Abstract
Low back pain in patients with myofascial pain syndrome is characterized by painful active myofascial trigger points (ATPs) in muscles. This article reviews a novel, noninvasive modality that combines simultaneous imaging and treatment, thus taking advantage of the electrodermal information available from imaged ATPs to deliver localized neurostimulation, to stimulate peripheral nerve endings (Aδ fibers) and in turn, to release endogenous endorphins. “Hyperstimulation analgesia” with localized, intense, low-rate electrical pulses applied to painful ATPs was found to be effective in 95% patients with chronic nonspecific low back pain, in a clinical validation study.
Keywords:
Introduction
Low back pain (LBP) is one of the most common complaints in the Western society.Citation1,Citation2 Ninety percent of the population in the United States suffer from low back pain at one or multiple points in time in their lifetime.Citation2
Although LBP is a common chronic pain syndrome, in most cases a specific diagnosis cannot be established. It can arise due to spinal injury spinal disc problems, osteoarthritis, spinal stenosis, compression fractures, spinal tumors, etc.Citation3
Nonsteroidal anti-inflammatory drugs (NSAIDS) are generally used for acute LBP. Opioids are the alkaloid analgesics used for the treatment of moderate to severe pain syndromes. These opioids work on the µ, κ, and α receptors in the central nervous system;Citation4 however, due to the wide presence of these receptors in the body, opioids not only suppress the noxious stimuli effects, but also have undesirable side effects.Citation5,Citation6 The concerns arising from the use of analgesic medications have increased the interest in nonpharmacological therapies for LBP. Nonpharmacological treatment modalities for pain relief include heat, cold acupuncture, electrotherapy, and massage.Citation7 The use of cold and hot methods has been shown to cause tissue and nerve injury.Citation8–Citation10 Among the electrotherapy modalities are transcutaneous electrical nerve stimulation (TENS) with various pulse modulation, electroacupuncture, and percutaneous electrical nerve stimulation (PENS).Citation11–Citation13 Unfortunately, these modalities go amiss with respect to their provided duration and magnitude of analgesia. Study of TENS has produced results with limited statistical significance,Citation14 and the American Academy of Neurology has advised against the use of TENS in chronic LBP, stating that the strongest evidence indicates that it is ineffective for this syndrome.Citation15 One accepted explanation for LBP symptoms is that patients have myofascial pain syndrome, a condition characterized by painful active myofascial trigger points (ATPs) in muscles.Citation16,Citation17
ATP pathophysiology
In the last 10–15 years, much clinical and basic science research into ATPs has been published, including epidemiological, diagnostic, therapeutic, and pathophysiological studies.Citation17–Citation21 The pathogenesis of ATPs is probably related to sensitized sensory peripheral free nerve endings (nociceptors) associated with dysfunctional endplates.Citation16 In a histological study, small nerve fibers were commonly found near the sensitive ATPs.Citation22 Therefore, the sensitive loci in the region of muscle ATPs are probably related to sensitized nerve fibers (nociceptors).
Local pain could be explained by the tissue ischemia resulting from prolonged muscle contraction, with accumulation of acids and chemicals such as serotonin, histamine, kinins, and prostaglandins.Citation23
Studies have suggested that the development of ATPs is dependent on an integrative mechanism in the spinal cord. When the input from the nociceptors in an original receptive field (pain from ATPs) persists, central sensitization in the spinal cord may develop, and the receptive field corresponding to the original dorsal horn neuron may be expanded (referred pain). Through this mechanism, new “satellite ATPs” may develop in the referred zone of the original ATPs.Citation16
“Hyperstimulation analgesia” of ATPs
Common treatments of ATPs typically include minimally invasive intervention, such as injections with local anesthetics, corticosteroids, botulinum toxin, or dry needling.Citation24 Serious complications, although of rare occurrence, have been reported (eg, pneumothorax, hematoma, intravascular injection of local anesthetics, and intrathecal injections).Citation25
