 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Patients with chronic pain have impaired cognitive functions, including decision making, as shown with the Iowa gambling task (IGT). The main aim of this study was to elucidate whether patients’ decision making is associated with a lack of the anticipatory skin conductance response (SCR). An increase in anticipatory SCR before making unfavorable choices is known to guide decisions in healthy controls during the IGT. Since several brain regions involved in decision making are reported to have altered morphology in patients with chronic pain, the second aim was to explore the associations between IGT performance and brain structure volumes. Eighteen patients with chronic pain of mixed etiology and 19 healthy controls matched in terms of age, sex, and education were investigated with a computerized IGT during the recording of SCR, heart rate, and blood pressure. The participants also underwent neuropsychological testing, and three-dimensional T1-weighted cerebral magnetic resonance images were obtained. Contrary to controls, patients did not generate anticipatory SCRs before making unfavorable choices, and they switched between decks of cards during the late phase of the IGT significantly more often, and this was still observed after adjusting for depression scores. None of the other autonomic measures differed during IGT performance in either group or between groups. In patients, IGT scores correlated positively with total cortical grey matter volume. In controls, there was no such association, but their IGT scores correlated with the anticipatory SCR. It may be speculated that the reduction in anticipatory SCRs makes the chronic pain patients rely more on cortical resources during decision making.
Introduction
Patients with chronic pain have impaired cognitive functions;Citation1 among those are impaired decision making,Citation2–Citation4 as demonstrated in the Iowa gambling task (IGT), a test that simulates real-life decision making.Citation5 In this test, the goal is to win as much as possible by discerning which of the two decks of cards are advantageous and which two are disadvantageous. Previous research on the IGT has shown that patients with chronic pain score lower and/or switch more frequently between decks compared to healthy controls (HCs).Citation2–Citation4
The IGT was introduced as a test of the somatic marker hypothesis.Citation5 This hypothesis states that when facing ambiguous decisions, cognitive processes are insufficient in guiding choices.Citation6,Citation7 Instead, autonomic physiological reactions, such as skin conductance responses (SCRs) that are learned to be associated with a specific outcome, are engendered in the body and are relayed to the brain where they give rise to an emotion-guided decision.Citation7 This is called a somatic marker. In healthy subjects, increased anticipatory SCRs are present before choosing cards from the disadvantageous decks of cards in the IGT (see and for an illustration of the model).Citation8 This is not found in subjects with lesions in the ventromedial prefrontal cortex, who have impaired decision-making skills, but who are otherwise spared intellect.Citation8,Citation9 Similarly, the reduced decision-making ability in patients with chronic pain may arise from a lack of anticipatory SCR generation, but this has not yet been investigated.
Figure 1 Decision-making structures important during the Iowa gambling task.
Abbreviations: P, precuneus; B, brainstem; CC, cingulated cortex; V, ventromedial prefrontal cortex; H, hippocampus; A, amygdala; I, insula.
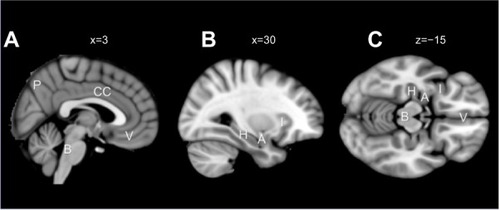
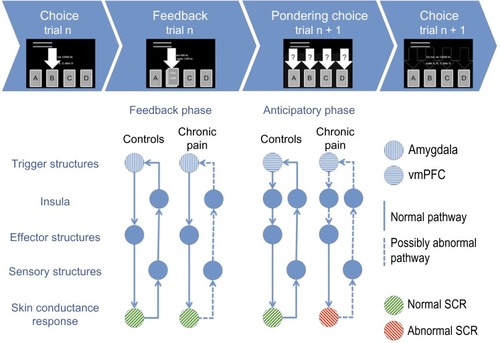
Figure 2 The generation of SCR during the IGT in the PCP and HC groups in the present study in light of the somatic marker hypothesis.
Abbreviations: SCR, skin conductance response; IGT, Iowa gambling task; PCP, patients with chronic pain; HC, healthy control; n, choice number; vmPFC, ventromedial prefrontal cortex.
