Abstract
Purpose
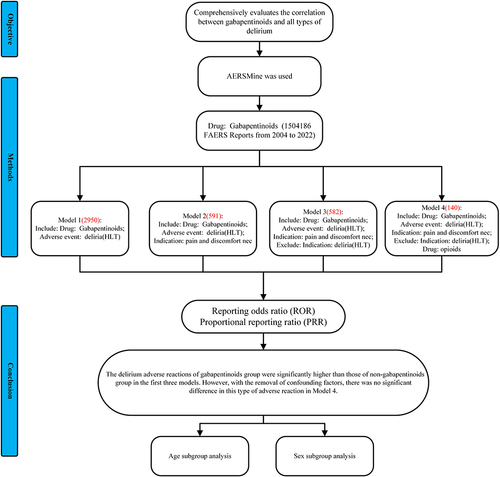
This study comprehensively describes and evaluates the correlation between gabapentinoids and all types of delirium.
Methods
We used AERSMine to select all adverse reaction data from 2004 Q1 to the 2022 Q4 in the FDA Adverse Event Reporting System (FAERS) database, and delirium events reported by gabapentinoids drugs were included in this study. Collected and analyzed the clinical details of these reports. We have developed four models. Among the four models, reporting odds ratio (ROR) and proportional reporting ratio (PRR) were used to evaluate the potential association between and delirium. We undertook a subgroup analysis for the age and sex cohorts.
Results
A total of 2950 reports of gabapentinoids-related delirium was collected. Excluding cases with a history of delirium (Model 2), opioid drugs (Model 3), and other adverse events related to gabapentinoids drugs (Model 4), pain cases with gabapentin drugs as the main suspected drug were selected. In model 1, the reporting rates of delirium at the delirium and delirium tremens levels were higher in the gabapentinoids group than in the non-gabapentinoids group (ROR 1.09(1.05,1.13); ROR 1.54(1.16,2.04)). In model 2.3 the delira and the delirium level were higher in the gabapentinoids group (ROR 1.42(1.29,1.56), ROR 1.44(1.31,1.59); ROR 1.43(1.30,1.58), ROR 1.46(1.33,1.61)). There is no difference in delirium levels in Model 4. Delirium levels were higher in the gabapentinoids group than in the non-gabapentinoids group in ≥65 years old. The delirium and deliria levels were higher in the male group than in the female group.
Conclusion
The delirium adverse reactions of the gabapentinoids group were significantly higher than those of non-gabapentinoids group in the first three models. However, with the removal of confounding factors, there was no significant difference in this type of adverse reaction in Model 4. In elderly and male patients, the incidence of delirium with gabapentinoids was significantly increased.
Introduction
Prescribed for neuropathic pain, epilepsy, and anxiety, gabapentinoids (pregabalin, gabapentin) are a class of medications with a long history of use.Citation1–3 Both gabapentinoids have received widespread prescriptions in the US since their initial introduction.Citation4 The American Pain Society recommends such drugs as a component of multimodal analgesia in the perioperative.Citation5 Due to the increasing use of it, the potential for negative impacts warrants worry. In 2022, Park et alCitation6 published in the JAMA Intern Med, conducted a retrospective cohort study and found that the use of gabapentin during surgery was linked to a slightly higher risk of delirium, considering the negative consequences of perioperative delirium, this finding raises concern about an increasingly adopted clinical practice.Citation6 To the best of our knowledge, the current clinical research is mainly focuses on whether they can reduce the incidence of postoperative delirium, rather than investigating whether it will cause delirium.Citation7,Citation8
Delirium is identified when there is a short-term, persistent disturbance of attention and awareness that differs from baseline and has a tendency to fluctuate.Citation9–11 The link between gabapentinoids and delirium is controversial, even Leung et al confirm that gabapentin could decrease postoperative delirium in older patients through clinical trial.Citation6,Citation12–14 Rare adverse events (AEs) may best be discovered through spontaneous reporting, and there is currently less empirical support for gabapentinoids’ potential to increase the incidence of delirium. It is important to highlight that meta-analysis depends mostly on data from published studies, which may not always provide a large enough dataset. However, millions of willingly submitted adverse event reports from consumers, manufacturers, healthcare professionals, and other stakeholders are housed in the FDA Adverse Event Reporting System (FAERS), a publicly available database in the United States. Its main objective is to make the FDA’s post-marketing safety surveillance of biological and pharmaceutical products easier. Therefore, adverse reaction database mining, which makes use of information from the FAERS database, is a great way to determine whether a medicine and an adverse event are related. Due to FAERS’s extensive and continuously updated repository, adverse reaction database mining grounded in FAERS can more accurately capture the dynamics of real-world research. In pharmacovigilance research, data mining in a large spontaneous adverse event reporting system database has become an essential method for doing medication safety evaluations.Citation15–17 We investigated the link between gabapentinoids and delirium by mining the FAERS database to respond to these questions and better comprehend the possible risk of all deliriums related to gabapentinoids.