“Hyperstimulation analgesia” is an alternative modality, in which localized, intense, low-rate electrical pulses are applied to small surface areas at ATP locations to stimulate peripheral nerve endings (Aδ fibers), thus causing the release of endogenous endorphins.Citation26,Citation27 Hyperstimulation anesthesia has been investigated in several controlled studies, showing a positive response in 87% of patients.Citation26,Citation28–Citation30 Considerable evidence suggests that this type of neurostimulation analgesia is achieved through the activation of extra-segmental antinociceptive mechanisms, which accelerate the release of endogenous endorphins, serotonin, and cortisol.Citation27,Citation31–Citation34
Identification of ATPs
While the most common physical finding of ATPs has been considered the palpation of a hypersensitive nodule of muscle fiber of harder-than-normal consistency the identification of such nodule appears to be very dependent on the subjective experience of the physician. There is no accepted reference standard for the clinical diagnosis of ATPs, and data on the reliability of physical examination are conflicting, and a 2009 review of nine studies examining the reliability of ATP diagnosis found that physical examination could not be recommended as reliable for the diagnosis of ATPs.Citation24 Attempts to confirm the presence of myofascial trigger points using magnetic resonance elastography have been described.Citation35 Recently, Sikdar et al have tried to use ultrasound to visualize and characterize ATPs.Citation36 They found that ATPs appeared as focal, hypoechoic regions of elliptical shape, with a size of 0.16 cm.Citation36
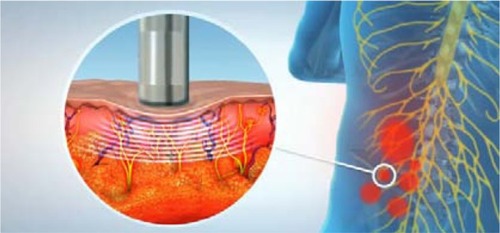
The presence of ATPs causes a localized decrease in skin resistance compared with the surrounding area.Citation37,Citation38 The hypoxic state in the pain area increases nociceptors and other sensitizing substances in the area, and this biochemical change induces greater blood flow and secretion from sweat glands, via stimulation of the autonomic nervous system.Citation39 These physiologic differences may account for acute variations in electrodermal measurements at the pathologic site. ATPs are defined as small-diameter (3–4 mm), circumscribed low-skin-resistance areas.Citation38 Localized decrease in skin resistance is frequently associated with clinical ATPs that are richly innervated by myelinated Aδ fibersCitation40,Citation41 the smallest in diameter (0.2–1.5 μm) and most commonly present myelinated axons in peripheral nerves. Their extremely small size prevents their identification by any imaging modalityCitation38
Electrical skin impedance measurements are considered to be vulnerable to certain sources of imprecision, including instrument error resulting from the size, pressure, and the duration of probe application as well as from local skin conditions, such as variable thickness, hydration, and integrity of the stratum corneum.Citation38,Citation42
Auto-targeting hyperstimulation of painful ATPs
The hyperstimulation analgesia procedure is not extensively utilized in the clinical setting due to the necessity of locating appropriate ATPs. This necessity requires previous knowledge of the potential locations and the identification of ATPs associated with LBP and makes such treatments time consuming and cumbersome.Citation26–Citation28 Some devices offer the capability of measuring skin impedance for the location of ATPs, with the aim of applying hyperstimulation to them;Citation43 however these are manually held devices that prolong the procedure, by firstly allowing application of hyperstimulation to a single point and secondly, by offering limited accuracy (due to their measurement of a single point at a time) that does not take advantage of the electrodermal information of the entire region of interest.
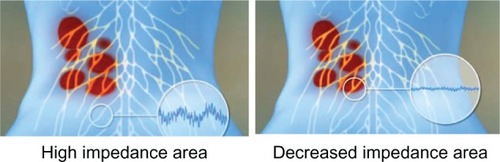
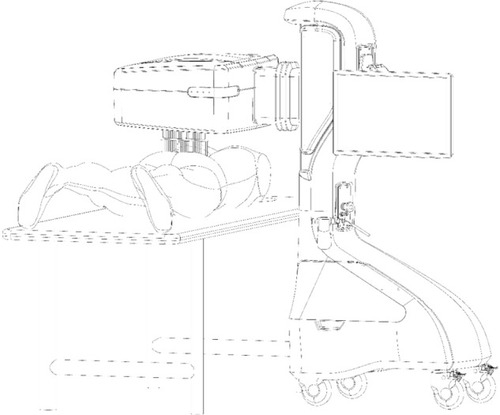
A novel automated robotic system, the Soleve™ (Nervomatrix Ltd, Netanya, Israel) (), utilizes an array of miniature probes (), allowing the measurement of skin impedance over the back at 1000 points in less than a couple of minutes.Citation44 The system visualizes and analyzes the data to locate areas of low impedance compared with surrounding areas, thus indicating ATPs appropriate for hyperstimulation (). Therapeutic neurostimulation, using modulated, intense electrical pulses, is then applied locally to specific painful ATPs, providing highly effective pain relief by stimulating the release of endorphins.Citation31–Citation34
Figure 1 A novel automated robotic system, the Soleve™ (Nervomatrix Ltd, Netanya, Israel).
