi:10.1055/s-0031-1284213
- Greve T, Tonn JC, Mehrkens JH. Microvascular decompression for trigeminal neuralgia in the elderly: efficacy and safety. J Neurol. 2021;268:532–540. doi:10.1007/s00415-020-10187-w
- Müller S, Khadhraoui E, Khanafer A, Psychogios M, Rohde V, Tanrikulu L. Differentiation of arterial and venous neurovascular conflicts estimates the clinical outcome after microvascular decompression in trigeminal neuralgia. BMC Neurol. 2020;20:1–8. doi:10.1186/s12883-020-01860-8
- Kasuya H, Tani S, Kubota Y, et al. Characteristics and management of the offending veins in microvascular decompression surgery for trigeminal neuralgia. Neurosurgical Review. 2021;44:2337–2347. doi:10.1007/s10143-020-01411-2
- Go KO, Hwang K, Han JH. Surgical nuances to reduce and manage cerebrospinal fluid leaks after microvascular decompression. J Clin Med. 2020;9(4):902. doi:10.3390/jcm9040902
- Liang C, Yang L, Zhang BB, Guo SW, Li RC. Three-dimensional time-of-flight magnetic resonance angiography combined with high resolution T2-weighted imaging in preoperative evaluation of microvascular decompression. World J Clini Cases. 2022;10(34):12594. doi:10.12998/wjcc.v10.i34.12594
- Chen SR. Neurological imaging for hemifacial spasm. Int Ophthal Clinics. 2018;58(1):97–109. doi:10.1097/IIO.0000000000000212
- Woo SR, Seo MW, Kim YH, et al. Extreme duplication-type, nonseparated fenestration of the basilar artery in a patient with pontine infarction: confirmation with virtual arterial endoscopy. J Clin Neurol. 2006;2(1):74–77. doi:10.3988/jcn.2006.2.1.74
- van der Paardt MP, Stoker J, van der Paardt MP. Magnetic resonance colonography for screening and diagnosis of colorectal cancer. Mag Reson Imag Clin. 2014;22(1):67–83. doi:10.1016/j.mric.2013.07.006
- Incesu L, Aslan K, Polat A, et al. Intravascular Virtual MR Endoscopy Evaluation of Cerebral Aneurysms. Turk Neurosurg. 2014;24(2).
- Azuma T, Yamaguchi K, Iida T, Oouhida J, Suzuki M. MR virtual endoscopy for biliary tract and pancreatic duct. Magn Reson Med Sci. 2007;6(4):249–257. doi:10.2463/mrms.6.249
- Neubauer A, Wolfsberger S. Virtual endoscopy in neurosurgery: a review. Neurosurgery. 2013;72:A97–A106.
- Wei W, Liu Z, Zhang W, Wang Y, Chen M. Application of virtual endoscopy in microvascular decompression of trigeminal neuralgia. J Craniofacial Surgery. 2021;32(5):1696–1699. doi:10.1097/SCS.0000000000007347
- Shen J, Shen W, Su Z, Gu X. The evaluation of MR virtual endoscopy in microvascular decompression of primary trigeminal neuralgia. J Med Imag. 2020;30(7):1141–1144.
- Chai X, Xiao C, Huang Q, Wang X, Li C, Luo Z. Application of 3D high-resolution imaging sequences combined with MRVE in preoperative assessment of trigeminal neuralgia in a 3.0 T MRI system. Inter J Med Rad. 2019;42(4):381–384. doi:10.19300/j.2019.L6369