As far as we know, there is also a lack of knowledge as to whether impaired performance on the IGT correlates with changes in the cerebral morphology of patients with chronic pain. This may be expected, since several brain regions involved in decision making are reported to have altered morphology in chronic pain patients.Citation10 Decision making relies on many brain regions for generating, relaying, and interpreting SCR and/or other somatic signals. The winner of any competing signals influences the final choice.Citation7 This process is dependent on trigger structures (the amygdala and ventromedial prefrontal cortex), effector structures (such as the hypothalamus, periaqueductal gray area, nucleus accumbens, and neurotransmitter brainstem nuclei), sensory structures (sensory brain stem nuclei), and processing structures (such as the insula and somatosensory cortices), as well as memory structures (for instance, the hippocampus and dorsolateral prefrontal cortex).Citation7 In patients with chronic pain, gray matter volume reductions have been reported in all these mentioned structure groups, most consistently in the trigger (ventromedial prefrontal cortex), processing (insula), and memory (dorsolateral prefrontal cortex) structures.Citation10–Citation12
The overall intention of this study was to explore and compare differences and associations between IGT behavior, autonomic signals, and brain structure volumes in patients with chronic pain and matched HCs. The main aim was to examine whether patients’ impaired decision making is associated with a lack of anticipatory autonomic physiological reactions. We predicted, based on the previously reported deficits in performance on the IGT in chronic pain patients,Citation2–Citation4 and on the somatic marker hypothesis, that the patient group would lack the anticipatory SCR before picking cards from the disadvantageous decks. To elucidate possible contributions of other autonomic physiological reactions on IGT performance in the patient group, heart rate (HR) and blood pressure were also measured. The secondary aim was to explore the associations between IGT performance and brain structure volumes. We anticipated that reduced brain structure volumes would be related to impaired IGT performance in patients with chronic pain.
Methods
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Social Sciences Data Service, and was performed in accordance with their requirements and the Declaration of Helsinki. Written informed consent was obtained from all participants.
Materials
Twenty subjects (16 females) with chronic pain were recruited during consultations in a university pain clinic, and 20 (18 females) age- and education-matched HCs were recruited from the local community. Prior to inclusion, the patients had to report a 6-month average pain intensity of ≥4 on the Verbal Rating Scale, with scores ranging from 0–10.Citation13 The included patients also had to be in a chronic pain state, which corresponds to Verbal Rating Scale scores ≥4 for at least 6 months.Citation14
All participants were offered a monetary compensation of 400 Norwegian Kroner (NOK) (approximately USD $65) and pictures from their morphological brain scan. Psychiatric and neurological disorders, known traumatic brain injuries (13 Glasgow coma scale score at the time of injury), or magnetic resonance imaging (MRI) contraindications were used as exclusion criteria. A diagnosis of mild or moderate depression did not warrant exclusion in any of the groups. Furthermore, patients with a high consumption of analgesics were excluded (>180 mg of codeine or its equivalent per 24 hours, 24-hour continuous benzodiazepine treatment, or use of carisoprodol). All participants reported being right-handed, and they were assessed with the Edinburgh Handedness InventoryCitation15 (patients: 0.82±0.21 [mean±standard deviation]; controls: 0.91±0.16).
One patient was excluded due to a neurological disease that was discovered after inclusion, and one patient and one control were excluded due to technical problems during the IGT presentation. Thus, the groups included in the final analysis consisted of 18 patients with chronic pain (15 females) and 19 HCs (17 females).
Of the 18 included chronic pain patients, eleven were classified as having pain of musculoskeletal etiology, five of idiopathic etiology, two with visceral etiology, and none with neuropathic pain.
Data collection
Procedure
On day 1, the participants underwent MRI scanning. On day 2, the participants filled out questionnaires measuring pain and completed neuropsychological tests to assess their general intelligence, depression level, working memory and effort, and finally performance on the IGT during neurophysiological monitoring.
The IGT testing began with the subjects visiting the lavatory and washing their hands to ensure good SCR readings. Following that, various autonomic measuring equipment were attached (see below). Instructions for the computerized version of the IGTCitation16 were read to the subjects by the researcher (NAE) while the game was demonstrated. The subjects were then left alone in the room and monitored by closed-circuit camera and microphone by the researcher. They were instructed to relax while an on-screen clock counted down from five minutes, and they were then instructed to commence with the task. They began to choose cards by pressing keyboard keys labeled A, B, C, and D, which corresponded to the labeling of the card decks on the screen.
