Abstract
Objective
To determine the risk of postherpetic neuralgia (PHN) in patients with acute herpes zoster (HZ), this study developed and validated a novel clinical prediction model by incorporating a relevant peripheral blood inflammation indicator.
Methods
Between January 2019 and June 2023, 209 patients with acute HZ were categorized into the PHN group (n = 62) and the non-PHN group (n = 147). Univariate and multivariate logistic regression analyses were conducted to identify risk factors serving as independent predictors of PHN development. Subsequently, a nomogram prediction model was established, and the discriminative ability and calibration were evaluated using the receiver operating characteristic curve, calibration plots, and decision curve analysis (DCA). The nomogram model was internally verified through the bootstrap test method.
Results
According to univariate logistic regression analyses, five variables, namely age, hypertension, acute phase Numeric Rating Scale (NRS-11) score, platelet-to-lymphocyte ratio (PLR), and systemic immune inflammation index, were significantly associated with PHN development. Multifactorial analysis further unveiled that age (odds ratio (OR) [95% confidence interval (CI)]: 2.309 [1.163–4.660]), acute phase NRS-11 score (OR [95% CI]: 2.837 [1.294–6.275]), and PLR (OR [95% CI]: 1.015 [1.010–1.022]) were independent risk factors for PHN. These three predictors were integrated to establish the prediction model and construct the nomogram. The area under the receiver operating characteristic curve (AUC) for predicting the PHN risk was 0.787, and the AUC of internal validation determined using the bootstrap method was 0.776. The DCA and calibration curve also indicated that the predictive performance of the nomogram model was commendable.
Conclusion
In this study, a risk prediction model was developed and validated to accurately forecast the probability of PHN after HZ, thereby demonstrating favorable discrimination, calibration, and clinical applicability.
Introduction
Herpes zoster (HZ) is characterized by a neurocutaneous inflammatory reaction induced by latent varicella-zoster virus (VZV) reactivation within the host. It appears as a vesicular rash with a dermatomal distribution.Citation1 Postherpetic neuralgia (PHN) is the most prevalent and refractory complication of HZ that persists for over 3 months after the occurrence of shingles. Affected individuals may experience enduring and intense pain for numerous years, or even decades, which significantly affects their daily life and increases the economic burden.Citation2
HZ has a global incidence of 3–5/1000 person-years, with 5–35% of patients progressing to PHN.Citation3 PHN represents a intricate neuropathic pain disorder, and its underlying mechanisms remain enigmatic. Present therapeutic modalities for PHN predominantly encompass oral analgesics, nerve blocks, pulsed radiofrequency, and spinal cord stimulation.Citation4 Nevertheless, most of the therapeutic effects remain unsatisfactory.Citation5
Considering the profound effect of PHN on patient health and quality of life, the anticipation of PHN occurrence in patients with acute HZ has become the focal point of contemporary research, which promises preventive strategies for those with HZ. According to several studies, older age, severe acute pain, and an intense rash are key risk factors for PHN.Citation6,Citation7 Additionally, a limited number of studies have suggested that gender, immunosuppression, diabetes, systemic lupus erythematosus, and respiratory diseases (asthma, COPD, obstructive sleep apnea, and lung cancer) are linked to PHN.Citation8,Citation9 However, prevailing studies have often confined themselves to clinical factors rather than incorporating serological or virological tests, which has potentially led to inaccurate outcomes.
Inflammation is acknowledged as a pivotal facet of chronic pain.Citation10 Emerging research speculates that heightened peripheral blood inflammation contributes to chronic pain.Citation11,Citation12 Indicators of peripheral blood inflammation, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), C-reactive protein-to-albumin ratio (CAR), and systemic immune inflammation index (SII), are used for the diagnosis, treatment, follow-up management, and prognosis prediction of various diseases, including cancer, acute pulmonary embolism, diabetic foot-induced sepsis, and coronary artery disease. This is because of the accessibility, reproducibility, non-invasiveness, and cost-effectiveness of these indicators.Citation13–17 Furthermore, some studies have examined the correlation between indicators of peripheral blood inflammation and chronic pain, such as postsurgical pain, low back pain, fibromyalgia, and neuropathic pain, in diabetes.Citation11,Citation18–20 However, studies exploring the role of these indicators in the context of HZ are limited.
