Abstract
Purpose
The treatment of central neuropathic pain remains amongst the biggest challenges for pain specialists. The main objective of this study was to assess gabapentin (GBP), amitriptyline (AMI), and carbamazepine (CARBA) for the treatment of a rodent central neuropathic pain model.
Methods
Male Sprague Dawley rats were trained on the rotarod, Hargreaves, Von Frey and acetone behavioral tests, and baseline values were obtained prior to surgery. A stereotaxic injection of either a collagenase solution or saline was made in the right ventral posterolateral thalamic nucleus. The rats were tested on days 2, 4, 8, and 11 postsurgery. They were retested at regular intervals from day 15 to day 25 postsurgery, after oral administration of either the vehicle (n=7 and n=8 rats with intracerebral injections of collagenase and saline, respectively) or the different drugs (GBP [60 mg/kg], AMI [10 mg/kg], CARBA [100 mg/kg]; n=8 rats/drug).
Results
A significant decrease in the mechanical thresholds and no change in heat threshold were observed in both hind limbs in the collagenase group, as we had previously shown elsewhere. Reversal of the mechanical hypersensitivity was achieved only with GBP (P<0.05). AMI and CARBA, at the dosages used, failed to show any effect on mechanical thresholds. Transient cold allodynia was observed in some collagenase-injected rats but failed to be statistically significant.
Conclusion
Intrathalamic hemorrhaging in the ventrolateral thalamic nucleus induced a bilateral mechanical allodynia, which was reversed by GBP but not AMI or CARBA.
Introduction
Anticonvulsants and antidepressants are commonly used for the treatment of chronic neuropathic pain.Citation1 Among the various types of chronic neuropathic pain, central poststroke pain (CPSP) is a condition caused by a vascular lesion in the central nervous system, involving the spinothalamocortical pathway responsible for the transmission of thermal and pain sensation.Citation2 According to population-based studies, the incidence of CPSP varies around 10% but may be higher following thalamic lesions, particularly if right sided.Citation3–Citation6 The ventroposterior nuclei of the thalamus are reported to play a key role in central pain.Citation7,Citation8
CPSP is characterized by sensory deficits, such as allodynia to thermal or mechanical stimuli, dysesthesia, and hyperalgesia.Citation5,Citation9 The pain can be spontaneous or evoked by stimuli, such as thermal stimuli (especially cold); mechanical stimuli, such as touch or movement, or even by stress.Citation10,Citation11 The development of pain in stroke patients is of great concern since it lessens their quality of life by interfering with daily activities, mood, walking ability, normal work, social interaction, sleep, and enjoyment of life, eventually leading to depression.Citation12,Citation13 Therefore, the diagnosis and management of CPSP represents a major concern and a real medical challenge.
In terms of treatment recommendations, the use of tricyclic antidepressants, such as amitriptyline (AMI), and anticonvulsants, such as carbamazepine (CARBA) or gabapentin (GBP), are often the first steps of the therapeutic plan to treat CPSP.Citation14 AMI has been used for the treatment of diabetic neuropathy, cancer neuropathic pain, and thalamic pain.Citation15–Citation17 GBP was first used for the treatment of epilepsy;Citation18 however, more recently, it has shown to be effective in the treatment of neuropathic pain of either peripheral or central origin.Citation18,Citation19 CARBA has inhibitory effects on spontaneous spinal neural activity as well as on noxious and nonnoxious evoked responses, in a spinal nerve ligation model.Citation20 Spontaneous activity, characterized by low-threshold calcium burst spikes within the thalamus, has been reported in patients with central pain.Citation21,Citation22 Thus, there is a rationale that CARBA might also decrease central pain in a rodent model. AMI, CARBA, and GBP are therefore, proposed therapeutic strategies for the treatment of central thalamic pain.