Clinical validation
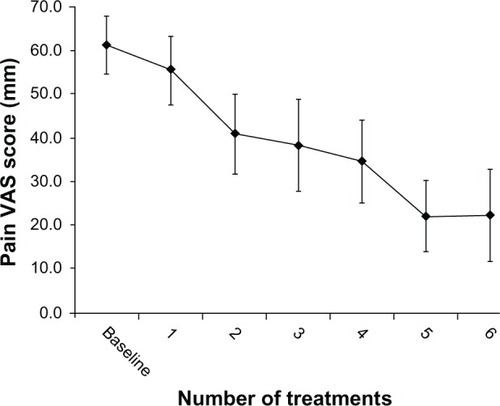
The effectiveness of the Soleve™ system was investigated in 19 patients diagnosed with nonspecific chronic LBP.Citation44 Fifteen of the patients were female (79%), and four were men (21%), with a mean age of 52.1 ± 10.8 years. The protocol consisted of six treatment sessions, 2–4 days apart. Each session included a <1-minute, automatic impedance screening, followed by a 20-minute treatment. The primary outcome measurement consisted of changes in pain intensity, as measured on a 100 mm-long pain visual analog scale (VAS) obtained at enrollment, pre-, and 2 hours posttreatment. The mean ± standard deviation (SD) baseline VAS score for all patients was 61 ± 14 mm (). Following treatment, VAS scores decreased by 39 ± 17 mm (P < 0.001) compared with the baseline scores. Notably, the VAS scores of all but one patient decreased by more than 20 mm after the fourth treatment, representing a marked improvement in 95% of enrolled patients.
Figure 4 Pain VAS scores.
Abbreviations: SD, standard deviation; VAS, visual analog scale.
Conclusion
A novel, noninvasive, image-guided, targeted neurostimulation modality that combines impedance imaging to locate ATPs and treatment based on the image analysis was found very effective clinically in 95% of patients after a series of four treatments. This promising result warrants future investigation and randomized, controlled, longitudinal studies in the treatment of LBP.
Acknowledgment
This paper was supported by a grant from Nervomatrix, Israel.
Disclosure
Dr Gorenberg is a stockholder at Nervomatrix, Israel. The authors report no other conflicts of interest in this work.
References
- KatzRTImpairment and disability rating in low back painPhys Med Rehabil Clin N Am200112368169411478198
- SteenstraIAAnemaJRvan TulderMWBongersPMde VetHCvan MechelenWEconomic evaluation of a multi-stage return to work program for workers on sick-leave due to low back painJ Occup Rehabil200616455757817086503
- AtlasSJDeyoRAEvaluating and managing acute low back pain in the primary care SettingJ Gen Intern Med200116212013111251764
- PappagalloMAggressive pharmacologic treatment of painRheum Dis Clin North Am199925119321310083964
- RainsfordKDProfile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs)Am J Med19991076A27S35S10628591
- FurlanADSandovalJAMailis-GagnonATunksEOpioids for chronic noncancer pain: a meta-analysis of effectiveness and side effectsCMAJ2006174111589159416717269
- GodfreyHUnderstanding pain, part 2: pain managementBr J Nurs2005141790490916224328
- McLeanDAThe use of cold and superficial heat in the treatment of soft tissue injuriesBr J Sports Med198923153542731001
- OchoaJLYarnitskyDThe triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve diseaseBrain1994117Pt 11851978149211
- SlukaKWalshDTranscutaneous electrical nerve stimulation: basic science mechanisms and clinical effectivenessJ Pain20034310912114622708
- GhonameEACraigWFWhitePFPercutaneous electrical nerve stimulation for low back pain: a randomized crossover studyJAMA199928981882310071003
- GhonameESCraigWFWhitePFThe effect of stimulus frequency on the analgesic response to percutaneous electrical nerve stimulation in patients with chronic low back painAnesth Analg199988484184610195535
- HamzaMAGhonameEAWhitePFEffect of the duration of electrical stimulation on the analgesic response in patients with low back painAnesthesiology19999161622162710598602
- KhadilkarAOdebiyiDOBrosseauLWellsGATranscutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain [review]Cochrane Database Syst Rev200884CD00300818843638
- SimonsDGTravellJGMyofascial origins of low back pain. 1. Principles of diagnosis and treatmentPostgrad Med1983732666870736218489
- SimonsDGTravellJGMyofascial origins of low back pain. Principles of diagnosis and treatmentPostgrad Med1983732666870736218489