The models currently employed for predicting PHN risk after acute HZ have limitations. Therefore, we here retrospectively analyzed 209 HZ patients to establish and validate a novel clinical prognostic model by incorporating clinical factors and markers of peripheral blood inflammation (NLR, PLR, LMR, CAR, and SII) for predicting PHN development.
Materials and Methods
Patient Population
The electronic health records of 209 patients diagnosed as having HZ during the acute phase were extracted from the archives spanning January 2019 to June 2023 at Jinling Hospital, Nanjing University School of Medicine, People’s Republic of China. The study was approved by the medical ethics committee (2019NZKY-030-02), and explicit informed consent forms were procured from all patients. Our study complies with the principles of the Declaration of Helsinki (October 2013).
The study inclusion criteria were as follows: (1) diagnoses of HZ and PHN were established based on clinical symptoms and supplementary examinations; (2) within a 14-day timeframe from onset; and (3) comprehensive clinical records were available. The exclusion criteria were as follows: (1) absence of 3-month follow-up data; (2) presence of severe organ dysfunction, including the heart, lung, or kidney; and (3) recent occurrence of acute severe infection.
Data Collection and Definition
Clinicopathological data were systematically extracted from the patient’s electronic medical records, including age, gender, body mass index (BMI), distributional side of the rash, Numeric Rating Scale (NRS-11) score, hypertension, and diabetes mellitus. The assessment of pain intensity was conducted utilizing the NRS scale, which ranges from 0 to 10, with a delineation of severe pain corresponding to a score of ≥7 on the NRS.
A peripheral blood count was performed before any treatment modality was initiated. The indices NLR, PLR, LMR, CAR, and SII were calculated and documented using the prescribed formulas: NLR = neutrophil/lymphocyte, PLR = platelet/lymphocyte, LMR = lymphocyte/monocyte, CAR = C-reactive protein/albumin, and SII = platelet × neutrophil/lymphocyte. The last interview was conducted in July 2023.
Statistical Analysis
Continuous variables were analyzed using t-tests, while categorical variables were analyzed using chi-square tests. Univariate and multivariate logistic regression analyses were conducted to determine independent predictors of PHN in patients with acute HZ, with odds ratio (OR) and corresponding 95% confidence intervals (CI) employed as effect estimates.
Based on the results of multivariate logistic regression analyses, we established the risk prediction model and nomogram model. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated using the pROC package. The calibration of our model was assessed using the Hosmer–Lemeshow goodness-of-fit test and calibration curve.
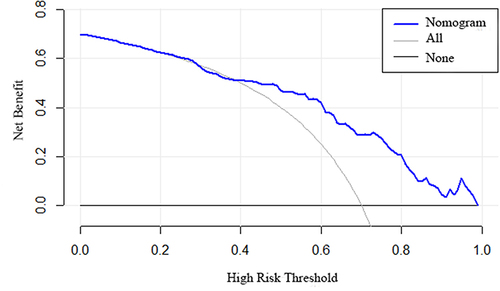
Moreover, the clinical utility of the nomogram was validated by conducting the decision curve analysis (DCA) to quantify the net benefit under different threshold probabilities. The model was internally validated using the repeated sampling method (Bootstrap method). Statistical analyses were conducted using R software (version 4.3.2), with statistical significance set at p < 0.05.