Until recently, the lack of a reliable animal model has prevented a true understanding of the underlying mechanisms of CPSP. In 2009, Wasserman and KoeberleCitation23 produced a model of this condition by injecting a collagenase solution within the ventroposterolateral (VPL) thalamic nucleus of rats. The collagenase acts by disrupting the blood vessels, thus inducing a well-localized hemorrhage, with the extent of the lesion depending on the amount of collagenase injected.Citation24 In a recent study from our lab, a collagenase injection within the right VPL thalamic nucleus of rats produced persistent bilateral mechanical allodynia, which developed from the second day after the injection, and transient cold allodynia.Citation25 Animal models can help to further understand the mechanisms involved in CPSP and allow experimentation with different therapeutic approaches for this condition. The main objective of the present study was to evaluate the effects of AMI, GBP, and CARBA on central neuropathic pain, using a rodent model of intrathalamic collagenase-induced hemorrhage.
Materials and methods
Animals
Forty Sprague Dawley® rats (Charles River, Wilmington, MA, USA), between 7 and 8 weeks of age (body weight: 300–350 g), were used for this study. They were housed in a standard research environment (temperature: 21°C±3°C; fresh filtered air [15 changes/h]; humidity 40%–60%; light–dark cycle [12 hours:12 hours]). The rats were pair-housed in polycarbonate cages (Ancare Corp, Bellmore, NY, USA) with hardwood bedding (Beta Chip®, Northeastern Products Corp, Warrensburg, NY, USA) and acclimated for 7 days prior to the study. The animals were fed a rodent chow (Rodent Chow 5075; Charles River) and received tap water, both, ad libitum. The Animal Care and Use Committee of the University of Montreal Faculty of Veterinary Medicine approved the protocol prior to animal use. All procedures were conducted in accordance with the guidelines of the Canadian Council on Animal Care.
Surgical techniques
The rats were divided in two groups (n=8 in the sham [SHAM] group; n=32 in the collagenase group).The surgical details have previously been described by Wasserman and Koeberle.Citation23 The animals were anesthetized using vaporized isoflurane (3%) (AErrane; Baxter Healthcare Corp, Deerfield, IL, USA) in oxygen. A regulated heating blanket was used to keep the temperature within normal limits (36°C–37°C). Body temperature was monitored using a rectal probe (TH-8 Thermalert Monitoring Thermometer; Physitemp, Clifton, NJ, USA). The skin hair of the skull was clipped and cleaned with a Proviodine® solution. A sagittal skin incision was made from the frontal to the occipital bone, and the periosteum was gently detached. The area of interest (3.5 mm anterior and posterior, and 3.5 mm lateral to the bregma)Citation26 was localized using a stereotaxic apparatus (Small Animal Stereotaxic Instrument; David Kopf Instruments, Tujunga, CA, USA). A 1.5 mm burr hole (diameter 1.5 mm) was made using a stereotaxic drill, (Foredorn flex shaft drill, Stoelting Co., Wood Dale, IL, USA) and injections were done using a Hamilton syringe (Hamilton Company, Reno, NV, USA). The collagenase group received 0.25 μL of a solution of 0.025 UI of collagenase Type IV (Sigma-Aldrich Corp, St Louis, MO, USA) in sterile saline. The syringe was lowered (6 mm ventral) so that the injection site would be within the right ventral posterolateral thalamic nucleus. Similar surgical and injection procedures were performed in “SHAM” animals receiving an equal volume of sterile saline (0.9% NaCl). The total injection time was 2 minutes, and the needle was left in place another 5 minutes to prevent any reflux. Following the injection, the skin was closed using simple discontinuous Monocryl 4.0 sutures (Ethicon Inc., Cincinnati, OH, USA). The animals were caged singly for the duration of the study.
One animal died of isoflurane overdose during surgery, leaving 31 animals in the collagenase group.