Room temperature was consistently maintained between 22°C–26°C, and this was confirmed before testing with an electronic thermometer (Digitron 2088T, Elektron Technology, Cambridge, UK).
Iowa gambling task (IGT)
We used a modified computerized version of the original IGT, which is similar to the version described by Bechara et al.Citation16 To avoid confusion about the value of foreign currencies, all USD values from the original IGT were converted to local currency (NOK). Subjects chose cards from four decks (A, B, C, and D) with the goal of winning as much as possible. In each deck, there are varying amounts of rewards and punishments, with decks A and B offering a fixed gain of $100, and decks C and D offering a fixed gain of $50. The losses vary in frequency, with a 10% loss in decks B and D, and a 50% loss in decks A and C. This results in an average gain or loss after ten cards, with a $250 loss for cards from decks A and B, and a $250 gain for decks C and D. Decks C and D were thus the advantageous decks, and A and B were the disadvantageous decks. On the computer display of the four card decks, a red bar indicating the amount of debt and a green bar indicating the amount of winnings were presented. On the same screen, updated instructions were presented to the subjects in Norwegian (“You now have X NOK”, “Press A, B, C, or D”, “You won Y NOK”, and on some trials “but you lost Z NOK”, where X, Y and Z were currency amounts) (). The IGT has been described in more detail elsewhere.Citation16
Figure 3 The Iowa gambling task.
We randomized the on-screen position and naming of the different decks to avoid bias from naming or placement on the screen (see ).Citation17 Furthermore, we shuffled the decks of cards between subjects. Randomization of the card order increases the robustness of averaging the autonomic signal, and it also rules out the effect of a specific card order on somatic marker generation. Randomization was obtained by block randomization in blocks of ten cards, keeping the original punishment and reward frequencies as described by Bechara et al.Citation5
The interval from one choice to the next was set to a minimum of 6.5 seconds, and it ended when the subject chose a card. The IGT has been criticized for allowing certain decks to run out of cards,Citation18 thus reducing its sensitivity to detecting impairment in decision making. Because of this, we set all decks to contain 100 cards, which is equal to the total amount of cards that the subject was allowed to draw.
Autonomic measures
As a measure of the state and balance of the autonomic nervous system, several autonomic measurements were made. Skin sweat gland activity (ie, SCR), HR (ie, R wave to R wave intervals [RR]) from electrocardiogram (ECG) (PowerLab unit, ADInstruments, Dunedin, New Zealand) recordings, and blood pressure from the finger manometer were recorded during IGT performance. The ECG data was used to calculate both HR and HR variability. HR variability was calculated based on the HR, according to current recommendations.Citation19 SCR, RR intervals, and blood pressure were used in event-related analyses (autonomic activity directly preceding or following choices). The average HR, HR variability, and blood pressure were calculated separately for the 5-minute baseline period before the IGT (“Baseline”) and for the entire IGT period (“Activity”). In addition the individual’s change (Δ) in these measurements from Baseline to Activity was calculated, for example:
(1)
Sample characteristics
Pain
Pain intensity was assessed using the validated Norwegian translation of the Brief Pain Inventory (BPI).Citation20,Citation21 The BPI assesses the intensity of pain during the last 24 hours using a 0–10 numerical rating scale.
Depression level
Since major depressive disorder is known to affect decision making,Citation22 depression levels were scored with the Beck Depression Inventory-II to enable the correction of IGT scores.Citation23
Working memory
Working memory function is necessary for normal IGT performance,Citation24 and reduced working memory performance has been reported in chronic pain samples.Citation25 Thus, all subjects completed the Letter–Number Sequencing subtest of the Wechsler Adult Intelligence Scale III, a standard two-back test, and a visual version of the Paced Auditory Serial Addition Test.Citation26 These three tasks cover various aspects of working memory, ranging from simple storage of information in working memory, to manipulation of the stored information.
Hardware
For the autonomic measurements, all data were collected using Chart (version 5.5; ADInstruments, Dunedin, New Zealand) and sampled at 1 kHz. The computer was fed data from PowerLab 16/30 (ADInstruments), which got user input data and card information data via a serial output/parallel input cable (Leteng AS, Oslo, Norway). The SCR was measured with finger electrodes with a dedicated preamplifier (MLT116F and ML135; ADInstruments). Respiration (Thermistor; Sleepmate® Technologies, Glen Burnie, MD, USA) and one-lead ECG (lead II) were also measured. Continuous finger blood pressure was measured (Finometer® PRO, internal sampling at 200 Hz; Finapres Medical Systems BV, Amsterdam, the Netherlands). Recordings of blood pressure and SCR were done on the left (nonmoving) hand to reduce motion artifacts.