Materials and Methods
Data Acquisition and Preprocessing
This project was approved by the Shanxi Bethune Hospital Committee (NO. YXLL-SL-2020-017). The FAERS database was used in this retrospective pharmacovigilance investigation. More than 19 million global case reports of possible medication side effects are available in this database. We used AERSMine,Citation18 a web-based analysis tool, to design the FAERS data from the 2004 Q1 to the 2022 Q4 on March 30, 2023.
We used the following neurological or psychiatric AEs terms to look for cases when gabapentinoids were prescribed and any of the AEs of interest were present. The primary outcome was delirium, identified using a validated claims-based algorithm.Citation19,Citation20 This algorithm consists of explicit (ie, delirium is directly mentioned) diagnosis codes for delirium. The FAERS database reports AEs from system organ class (SOC), high-level group terms (HLGT), high-level terms (HLT), and preferred terms (PT) according to the MedDRA (26.0).Citation21 We obtained reports of gabapentinoids-related deliria at the HLT level from AERSMine (eTables 1 and 2).
In four models, the reporting rate of gabapentinoid-related delirium was compared with different control drugs: Model 1: Other drugs excluding gabapentinoids (non-gabapentinoid drugs) without indication restrictions; Model 2: Non-gabapentinoid drugs in which pain is an indication, as pain is the risk factor of delirium;Citation22 Model 3: Exclude patients with a history of delirium before using gabapentinoids drugs based on model 2; Model 4: Number of reports of excluding combined use of opioid in all cases based on model 3.Citation23
Statistical Analysis
Disproportionality analysis was employed to discover relevant signals. The reporting odds ratio (ROR), proportional reporting ratio (PRR), and the Bayesian confidence propagation neural network (BCPNN) were used to calculate the degree of disproportionality,Citation24,Citation25 and the 95% CI for gabapentinoids-related deliriums was evaluated with various models.Citation26 We conducted a subgroup analysis of patients 65 and older, as well as those younger than 65. If the lower limit of the 95% CI was more than 1.0, the connection was judged statistically significant. Data analysis was performed using. The formula is as follows:
95% CI = e^(ln(ROR)±1.96
Note: a is the number of delirium AE records for gabapentinoids; b is the number of other AE for gabapentinoids; c is the number of delirium AE records for non-gabapentinoids; d is the number of other AE for non-gabapentinoids;
All data categorizations and statistics were performed using Microsoft Excel version 2023.
Results
After applying inclusion criteria, a total of 19,089,556 records (including 70,260 delirium reports) were evaluated ().
Gabapentinoids-Related Various Delirium in HLT, and PT in Model 1
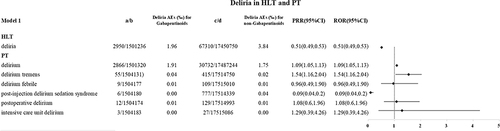
The FAERS files include 2950 gabapentinoids-related delirium reports from 2004 Q1 to 2022 Q4. In model 1, the reporting rates of delirium at the delirium, delirium tremens levels were higher in the gabapentinoids group than in the non-gabapentinoids group (1.91‰ vs 1.75‰, ROR 1.09(1.05,1.13) 0.04‰ vs 0.02‰, ROR 1.54(1.16,2.04)), see .
Gabapentinoids-Related Various Delirium in HLT, and PT in Model 2
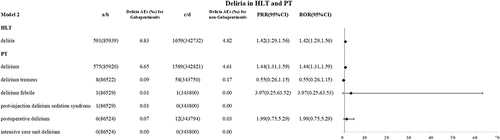
In model 2, 591 reports of delirium related to gabapentinoids-related drugs have been reported. The reporting rates of delira and the delirium level were higher in the gabapentinoids group (6.83‰ vs 4.82‰, ROR 1.42(1.29,1.56); 6.65‰ vs 4.61‰, ROR 1.44(1.31,1.59)), see .