Behavioral study
The animals were trained daily for 1 week prior to the beginning of the behavioral evaluation, in the following sequential order: rotarod treadmill, Von Frey filaments, acetone and Hargreaves tests. Baseline values were obtained 3 days prior to surgery. After surgery, the animals were given 1 day to recover and were then tested on days 2, 4, 8, and 11 postsurgery to evaluate their temperature and mechanical threshold as well as motor coordination. They were tested again on day 15, 18, 22, and 25 following the treatment administration. The tests were always performed in the morning (8–11 am), to avoid circadian variations. All the rats were acclimated to the room for 15 minutes and then in each apparatus for 15 minutes prior to testing (except in the case of the rotarod evaluation).
Rotarod test
Motor coordination was evaluated with the rotarod treadmill (Standard Rota-Rod for Rat ENV-576; Med Associates Inc., St Albans, VT, USA). The acceleration was set from 5 to 35 rpm over 5 minutes. The maximal time the animal was able to stay on the rotarod (up to a maximum of 5 minutes) without falling was recorded for each performance.
Von Frey mechanical sensitivity test
The mechanical threshold was evaluated with Von Frey filaments (Stoelting Co, Wood Dale, IL, USA) using the up–down paradigm.Citation27 The rats were placed in Plexiglas® chambers (Plantar Analgesic meter, IITC Life Science Inc., Woodland Hills, CA, USA) set on a customized platform made of wire mesh. The Von Frey filaments, ranging from 4 to 22 g, were applied, for up to 3 seconds, to the plantar surface of the rats’ hind paws. To avoid the effect of anticipation, a first paw was tested in all animals, and then, the opposite paw and sequence of testing (right or left paws) was alternated at each testing sequence. The force of the smallest filament causing a withdrawal reaction was recorded as the threshold. A force calibration was performed for each filament at the beginning and at the end of the study.
Acetone test
The cold threshold was evaluated according to the method previously described by Choi et al.Citation28 Briefly, a drop of acetone (25 μL) (laboratory grade) was applied with a syringe to the plantar surface of the hind paw. The number of movements of the hind leg and total duration of withdrawal behavior were recorded for 30 seconds. The responses to acetone were graded on the following scale: 0= no reaction 1= mild reaction, characterized by a quick withdrawal (fewer than three movements) or short duration of lifting paw (fewer than 3 seconds), 2= longer withdrawal or repeated movements (three or more movements or ≥3 seconds). To determine the percentage of animals that were reactive to acetone, only grade 2 were considered. The data were presented as the percentage of animals reacting to acetone, per group.
Hargreaves thermal sensitivity test
Thermal thresholds were evaluated with a Hargreaves apparatus (Plantar Analgesia Meter; IITC Life Science Inc.) as previously described.Citation29 Each animal was placed in a Plexiglas chamber with a glass floor heated between 28°C–31°C. The radiant heat from a high-intensity light bulb (40 W) was directed to the plantar surface of the hind paw. The withdrawal reaction time was recorded. To avoid the effect of anticipation, a first paw was tested in all animals, and then, the opposite paw and sequence of testing (right or left paws) was alternated at each testing sequence. A cutoff time of 20 seconds was preset to minimize tissue injury.
Treatments
The animals were fasted the night prior to gavage to avoid a food effect. They were given access to food pellets approximately 15 minutes following the gavage. The operator was blinded to the treatments (type of drug administered). The drugs and methylcellulose were purchased from Sigma-Aldrich Corp (St. Louis, MO, USA).
The rats from the collagenase group were further divided into four subgroups depending on the treatment received from days 15 to 25 postsurgery. These animals received either a daily oral administration of the vehicle (the control group [CTL]) (n=7) or one of the different drugs (AMI, GBP, or CARBA) (n=8/drug). The SHAM group received the vehicle daily by gavage, from days 15 to 25 postsurgery.