Results
Baseline Characteristics of the Study Population
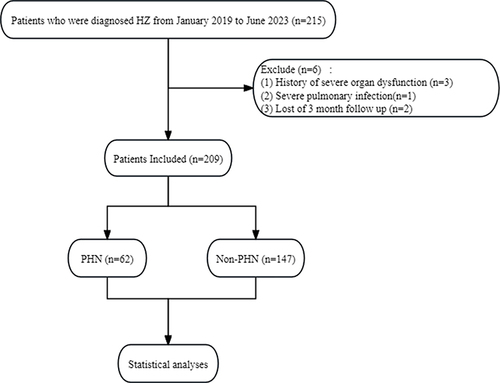
In total, 215 patients diagnosed as having acute phase HZ were included in this study, and of them, 6 patients were excluded (). Finally, 209 patients who met the inclusion criteria were analyzed in this study. Of the total patients enrolled, 62 patients (30%) developed PHN. All patients had received antiviral drug therapy before the baseline assessment. Among the participants, 130 (62%) and 75 (36%) patients had male gender and were aged ≥60 years, respectively. The patients with HZ were stratified into two groups based on the presence or absence of PHN. These patients revealed statistically significant differences in age, hypertension, NRS-11 score, PLR, and LMR (P < 0.05). Intergroup differences in gender, BMI, side, diabetes history, NLR, CAR, and SII were not significant. presents the demographic and clinical characteristics of the two groups.
Table 1 Baseline and Clinical Characteristics of the Study Population (N = 209)
Univariate and Multivariate Logistic Analyses
Logistic regression analysis was performed to ascertain factors contributing to PHN development. According to the univariate regression analysis, PHN occurrence in individuals was notably correlated with HZ and age (p = 0.010), hypertension (p = 0.012), acute phase NRS-11 score (p = 0.002), PLR (p < 0.001), and SII (p = 0.037). The five significant risk factors were then included in the multivariate logistic regression analyses. The results of this analysis disclosed that age (OR = 2.309, 95% CI: 1.163–4.660, p = 0.018), acute phase NRS-11 score (OR = 2.837, 95% CI: 1.294–6.275, p = 0.009), and PLR (OR = 1.015, 95% CI: 1.010–1.022, p < 0.001) were recognized as independent predictors of PHN ().
Table 2 Univariate and Multivariate Logistic Regression Analyses
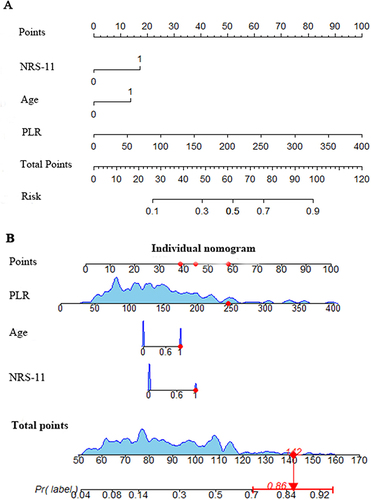
Construction of a Nomogram Model for Predicting PHN After Acute HZ
As depicted in , we here formulated a nomogram model after the multivariate logistic regression analyses to predict PHN occurrence from HZ. This model relies on the three identified independent predictors. A high total score indicated a high PHN probability. For example, an HZ patient aged ≥60 years and with a score of ≥7 on the NRS-11, PLR of 245, and has an estimated probability of PHN of 86%.
Predictive Model Validation
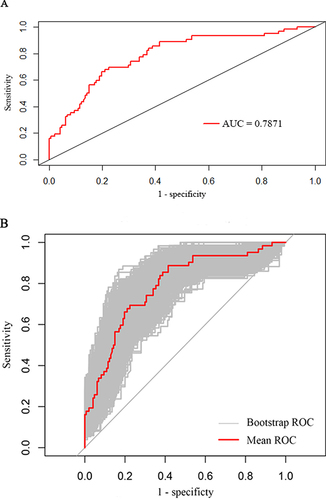
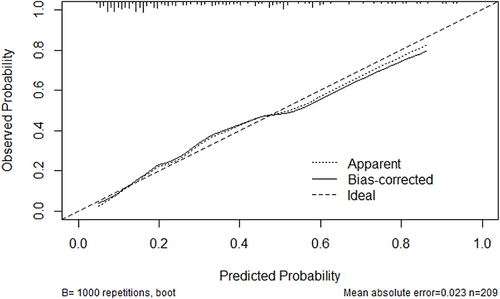
Subsequently, we conducted the ROC curve analysis to estimate the discriminative performance of our predictive model. The AUC was 0.7871 (95% CI: 0.6342–0.8155) and thus indicated commendable diagnostic performance (). The Bootstrap method was applied for internal validation, which resulted in an AUC of 0.7758 (). The calibration curve of our predictive model exhibited that the predicted and observed probabilities were highly consistent (), thereby confirming the model’s excellent calibration. Additionally, DCA was performed to evaluate the clinical application value. According to our DCA plot, the nomogram holds substantial clinical value, thus offering remarkable benefits to patients ().