Gabapentinoids-Related Various Delirium in HLT, and PT in Model 3
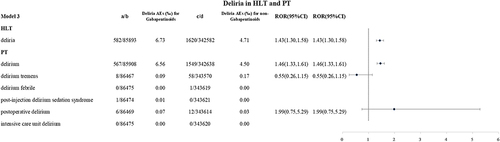
In model 3, 582 gabapentinoids-related delirium reports have been reported. The reporting rates of deliria and the delirium level were higher in the gabapentinoids group (6.73‰ vs 4.71‰, ROR 1.43(1.30,1.58); 6.56‰ vs 4.50‰, ROR 1.46(1.33,1.61)), see .
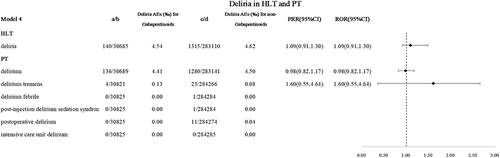
Gabapentinoids-Related Various Delirium in HLT, and PT in Model 4
In model 4, 140 gabapentinoids-related delirium reports have been reported. There is no difference in delirium levels between the gabapentinoids group and the non-gabapentinoid group, see .
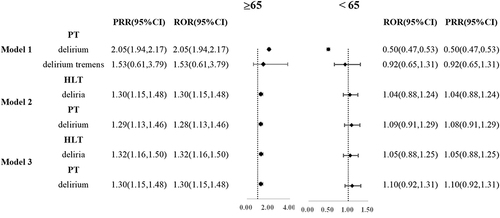
Delirium with Gabapentinoids Stratified by Age
We have added subgroup analysis on age (≥65 and <65) to the results of our study on four models ( and eTable 3). Among patients who model 1, 2 and 3, the delirium levels were higher in the gabapentinoids group than in the non-gabapentinoids group in ≥65 years old (ROR 2.04(1.93, 2.16); ROR 1.28(1.13, 1.46); ROR 1.32(1.16,1.50); ROR 1.30(1.15, 1.48)). There is no difference between gabapentinoids group and the non-gabapentinoids group in the <65 years old.
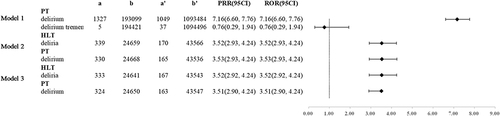
We performed a subgroup analysis by age of gabapentinoids patients on four models (). The results confirmed that among patients who model 1, 2 and 3, the delirium levels were higher in the ≥65 group than the <65 group () (ROR 7.16(6.60, 7.76); ROR 3.52(2.93, 4.24); ROR 3.53(2.93, 4.24); ROR 3.52(2.92, 4.24) ROR 3.51(2.90, 4.24)).
Figure 7 Delirium ROR and PRR occurred at different age in patients taking gabapentinoids.
Delirium with Gabapentinoids Stratified by Sex
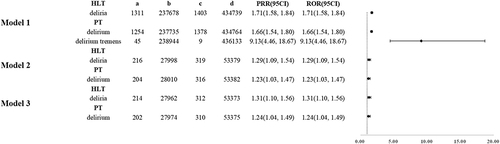
We have added stratified research on sex to the results of our study on four models (, eTable 4 and eFigure 1). For gabapentinoids patients, the results confirmed that among patients who model 1, 2 and 3, the delirium and deliria levels were higher in the male group than in the female group (ROR 1.71(1.58, 1.84); ROR 1.66(1.54, 1.80); ROR 9.13(4.46, 18.67); ROR 1.29(1.09, 1.54); ROR 1.23(1.03, 1.47); ROR 1.31(1.10, 1.56); ROR 1.24(1.04, 1.49)).
Figure 8 Delirium ROR and PRR occurred at different sex in patients taking gabapentinoids.
Discussion
The connection of gabapentinoids with all forms of delirium was investigated in the FAERS database by continuously removing influencing factors in four models, and a thorough pharmacovigilance analysis of delirium AEs was carried out at the HLT/PT levels. According to disproportionality studies, gabapentinoids are linked to delirium at the PT level in the first four models, and when we finally attempted to rule out the effects of opioids, there was no significant difference in delirium adverse reactions between the gabapentinoids group.