The dose concentrations for the three different drugs were chosen according to previous studies showing efficacy in the reduction of neuropathic pain in rodent models.Citation30,Citation31 The following doses were administered: AMI 10 mg/kg (in an emulsion consisting of 0.5 g/100 mL of the vehicle, for a concentration of 5 mg/mL); GBP 60 mg/kg (3 g GBP in 100 mL of the vehicle, for a concentration of 30 mg/mL); and CARBA 100 mg/kg (in an emulsion of 5 g dissolved in 100 mL of vehicle, for a concentration of 50 mg/mL). For the SHAM and CTL groups, 0.5 mL of the solution (composition: 5% weight/volume [w/v] sucrose and 1.5% [w/v] methylcellulose in water) was given orally. The behavioral tests started approximately 1 hour after the gavage.
Histological methods
Histology was mainly performed to confirm the location of the hemorrhagic lesion. The methods of animal perfusion, tissue collection, and histological staining have previously been described.Citation25
Statistical analyses
An analysis of variance (ANOVA) linear model with repeated measures and post hoc Tukey’s tests performed with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) were used to analyze the data. A priori contrasts with Bonferroni sequential adjustment for multiple comparisons were performed. Comparisons were made between the baseline and the postsurgery and posttreatment values. The SHAM group was also compared with the treatment groups. The results were presented as a mean and standard error (SE). P-values,0.05 were considered significant. Cochran–Mantel–Haenszel tests were used for the evaluation of cold allodynia.
Results
Motor coordination evaluated with the rotarod test
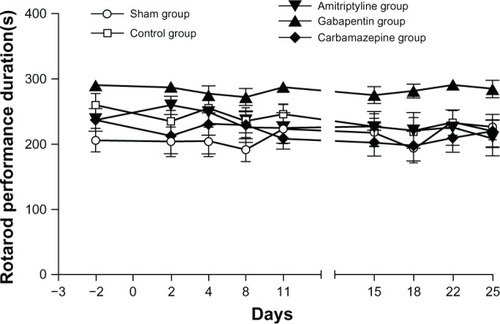
There was no significant difference in motor coordination between the treatment groups (F4, 31=3.29) (nonsignificant [ns]). For each group, there was no significant difference when comparing the baseline values and all post surgical evaluations, suggesting that neither the CTLs nor the different treatments had an effect on motor coordination ().
Figure 1 Results from the rotarod test (motor coordination) evaluated in Sprague Dawley® rats.
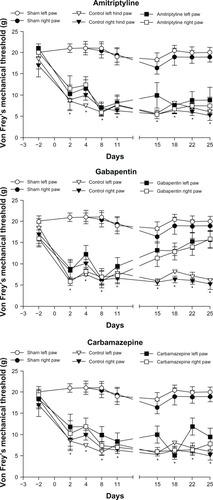
Mechanical sensitivity evaluated with Von Frey filaments
A significant effect of treatment occurred for mechanical sensitivity (right hind limb: F 3, 28.1=8.33 [P=0.0004]; left: F3, 28.1=22.68 [P<0.0001]) (). Prior to the surgery, the baseline values were not significantly different between the groups, for both hind limbs. For the SHAM group, no difference was seen between the baseline values and postsurgical results. Following surgery, from days 2 to 11, a significant decrease in the mechanical threshold was noted in the contralateral and ipsilateral hind limbs in the CTL group (P<0.05) compared with that in the SHAM group. This persisted in the CTL group even after treatment with the vehicle. Following treatment, AMI and CARB were showed no effect on mechanical sensitivity. Conversely, GBP reduced mechanical sensitivity, values increasing progressively near SHAM results starting from day 15 onwards.
Figure 2 Results of the mechanical allodynia test using Von Frey filaments.
Abbreviations: AMI, amitriptyline; CARBA, carbamazepine; CTL, control; GBP, gabapentin; ns, nonsignificant.