- SimonsDGHongCZSimonsLSEndplate potentials are common to midfiber myofacial trigger pointsAm J Phys Med Rehabil200281321222211989519
- SimonsDGTravellJGSimonsLSTravell and Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual Vol. 12nd edBaltimore, MDWilliams & Wilkins1999
- SimonsDGTravellJGSimonsLSTravell & Simon`s Myofascial Pain and Dysfunction: The trigger point manual12nd edBaltimoreWilliams & Wilkins1999
- SimonsDGMyofascial trigger points: the critical experimentJ Musculoskelet Pain199754111118
- SimonsDGDiagnostic criteria of myofascial pain due to trigger pointsJ Musculoskelet Pain199971/2111120
- HongCZChenJTChenSMYanJJSuYJHistological findings of responsive loci in a myofascial trigger spot of rabbit skeletal muscle from where localized twitch responses could be elicitedArch Phys Med Rehabil199677962
- TravellJRinzlerSHThe myofascial genesis of painPostgrad Med195211542543414920327
- LucasNMacaskillPIrwigLMoranRBogdukNReliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literatureClin J Pain2009251808919158550
- NelsonLSHoffmanRSIntrathecal injection: unusual complication of trigger-point injection therapyAnn Emerg Med19983245065089774938
- FlowerdewMWGadsbyJGA review of the treatment of chronic low back pain with acupuncture-like transcutaneous electrical nerve stimulation and transcutaneous electrical nerve stimulationComplementary Therapies in Medicine199754193201
- SjölundBTereniusLErikssonMIncreased cerebrospinal fluid levels of endorphins after electro-acupunctureActa Physiol Scand19771003382384920207
- ChengRPomeranzBElectrotherapy of chronic musculoskeletal pain: comparison of electroacupuncture and acupuncture-like transcutaneous electrical nerve stimulationClin J Pain198623143150
- ChengRPomeranzBElectroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systemLife Sci1979252319571962160969
- ChengRMcKibbinLRoyBPomeranzBElectroacupuncture elevates blood cortisol levels in naive horses; sham treatment has no effectInt J Neurosci1980102–395976245041
- BenderTNagyGBarmaIThe effect of physical therapy on beta-endorphin levelsEuropean Journal of Applied Physiology Volume2007100437138
- SjölundBHErikssonMBEEndorphins and analgesia produced by peripheral conditioning stimulationAdv Pain Res Ther19793587592
- SjölundBErikssonMElectro-acupuncture and endogenous endorphinsLancet19763087994108562925
- HughesGSJrLichsteinPRWhitlockDHarkerCResponse of plasma beta-endorphins to transcutaneous electric nerve stimulation in healthy subjectsPhys Ther1984647106210666330773
- BasfordJRAnKNNew techniques for the quantification of fibromyalgia and myofascial painCurr Pain Headache Rep200913537637819728964
- SikdarSShahJPGebreabTNovel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissueArch Phys Med Rehabil200990111829183819887205
- DommerholtJBronCFranssenJMyofascial trigger points: an evidence-informed reviewJ Man Manip Ther2006144203221
- ShultzSPDribanJBSwanikCBThe evaluation of electrodermal properties in the identification of myofascial trigger pointsArch Phys Med Rehabil200788678078417532902
- SimonsDGMenseSUnderstanding and measurement of muscle tone as related to clinical muscle painPain19987511179539669
- DommerholtJDry needling in orthopedic physical therapy practiceOrthopaedic Practice20041631520
- NakataniYA Guide for the Application of Ryodoraku Autonomous Nerve Regulatory TherapyAlhambra, CAChan’s Books and Products1972
- InuiKTranTDHoshiyamaMKakigiRPreferential stimulation of Adelta fibers by intra-epidermal needle electrode in humansPain200296324725211972996
- ColbertAHammerschlagRAickinMMcNamesJReliability of the Prognos electrodermal device for measurements of electrical skin resistance at acupuncture pointsJ Altern Complement Med200410461061615353016
- GorenbergMSchiffESchwartzKEizenbergEA novel image-guided, automatic, high-intensity neurostimulation device for the treatment of nonspecific low back painPain Res Treat2011201115230722110920