In the current clinical practice, anticonvulsants (such as sodium valproate and gabapentin) are often used to treat delirium, but there are limited data on their use.Citation27,Citation28 Previous researchers believe that gabapentin may prevent delirium by improving pain control and reducing opioid dose.Citation29 To date, several studies investigated whether gabapentin could reduce perioperative delirium. Pinto F W’s study found that gabapentin could reduce the occurrence of delirium in Analgesia in Oncologic Pediatric Patients.Citation12 In 2006, Leung et al confirmed that adding gabapentin to the treatment of postoperative pain decreased the incidence of postoperative delirium through the pilot research trial.Citation14 However, he proposed an opposite viewpoint with an RCT in 2017.Citation30 In a post-hoc analysis of an RCT of 161 patients (mean age, 63 years), Dighe et alCitation31 reported that the incidence of delirium in the gabapentin group and the placebo group were 12% and 9%. In another RCT of 697 patients (mean age, 73 years) undergoing orthopedic surgery, Leung et alCitation30 showed that 24.0% in the gabapentin group and 20.8% in the placebo group had delirium, although the difference in the delirium incidence was not statistically significant. More and more evidence has questioned the ability of gabapentin to reduce postoperative delirium, our study found that although the risk of delirium was not demonstrated in model 4, an increased risk of delirium was found in models 1, 2, and 3, and it can also provide evidence support for future research.
The 2-subunit of calcium channels, which are present in both the peripheral and central nervous systems, is the primary target of gabapentin’s action,Citation13,Citation32,Citation33 which may explain its delirium adverse effects. Delirium is associated with various underlying factors including preexisting diseases, metabolic or sleep disorders, psychotropic drug use, and altered sensory function.Citation34 This may explain the delirium adverse effects of gabapentinoids in models 1, 2, and 3 found in our study.
Our study indicates that there is no difference in ROR between gabapentinoids and non-gabapentinoids drugs when opioid drugs are excluded (model 4). Opioids are the most likely drugs to induce delirium and may have a significant confounding effect on reports of delirium response to gabapentinoids drugs in the real world.Citation35,Citation36
Research suggests that age and sex may be risk factors for delirium. Some studies reported that patients aged ≥65 years and men had a significantly increased incidence of delirium than those aged 40 to 65 years and women, respectively,Citation37,Citation38. Park et alCitation6 research subjects are elderly people, and older age is a common risk factor for delirium among rehabilitation inpatients.Citation39–41 Our results showed that the age difference in delirium adverse reactions between gabapentin group and non-gabapentin group was significant, but the gender difference was not significant. Therefore, we stratified the age and sex of the patients taking gabapentin, and the results showed that the incidence of gabapentinoids-induced delirium increases significantly in older and male patients. However, there is a large amount of data with unknown gender in the database, which also reduces the reliability of our study results (eTable 3).
In order to ensure the reliability of the results, we gradually designed four models. However, due to the complexity of post-operative medication, it is difficult for us to control unexpected bias, which will lead to the ambiguity of our results, so this is also part of the limitations of our study.
Limitations
FAERS cannot establish a causal relationship between gabapentinoids and delirium. The reporting habits may be impacted by recent publications of AEs in the literature and media attention. Comorbidities and concurrent medications confuse the link between a drug and an adverse event. FDA claims that the submitted information has not been examined by a medical expert. Manufacturers, consumers, and healthcare professionals may all submit FAERS data. A submission’s source must be taken into account. Incomplete or missing data can be found in FAERS. In other instances, the age was not provided or the medicine names were spelled incorrectly. Due to the inability to obtain the patient’s medication dose, it is impossible to rule out the bias in delirium caused by different medication doses. Not every adverse event or medication mistake involving a product is reported to FDA. Additionally, ROR only looks into a risk of AEs reporting that is elevated rather than a risk of AEs incidence in general.Citation42 Due to the small number of ROR reports, several confidence intervals, such as delirium febrile 3.97(0.25,63.53) in model 2, are quite large. The number of cases in Model 2–4 and the age and sex stratified analyses was small, and it may address the reliability of the conclusions. The FAERS database has the advantage of having a huge sample size, although there are some flaws, it is very important for discovering new and rare AEs.