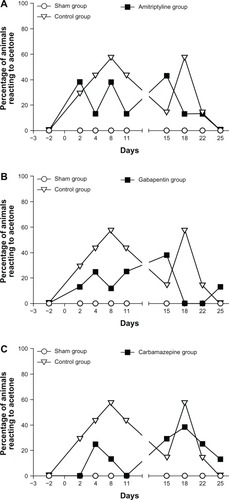
Evaluation of cold allodynia with the acetone test
The acetone test results are shown in . In the SHAM group, the animals never had significant reactions to acetone following surgery that were different from baseline. Conversely, an increase (graded 2) in the number (three or more movements) and duration of movements (≥3 seconds) occurred with acetone, in the animals in the collagenase group. In this group, 10%–30% of the animals reacted to acetone between days 2–22 following the surgery. Some animals showed severe allodynia, with duration of movement being around 20 seconds or number of movements being higher than 20, affecting mainly the left hind limb. However the results were not statistically significant because sensitivity to cold only affected a small number of animals. GBP seemed to reverse the cold allodynia on days 18 and 22 as none of the animals reacted to acetone compared with the other treatments.
Figure 3 Evaluation of cold allodynia with the acetone test.
Abbreviations: AMI, amitriptyline; CARBA, carbamazepine; CTL, control; GBP, gabapentin; SHAM, sham.
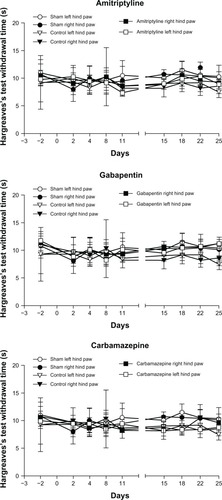
Heat sensitivity evaluated with the Hargreaves test
There was no significant difference in heat sensitivity for both hind limbs (right: F3, 28.2=1.36 [ns]; left: F3, 28.1=1.54 [ns]). No significant difference in the thermal threshold was observed when comparing the baseline and any of the postsurgical evaluations for the contralateral and ipsilateral hind limbs in all the groups ().
Figure 4 Thermal hyperalgesia using the Hargreaves test.
Abbreviation: ns, nonsignificant.
Histopathological evaluations
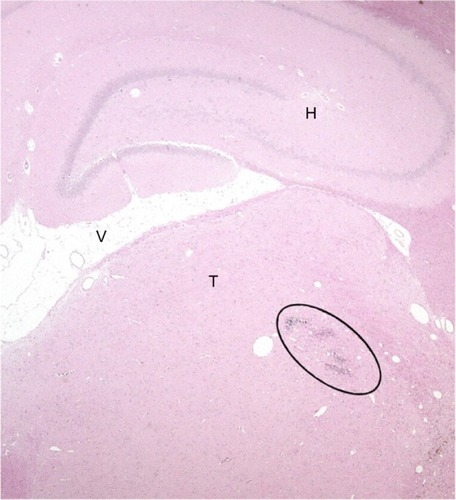
In the SHAM group, the microscopic evaluation of the brain slices was unremarkable. Conversely, in the collagenase group, small well circumscribed lesions were observed in the lateral thalamic nuclei (including the VPL) ().
Figure 5 Photomicrograph of a transverse rat brain section (4 μm) from the VPL nucleus of the thalamus.
Abbreviations: H, hippocampus; T, thalamus; V, ventricle; VPL, ventroposterolateral.
Discussion
A previously published,Citation25 an animal model of central pain induced by an intrathalamic hemorrhage was used to assess AMI, GBP, and CARBA as potential therapeutic drugs. Following administration of a collagenase solution in the VPL thalamic nucleus, we observed a significant bilateral mechanical allodynia up to 25 days after the intracerebral lesion, which developed as early as day 2 postsurgery. No sensitivity to heat developed in these animals, and a transient cold allodynia was observed only in some neuropathic animals. These results are similar to our previous findingsCitation25 and reflect the mechanical and cold allodynia that typically occur in humans following poststroke pain.Citation9,Citation10 At the dosages used, AMI and CARBA failed to show any significant effect on mechanical allodynia; however, GBP significantly alleviated mechanical sensitivity in both hind limbs. A putative effect of GBP on cold allodynia was only transient.