Discussion
A recent meta-analysis reported that the incidence of HZ for all age groups is 4.28 cases per 1000 population-years, with the estimated HZ case numbers exceeding 6 million in China.Citation21 Among these cases, 12.6% of patients are expected to develop PHN. Once PHN ensues, patients may experience severe and intractable pain of diverse nature, such as burning, throbbing, cutting, or tearing, which detrimentally affects their quality of life and imposes a substantial economic burden on them, their families, and their society at large.Citation3 Therefore, a clinically practical assessment tool that accurately predicts PHN development must be explored, thereby guiding prevention and intervention strategies during acute phase HZ to mitigate PHN incidence. However, predicting the emergence of PHN is a formidable challenge.
In our study, univariate logistic regression analyses were performed to identify potential risk factors for PHN. Five variables, namely age, hypertension, acute phase NRS-11 score, PLR, and SII, were statistically significant (P < 0.05). Subsequently, these significant variables were included in the multivariate analyses with the stepwise selection. The results of the aforementioned analyses unveiled age, acute phase NRS-11 score, and PLR as independent influencing factors. Based on these three predictors, the nomogram model was established. Our model incorporated clinically pertinent variables and novel laboratory-associated predictive factors.
Older age is a risk factor known to influence concurrent PHN. The incidence of HZ and PHN markedly increases with age, particularly after the age of 60 years.Citation7,Citation22,Citation23 The overall PHN proportion among HZ patients aged ≥60 years is approximately 20.83%.Citation24 In our study, the incidence of PHN among HZ patients aged ≥60 years was 29.33%, whereas that among patients aged <60 years was 22.76%. The PHN risk increases with older age, and older age is substantially relevant as an independent risk factor for PHN, which aligns with previous findings.Citation5,Citation25 This age-related increase in disease is closely related to the weakening of VZV-specific T cell mediated immunity and elevated virus levels after zoster reactivation.Citation26–28
Pain is the predominant clinical manifestation of HZ. The pain intensity during the acute phase is positively correlated with the severity of nerve injury, which directly influences PHN incidence.Citation29 In this study, PHN incidence significantly differed between the high- and low-NRS-11 groups (50% vs 25%, P < 0.05). This indicated that acute phase NRS-11 is also a pivotal risk factor for PHN. A higher NRS-11 score usually indicates more reactivation of varicella zoster virus, which results in a more severe inflammatory response and nerve fiber damage, and then augments the PHN likelihood.Citation30,Citation31 Fujiwara et al further examined the association between pain catastrophizing (Pain Catastrophizing Scale score ≥ 30) in the acute phase of HZ and PHN development, but no significant correlation was noted.Citation32
PHN primarily manifests as neuropathic pain, and its exact pathogenesis remains complex. Immune-mediated inflammation plays a crucial role in neuropathic pain development by generating nerve cell-damaging pro-inflammatory cytokines and molecules, thereby leading to persistent pain.Citation33 The intricate balance between pro- and anti-inflammatory cytokines regulates neuropathic pain development.Citation34
Several new composite parameters reflecting inflammatory indices including NLR, PLR, LMR, CAR, and SII have recently been used to evaluate the body’s inflammatory response. These parameters have been considered novel, accurate inflammatory biomarkers in many chronic or acute inflammation-related diseases.Citation35,Citation36 In the present study, these inflammatory indices, along with traditional clinicopathological variables were subjected to univariate and multivariate analyses, ultimately revealing PLR as an independent risk factor for PHN. This is the first study identifying PLR as an inflammatory marker in HZ and underscoring its utility