Conclusion
Because gabapentinoids are so widely used, there are worries regarding safety, particularly in delirium. Our research offered fresh, practical proof of the safety of gabapentinoids for treating delirium. Using the FAERS database reveals that the reporting rates of delirium were higher in the gabapentinoids group than in the non-gabapentinoids group at the delirium levels in model 1,2,3, but no difference in delirium levels between the gabapentin group and the non-gabapentinoid group in the model 4. In elderly and male patients, the incidence of delirium with gabapentinoids was significantly increased. Given the advantages of the gabapentinoids group, the increased ROR does not suggest that doctors should limit the use of gabapentinoids, but rather that they should maintain the necessary level of awareness for any potential side effects.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Data Sharing Statement
This study analyzed publicly available datasets. These data can be found in the following locations: 1) https://research.cchmc.org/aers/explore.jsp; 2) https://fis.fda.gov/sense /app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-D58b-4203-a8aa-6d3021737452/state/analysis.
Additional information
Funding
References
- Di Nicola M, Martinotti G, Tedeschi D, et al. Pregabalin in outpatient detoxification of subjects with mild-to-moderate alcohol withdrawal syndrome. Hum Psychopharmacol. 2010;25(3):268–275. doi:10.1002/hup.1098
- Krupitsky EM, Rybakova KV, Skurat EP, Semenova NV, Neznanov NG. A double blind placebo controlled randomized clinical trial of the efficacy and safety of pregabalin in induction of remission in patients with alcohol dependence. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120(1):33–43. doi:10.17116/jnevro202012001133
- Tzellos TG, Papazisis G, Toulis KA, Sardeli C, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia. 2010;14(2):71–75.
- ClinCalk. Gabapentin Drug Usage Statistics. United States. Updated December 8, 2020. Available from: https://clincalc.com/DrugStats/Drugs/Gabapentin. Accessed July 30, 2024.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):268–275. doi:10.1002/hup.1098
- Park CM, Inouye SK, Marcantonio ER, et al. Perioperative gabapentin use and in-hospital adverse clinical events among older adults after major surgery. JAMA Intern Med. 2022;182(11):1117–1127. doi:10.1001/jamainternmed.2022.3680
- Ifuku M, Iseki M, Hidaka I, Morita Y, Komatus S, Inada E. Replacement of gabapentin with pregabalin in postherpetic neuralgia therapy. Pain Med. 2011;12(7):1112–1116. doi:10.1111/j.1526-4637.2011.01162.x
- Toth C. Substitution of gabapentin therapy with pregabalin therapy in neuropathic pain due to peripheral neuropathy. Pain Med. 2010;11(3):456–465. doi:10.1111/j.1526-4637.2009.00796.x
- Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. 2013;25(2):191–192. doi:10.1590/s2317-17822013000200017
- Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. doi:10.1056/NEJMoa1808217
- Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Pinto Filho WA, Silveira LHJ, Vale ML, Fernandes CR, Gomes JA. Gabapentin in improvement of procedural sedation and analgesia in oncologic pediatric patients: a clinical trial. Anesth Pain Med. 2019;9(5):e91197. doi:10.5812/aapm.91197
- Gupta A, Joshi P, Bhattacharya G, et al. Is there evidence for using anticonvulsants in the prevention and/or treatment of delirium among older adults? Int Psychogeriatr. 2022;34(10):889–903. doi:10.1017/S1041610221000235
- Leung JM, Sands LP, Rico M, et al. Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology. 2006;67(7):1251–1253. doi:10.1212/01.wnl.0000233831.87781.a9
- Chen C, Wu B, Zhang C, Xu T. Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int Immunopharmacol. 2021;95:107498. doi:10.1016/j.intimp.2021.107498
- Zhang J, Guo Q, Zhang R, Wei M, Nie Z, Raza F. Gender differences in adverse events of ketamine drugs: a real-world study based on FAERS. Journal of Clinical Pharmacy and Therapeutics. 2024;2024(1):4898082. doi:10.1155/2024/4898082
- Chen Y, Fan Q, Liu Y, Shi Y, Luo H. Cardiovascular toxicity induced by SSRIs: analysis of spontaneous reports submitted to FAERS. Psychiatry Res. 2023;326:115300. doi:10.1016/j.psychres.2023.115300