Mechanical allodynia is a clinical finding often reported in patients with CPSP.Citation9,Citation11,Citation32 Tactile hypoesthesia may also occur in the absence of mechanical allodynia.Citation32 Our results show a persistent bilateral mechanical allodynia following the occurrence of a unilateral thalamic lesion, as was reported from previous findings.Citation25 Increased thermal thresholds, either to cold or hot temperatures, are frequently seen in CPSP patients.Citation2 These changes can be characterized by either hyper-or hyposensitivity to cold, or warm temperature hyperalgesia.Citation32 In the present study, we showed that the thermal heat sensitivity was unchanged in the animal with a thalamic lesion, which is concordant with our previous findings.Citation25 In stroke patients, an increased sensitivity to cold and a decreased sensitivity to warmth occur frequently.Citation9,Citation32 Our results reflect the apparent low prevalence of hypersensitivity to noxious cold.
A possible mechanism of CPSP could be a loss of inhibition, plausibly following loss of gamma-aminobutyric acid (GABA)-Eric neurons or inhibition, particularly in the reticular nucleus surrounding the thalamus.Citation33 It is also possible that allodynia and hyperalgesia in CPSP patients may result from glutamatergic neuronal hyperexcitability, which may be related to central sensitization.Citation34,Citation35 However, the precise pathophysiology of CPSP remains unclear. Although the mechanisms of CPSP are still poorly understood, the effect of GBP in reversing the mechanical allodynia associated with intrathalamic hemorrhage may allow us to gain some insight into the complexity of this condition. However, the mechanism of action of GBP is not fully defined. A synergy between increased GABA synthesis, non-N-methyl-D-aspartate (NMDA) receptor antagonism, and binding to the α2δ subunit of voltage-dependent calcium channels (CavCs) is suspected.Citation18 The binding to the α2δ subunit of CavC by GBP appears to be its most important mechanism of action to relieve neuropathic pain.Citation18 It seems that this α2δ subunit is involved in the inhibitory effects of GBP on stimulus-evoked glutamate release.Citation36 Repeated studies showed that GBP reduces glutamate release both in vitro and in vivo, in a model of peripheral neuropathic pain in rodents.Citation36,Citation37 GBP-binding on α2δ-1 was shown to reduce calcium influx into the nerve terminals in response to action potential–mediated excitation, which would presumably diminish neurotransmitter release.Citation38,Citation39 Thus, GBP could influence central sensitization by decreasing NMDA receptor activity as its binding on the calcium channel would inhibit the release of excitatory neurotransmitters, such as glutamate.Citation37 Importantly it is well recognized that CavCs modulate the central pain pathways by influencing fast synaptic transmission and neuronal excitability in the thalamus.Citation40 In conclusion, GBP could modulate thalamic pain, both presynaptically and synaptically.
The efficacy of antidepressants in some CPSP patients suggests the involvement of noradrenergic, serotoninergic, and dopaminergic mechanisms.Citation41 However, in our study, AMI failed to show any significant effect on mechanical allodynia. AMI has previously shown some degree of efficacy to relieve pain, at least partially, in CPSP patients, even in the absence of depression.Citation11,Citation42 The difference between our results and the response to AMI in human patients may be explained by some difference in methodology. CPSP patients achieved relief with AMI, and the difference in the rating of pain was 1 point on a pain scale when compared with placebo.Citation42 Although this small difference might be appreciated by human patients, it may be too subtle to be reflected in an animal study. Furthermore, the dose we administered to the animals was not titrated to effect and was given only for a short period of time (10 days). In one clinical case, mechanical allodynia was reduced by about 50%, but the AMI was titrated to effect and administered over a longer period of time.Citation42 Important side effects occurred (sedation, dizziness, and difficulty concentrating), and the final recommendation was to take the drug only during particularly painful episodes. In our study, AMI was not given to effect, although the dose used in the rats (10 mg/kg) was much higher than the human dose (75 mg, or 1 mg/kg for a 70 kg person). We therefore expected a treatment effect with AMI, and an adjustment of the dose could be performed in future studies. We do not suggest that AMI is an unjustified choice for the treatment of central pain but rather, that a therapeutic dose without apparent side effects was ineffective to treat this condition.