in PHN prediction. An elevated PLR indicates a relatively decreased lymphocyte, whereas increased platelet counts. Lymphocytes reflect cell-mediated adaptive immunity.Citation37 T lymphocytes are the primary components of lymphocytes and account for 65–75% of the total lymphocytes in peripheral blood.Citation38,Citation39 In the HZ pathogenesis, the body’s defense chiefly relies on cellular immune responses, and the T lymphocyte’s function is pivotal in maintaining normal immune function.Citation40,Citation41 T lymphocytes decreased and immune function was impaired in HZ patients,Citation42,Citation43 which accelerated VZV reactivation and resulted in neuropathic pain. Platelets, a typical blood cell component, are produced by mature megakaryocytes in bone marrow responsible for the systemic inflammatory response and immune regulation in the body.Citation44,Citation45
Other possible predictors of PHN were also evaluated. Underlying conditions including diabetes and cardiovascular diseases serve as an additional zoster severity-related burden.Citation46 In the present study, diabetes and hypertension were included in the analysis. The two groups exhibited significant differences in terms of hypertension, although hypertension was excluded from the following multivariate logistic regression analyses. The role of sex in PHN remains controversial, with published results being conflicting. Some studies have suggested a higher risk in female patients than in male patients, whereas some found no association with sex.Citation6 In the present study, sex was not significantly associated with PHN development. These conflicting results may warrant further investigation.
Limitations
Despite its many strengths, this study has some limitations. For instance, the primary constraint lies in the relatively small sample size and limited clinical data of HZ (n = 209), which potentially introduces bias to the results. In addition, to mitigate these limitations and enhance the robustness of the findings, further polycentric prospective large-scale studies are warranted to comprehensively investigate the influencing factors of PHN in patients with HZ.
Conclusions
Collectively, our findings suggest that age, acute phase NRS-11 Score, and PLR are independent predictors of PHN in patients with HZ.
Consent for Publishing
All the authors consent to publish the paper.
Disclosure
The authors declared no potential conflicts of interest in this work.
Data Sharing Statement
The original contributions outlined in the study are incorporated in the article. For additional inquiries, please direct your questions to the corresponding authors.
Additional information
Funding
References
- H-L L, Gong G, Wang J-L, F-B L. Analysis of risk factors for postherpetic neuralgia in patients with postmalignancy herpes zoster. Pain Physician. 2023;26(4):E397–E403.
- Harvey M, Prosser LA, Rose AM, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain. 2020;161(2):361–368. doi:10.1097/j.pain.0000000000001718
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833
- Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31(4):235–243. doi:10.3344/kjp.2018.31.4.235
- Gu J, Yuan Y, Wang J, Liu H, Zhang Z, Yan Y. Serum inflammatory cytokine levels in herpes zoster patients and their association with postherpetic neuralgia: a prospective study. Med Sci Monit. 2023;29:e941878.
- Forbes H, Thomas S, Smeeth L, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi:10.1097/j.pain.0000000000000307
- Chae J, Im J, Choi Y, Lee H, Kim W. Comparison of the severity of zoster-associated pain and incidence of postherpetic neuralgia in patients with and without pre-existing spinal disorders at the same spinal nerve level: a retrospective multicenter study. J Personal Med. 2023;13(9):1286. doi:10.3390/jpm13091286
- Morena D, Lumbreras S, Rodríguez J, et al. Chronic respiratory diseases as a risk factor for herpes zoster infection. Arch de Bronconeumol. 2023;59(12):797–804. doi:10.1016/j.arbres.2023.08.010
- Wang C, Hsieh W, Hsu T, Tsai F, Hsu C. Obstructive sleep apnea increases the risk of herpes zoster and postherpetic neuralgia. Postgraduate Med. 2023;2:1–8.
- Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159(3):595–602. doi:10.1097/j.pain.0000000000001122
- Shu B, Xu F, Zheng X, et al. Change in perioperative neutrophil-lymphocyte ratio as a potential predictive biomarker for chronic postsurgical pain and quality of life: an ambispective observational cohort study. Front Immunol. 2023;14:1177285. doi:10.3389/fimmu.2023.1177285
- Colgan D, Eddy A, Green K, Oken B. Adaptive body awareness predicts fewer central sensitization-related symptoms and explains relationship between central sensitization-related symptoms and pain intensity: a cross-sectional study among individuals with chronic pain. Pain Pract. 2022;22(2):222–232. doi:10.1111/papr.13083
- Moukas S, Kasimir-Bauer S, Tewes M, et al. Ratios of monocytes and neutrophils to lymphocytes in the blood predict benefit of CDK4/6 inhibitor treatment in metastatic breast cancer. Sci Rep. 2023;13(1):21262. doi:10.1038/s41598-023-47874-3
- Li L, Li Y, Lu M, et al. The combination of baseline neutrophil to lymphocyte ratio and dynamic changes during treatment can better predict the survival of osteosarcoma patients. Front Oncol. 2023;13:1235158. doi:10.3389/fonc.2023.1235158
- Elshahaat H, Zayed N, Ateya M, Safwat M, El Hawary A, Abozaid M. Role of serum biomarkers in predicting management strategies for acute pulmonary embolism. Heliyon. 2023;9(11):e21068. doi:10.1016/j.heliyon.2023.e21068
- Sun B, Chen Y, Man Y, Fu Y, Lin J, Chen Z. Clinical value of neutrophil-to-lymphocyte ratio and prognostic nutritional index on prediction of occurrence and development of diabetic foot-induced sepsis. Front Public Health. 2023;11:1181880. doi:10.3389/fpubh.2023.1181880
- He J, Song C, Zhang R, Yuan S, Li J, Dou K. Discordance between neutrophil to lymphocyte ratio and high sensitivity C-Reactive protein to predict clinical events in patients with stable coronary artery disease: a large-scale cohort study. J Inflamm Res. 2023;16:5439–5450. doi:10.2147/JIR.S428734
- Özcan-Ekşi E, Berikol G, Ekşi M. Potential blood markers as screening tools for subjects with low back pain: an age- and gender-matched cross-sectional analysis. Curr Med Res Opin. 2024;40(1):77–85. doi:10.1080/03007995.2023.2282646
- Aktürk S, Büyükavcı R. Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin Rheumatol. 2017;36(8):1885–1889. doi:10.1007/s10067-017-3647-0
- Allwright M, Karrasch J, O’Brien J, Guennewig B, Austin P. Machine learning analysis of the UK Biobank reveals prognostic and diagnostic immune biomarkers for polyneuropathy and neuropathic pain in diabetes. Diabetes Res Clin Pract. 2023;201:110725. doi:10.1016/j.diabres.2023.110725
- Zhang Z, Liu X, Suo L, Zhao D, Pan J, Lu L. The incidence of herpes zoster in China: a meta-analysis and evidence quality assessment. Hum Vaccines Immunother. 2023;19(2):2228169. doi:10.1080/21645515.2023.2228169
- Chen J, Lan K, Sheu M, Tseng S, Weng S, Hu M. Peptic ulcer as a risk factor for postherpetic neuralgia in adult patients with herpes zoster. J Med Virol. 2015;87(2):222–229. doi:10.1002/jmv.24051
- Li T, Wang J, Xie H, et al. Study on the related factors of post-herpetic neuralgia in hospitalized patients with herpes zoster in Sichuan Hospital of Traditional Chinese Medicine based on big data analysis. Dermatologic Therapy. 2020;33(6):e14410. doi:10.1111/dth.14410
- Takao Y, Miyazaki Y, Okeda M, et al. Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J Epidemiol. 2015;25(10):617–625. doi:10.2188/jea.JE20140210
- He Y, He J, Miao F, et al. A bibliometric and visualization analysis of global research on postherpetic neuralgia from 2000 to 2022: a review. Medicine. 2023;102(45):e34502. doi:10.1097/MD.0000000000034502
- Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30(6):582–587. doi:10.1212/WNL.30.6.582
- Berger R, Florent G, Just M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32(1):24–27. doi:10.1128/iai.32.1.24-27.1981
- Levin MJ, Murray M, Rotbart HA, Zerbe GO, White CJ, Hayward AR. Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis. 1992;166(2):253–259. doi:10.1093/infdis/166.2.253
- Zhou H, Wang Z, Jin H, Chen X, Lei L. A systematic review and meta-analysis of independent risk factors for postherpetic neuralgia. Ann Pall Med. 2021;10(12):12181–12189. doi:10.21037/apm-21-3028
- Ito S, Yasuda M, Kondo H, et al. Clinical courses of herpes simplex virus-induced urethritis in men. J Infect Chemother. 2017;23(10):717–719. doi:10.1016/j.jiac.2017.03.017
- Steain M, Sutherland J, Rodriguez M, Cunningham A, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88(5):2704–2716. doi:10.1128/JVI.03445-13
- Fujiwara A, Watanabe K, Yoshimura K, Yamamura Y, Ida M, Kawaguchi M. Correlation between pain catastrophizing in acute herpes zoster and postherpetic neuralgia: a retrospective analysis. J Anesth. 2023;37(4):589–595. doi:10.1007/s00540-023-03208-1
- Liang X, Fan Y. Bidirectional two-sample Mendelian randomization analysis reveals a causal effect of interleukin-18 levels on postherpetic neuralgia risk. Front Immunol. 2023;14:1183378. doi:10.3389/fimmu.2023.1183378
- Sun H, Yu Z. Effect of extracorporeal shock wave combined with pregabalin on patients with post-herpetic neuralgia. Medicine. 2023;102(30):e34361. doi:10.1097/MD.0000000000034361
- Xie S, Chen X. Red blood cell distribution width-to-platelet ratio as a disease activity-associated factor in systemic lupus erythematosus. Medicine. 2018;97(39):e12342. doi:10.1097/MD.0000000000012342
- Büyükaslan H, Asoğlu M. Evaluation of mean platelet volume, red cell distributed width and neutrophil to lymphocyte ratio in conversion disorder. Neuropsychiatr Dis Treat. 2019;15:2879–2884. doi:10.2147/NDT.S214392
- Rajakariar R, Lawrence T, Bystrom J, et al. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111(8):4184–4192. doi:10.1182/blood-2007-08-108936
- H-R A, Bai X-J, Liang J-Q, et al. The relationship between absolute counts of lymphocyte subsets and clinical features in patients with pulmonary tuberculosis. Clin Respir J. 2022;16(5):369–379. doi:10.1111/crj.13490
- Zhang L, Mao Z, Lai Y, Wan T, Zhang K, Zhou B. A review of the research progress in T-lymphocyte immunity and cervical cancer. Transl Cancer Res. 2020;9(3):2026–2036. doi:10.21037/tcr.2020.01.33
- Asada H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: the SHEZ study. Vaccine. 2019;37(44):6776–6781. doi:10.1016/j.vaccine.2019.09.031
- Yue J, Yao M. Humoral cytokine levels in patients with herpes zoster: a meta-analysis. J Pain Res. 2024;17:887–902. doi:10.2147/JPR.S449211
- Yuan Y, Zhang Y, Wang J, Liu H, Zhang H, Yan Y. Immune changes and their relationship with prognosis in patients with varicella-zoster virus encephalitis/meningitis. Am J Transl Res. 2023;15(2):1421–1429.
- Sei JJ, Cox KS, Dubey SA, et al. Effector and Central Memory Poly-Functional CD4(+) and CD8(+) T Cells are Boosted upon ZOSTAVAX(®) Vaccination. Front Immunol. 2015;6:553. doi:10.3389/fimmu.2015.00553
- Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(7):1321–1324. doi:10.1161/01.ATV.0000166521.90532.44
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi:10.1182/blood-2014-08-531582
- Torcel-Pagnon L, Bricout H, Bertrand I, et al. Impact of underlying conditions on zoster-related pain and on quality of life following zoster. J Gerontol a Biol Sci Med Sci. 2017;72(8):1091–1097. doi:10.1093/gerona/glw189