- Sarangdhar M, Tabar S, Schmidt C, et al. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat Biotechnol. 2016;34(7):697–700. doi:10.1038/nbt.3623
- Bui LN, Pham VP, Shirkey BA, Swan JT. Effect of delirium motoric subtypes on administrative documentation of delirium in the surgical intensive care unit. J Clin Monit Comput. 2017;31(3):631–640. doi:10.1007/s10877-016-9873-1
- Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945–953. doi:10.1002/pds.4226
- Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–117. doi:10.2165/00002018-199920020-00002
- Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102(4):1267–1273. doi:10.1213/01.ane.0000199156.59226.af
- Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi:10.1097/TA.0b013e31814b2c4d
- Ang PS, Chen Z, Chan CL, Tai BC. Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin Drug Saf. 2016;15(5):583–590. doi:10.1517/14740338.2016.1167184
- Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–486. doi:10.1002/pds.677
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. doi:10.5812/aapm.91197
- Bourgeois JA, Koike AK, Simmons JE, Telles S, Eggleston C. Adjunctive valproic acid for delirium and/or agitation on a consultation-liaison service: a report of six cases. J Neuropsychiatry Clin Neurosci. Spring. 2005;17(2):232–238. doi:10.1176/jnp.17.2.232
- Burry L, Hutton B, Williamson DR, et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9(9):CD011749. doi:10.1002/14651858.CD011749.pub2
- Hughes CG, Boncyk CS, Culley DJ, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg. 2020;130(6):1572–1590. doi:10.1213/ANE.0000000000004641
- Leung JM, Sands LP, Chen N, et al. perioperative gabapentin does not reduce postoperative delirium in older surgical patients: A randomized clinical trial. Anesthesiology. 2017;127(4):633–644. doi:10.1097/ALN.0000000000001804
- Dighe K, Clarke H, McCartney CJ, Wong CL. Perioperative gabapentin and delirium following total knee arthroplasty: a post-hoc analysis of a double-blind randomized placebo-controlled trial. Can J Anaesth. 2014;61(12):1136–1137. doi:10.1007/s12630-014-0235-5
- Fuzier R, Serres I, Guitton E, Lapeyre-Mestre M, Montastruc JL. French network of pharmacovigilance c. adverse drug reactions to gabapentin and pregabalin: a review of the French pharmacovigilance database. Drug Saf. 2013;36(1):55–62. doi:10.1007/s40264-012-0006-6
- Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805–1815. doi:10.1213/01.ane.0000287643.13410.5e
- Stollings JL, Kotfis K, Chanques G, Pun BT, Pandharipande PP, Ely EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–1103. doi:10.1007/s00134-021-06503-1
- Tuma R, DeAngelis LM. Altered mental status in patients with cancer. Arch Neurol. 2000;57(12):1727–1731. doi:10.1001/archneur.57.12.1727
- Connolly KP, Kleinman RS, Stevenson KL, Neuman MD, Mehta SN. Delirium Reduced With Intravenous Acetaminophen in Geriatric Hip Fracture Patients. J Am Acad Orthop Surg. 2020;28(8):325–331. doi:10.5435/JAAOS-D-17-00925
- He M, Zhu Z, Jiang M, et al. Risk Factors for Postanesthetic Emergence Delirium in Adults: a Systematic Review and Meta-analysis. J Neurosurg Anesthesiol. 2023;36:190–200. doi:10.1097/ANA.0000000000000942
- Kang X, Lin K, Tang H, et al. Risk Factors for Emergence Agitation in Adults Undergoing Thoracoscopic Lung Surgery: a Case-Control Study of 1950 Patients. J Cardiothorac Vasc Anesth. 2020;34(9):2403–2409. doi:10.1053/j.jvca.2020.02.046
- Bushi S, Barrett AM, Oh-Park M. Inpatient Rehabilitation Delirium Screening: impact on Acute Care Transfers and Functional Outcomes. PM R. 2020;12(8):766–774. doi:10.1002/pmrj.12304
- Heyman N, Nili F, Shahory R, Seleznev I, Ben Natan M. Prevalence of delirium in geriatric rehabilitation in Israel and its influence on rehabilitation outcomes in patients with Hip fractures. Int J Rehabil Res. 2015;38(3):233–237. doi:10.1097/MRR.0000000000000121
- Gual N, Morandi A, Perez LM, et al. Risk Factors and Outcomes of Delirium in Older Patients Admitted to Postacute Care with and without Dementia. Dement Geriatr Cogn Disord. 2018;45(1–2):121–129. doi:10.1159/000485794
- Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72(6):905–908. doi:10.1111/j.1365-2125.2011.04037.x