CARBA did not show a significant effect in reducing the mechanical allodynia associated with an intrathalamic hematoma. These results are less surprising given the poor effect of this drug in human CPSP patients.Citation11,Citation42 These findings may suggest that sodium channel blocking by CARBA does not represent a major target for the treatment of CPSP.
In conclusion, we found that a collagenase-induced hemorrhage within the VPL thalamic nucleus of rats induced bilateral mechanical allodynia that was fully reversed by GBP. No thermal hyperalgesia to heat occurred; however, cold allodynia was noted in some animals. AMI and CARBA, at the concentration administered, failed to show any significant effect on the mechanical allodynia.
Acknowledgments
This study was supported by the Fond de Développement pour la Médecine des Animaux de Laboratoire. We wish to acknowledge Guy Beauchamp for statistical analyses as well as Jacinthe Cardin and Nancy Veilleux for their assistance in the brain preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- GilronICoderreTJEmerging drugs in neuropathic painExpert Opin Emerg Drugs200712111312617355217
- BoivieJLeijonGJohanssonICentral post-stroke pain – a study of the mechanisms through analyses of the sensory abnormalitiesPain19893721731852748190
- FitzekSBaumgärtnerUFitzekCMechanisms and predictors of chronic facial pain in lateral medullary infarctionAnn Neurol200149449350011310627
- HansenAPMarcussenNSKlitHAndersenGFinnerupNBJensenTSPain following stroke: a prospective studyEur J Pain20121681128113622407963
- KlitHFinnerupNBAndersenGJensenTSCentral poststroke pain: a population-based studyPain2011152481882421272999
- NasreddineZSSaverJLPain after thalamic stroke: right diencephalic predominance and clinical features in 180 patientsNeurology1997485119611999153442
- BogousslavskyJRegliFUskeAThalamic infarcts: clinical syndromes, etiology, and prognosisNeurology19883868378483368064
- KrauseTBruneckerPPittlSThalamic sensory strokes with and without pain: differences in lesion patterns in the ventral posterior thalamusJ Neurol Neurosurg Psychiatry201283877678422696587
- AndersenGVestergaardKIngeman-NielsenMJensenTSIncidence of central post-stroke painPain19956121871937659428
- BowsherDCentral pain: clinical and physiological characteristicsJ Neurol Neurosurg Psychiatry199661162698676164
- LeijonGBoivieJJohanssonICentral post-stroke pain – neurological symptoms and pain characteristicsPain198936113252919091
- AppelrosPPrevalence and predictors of pain and fatigue after stroke: a population-based studyInt J Rehabil Res200629432933317106351
- WidarMAhlströmGDisability after a stroke and the influence of long-term pain on everyday lifeScand J Caring Sci200216330231012191043
- GordonABest practice guidelines for treatment of central pain after strokeHenryJLPanjuAYashpalKCentral Neuropathic Pain: Focus on Poststroke PainSeattle, WAIASP Press2007267273
- KaurHHotaDBhansaliADuttaPBansalDChakrabartiAA comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trialDiabetes Care201134481882221355098
- LeijonGBoivieJCentral post-stroke pain – a controlled trial of amitriptyline and carbamazepinePain198936127362465530
- MishraSBhatnagarSGoyalGNRanaSPUpadhyaSPA comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled studyAm J Hosp Palliat Care201229317718221745832
- BennettMISimpsonKHGabapentin in the treatment of neuropathic painPalliat Med200418151114982201
- AttalNBrasseurLParkerFChauvinMBouhassiraDEffects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot studyEur Neurol19984041912009813401
- ChapmanVSuzukiRChamaretteHLRyghLJDickensonAHEffects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated ratsPain1998752–32612729583762
- JeanmonodDMagninMMorelALow-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptomsBrain1996119Pt 23633758800933
- LenzFATaskerRRDostrovskyJOAbnormal single-unit activity recorded in the somatosensory thalamus of a quadriplegic patient with central painPain19873122252363501563
- WassermanJKKoeberlePDDevelopment and characterization of a hemorrhagic rat model of central post-stroke painNeuroscience2009161117318319324079
- RosenbergGAMun-BryceSWesleyMKornfeldMCollagenase-induced intracerebral hemorrhage in ratsStroke19902158018072160142
- CastelAHéliePBeaudryFVachonPBilateral central pain sensitization in rats following a unilateral thalamic lesion may be treated with high doses of ketamineBMC Vet Res201395923537119
- PaxinosGWatsonCThe Rat Brain: in Stereotaxic CoordinatesSan Diego, CAAcademic Press Inc1998
- ChaplanSRBachFWPogrelJWChungJMYakshTLQuantitative assessment of tactile allodynia in the rat pawJ Neurosci Methods199453155637990513
- ChoiYYoonYWNaHSKimSHChungJMBehavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic painPain19945933693767708411
- HargreavesKDubnerRBrownFFloresCJorisJA new and sensitive method for measuring thermal nociception in cutaneous hyperalgesiaPain198832177883340425
- FieldMJMcClearySHughesJSinghLGabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the ratPain1999801–239139810204753
- ChogtuBBairyKLSmithaDDharSHimabinduPComparison of the efficacy of carbamazepine, gabapentin and lamotrigine for neuropathic pain in ratsIndian J Pharmacol201143559659822022008
- GreenspanJDOharaSSarlaniELenzFAAllodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individualsPain2004109335736615157697
- LiaoYFTsaiMLChenCCYenCTInvolvement of the Cav3.2 T-type calcium channel in thalamic neuron discharge patternsMol Pain201174321639922
- SessleBJMechanisms of painHenryJLPanjuAYashpalKCentral Neuropathic Pain: Focus on Poststroke PainSeattle, WAIASP Press20076779
- VestergaardKNielsenJAndersenGIngeman-NielsenMArendt-NielsenLJensenTSSensory abnormalities in consecutive, unselected patients with central post-stroke painPain19956121771867659427
- QuinteroJEDooleyDJPomerleauFHuettlPGerhardtGAAmperometric measurement of glutamate release modulation by gabapentin and pregabalin in rat neocortical slices: role of voltage-sensitive Ca2+ α2δ-1 subunitJ Pharmacol Exp Ther2011338124024521464332
- CoderreTJKumarNLefebvreCDYuJSEvidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamateJ Neurochem20059441131113916092950
- FinkKDooleyDJMederWPInhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortexNeuropharmacology200242222923611804619
- TuchmanMBarrettJADonevanSHedbergTGTaylorCPCentral sensitization and Ca(V)α2δ ligands in chronic pain syndromes: pathologic processes and pharmacologic effectJ Pain201011121241124920472509
- TodorovicSMJevtovic-TodorovicVT-type voltage-gated calcium channels as targets for the development of novel pain therapiesBr J Pharmacol2011163348449521306582
- HenryJLCentral poststroke pain: an animal modelHenryJLPanjuAYashpalKCentral Neuropathic Pain: Focus on Poststroke PainSeattle, WAIASP Press2007171180
- HanssonPPost-stroke pain case study: clinical characteristics, therapeutic options and long-term follow-upEur J Neurol200411 Suppl 1223015061821