Image acquisition
Scanning was performed on a 3T Siemens Trio scanner with a 12-channel Head Matrix Coil (Siemens AG, Munich, Bavaria, Germany). Foam pads were used to minimize head motion. One T1-weighted three-dimensional volume measurement was acquired (repetition time [TR] =2,300 ms; echo time [TE] =2.88 ms; inversion time [TI] =900 ms; flip angle =9°; field of view [FOV] =526; slices =160; slice thickness =1.2 mm; in-plane resolution of 1.0 mm × 1.0 mm). No morphological abnormalities were revealed in any of the participants.
Analysis
IGT measurements
The IGT score was calculated as the number of advantageous (cards chosen from decks C and D) minus disadvantageous choices (cards chosen from decks A and B).
Patients with chronic pain have been shown to have reduced persistence during IGT performance (ie, they switch more often between the four decks of cards) than controls.Citation2,Citation4 IGT switching was calculated as the frequency at which a subject switched from drawing from an advantageous or disadvantageous deck choice, to drawing from the other type of deck choice. For example, “A, A” and “A, B” were not counted as a switch, but “A, C” and “A, D” were counted as a switch.
For IGT score and switching, total score and total switching was calculated for the whole test (cards 1 to 100). Additionally, score and switching was calculated for the learning phase of the test (cards 1 to 40) and the performance phase of the test (cards 41 to 100), as the processes underlying decision making have been shown to differ in the first 40 versus the last 60 cards in controlsCitation27 and in patients with chronic pain.Citation2
Event-related autonomic analysis
Previous research on anticipatory SCR during the IGT has used a variety of methods for calculating the SCR.Citation28–Citation31 We measured both anticipatory responses (5–0 seconds prior to making a choice) and feedback responses (0–5 seconds after making a choice) for SCR. SCRs were calculated in a manner similar to that reported by Bechara et alCitation32 (ie, the integral of the detrended skin conductance level curve, or the area under the curve, was obtained, with the skin conductance level recorded at the beginning of the integral serving as the baseline).
We used the same method to calculate the integral of systolic blood pressure and heart RR intervals in the 5 seconds prior to making a choice and after making a choice.
For the analysis of blood pressure levels, the delay from true aortic blood pressure to the pulse signals measured in the hand was assumed at a fixed 250 ms delay. Automatic calibration of the blood pressure monitoring equipment and ectopic heart beats were identified by manual inspection of the data, and any RR or blood pressure intervals that included such events were excluded from the analysis.
Postchoice SCRs were analyzed to assess whether SCR generation following punishment or reward was similar in the control and patient groups. For this analysis, SCRs after making a choice were grouped based on whether the card actually punished the subject or not (50% of cards in decks A and C and 10% of cards in decks B and D punished the subject). The integral was analyzed in the 5-second period after making a choice; otherwise, they were similar to anticipatory SCR calculations.
Analyses at the event level (ie, integrals for a period prior to making a card selection for a given subject) and HR variability analyses were done in Chart 7.0 (ADInstruments).
Cardiac autonomic regulation
Analysis of normalized low frequency (LF) power (0.04–0.15 Hz ms2), normalized high frequency (HF) power (0.15–0.4 Hz ms2), and the LF to HF ratio (LF/HF) were performed in the frequency domain (LabChart 7.0; ADInstruments) (Welch window, 1,024 data points, and segment overlap, 50%). The maximal frequency was set to 0.5 Hz.
Artifacts were excluded from the analyses, and RR intervals were estimated from the intervals noted before and after the ectopic heartbeats occurred. The recordings were inspected manually and corrected when necessary.
HR, HR variability, and blood pressure were calculated as the average for the 5-minute resting period before the start of the IGT (for example, “Baseline HR”), for the duration of the IGT (for example, “Activity HR”), and for each group’s mean absolute change from Baseline to Activity across all cardiac autonomic measures (for example, “ΔHR”).
MRI analysis
The volumetric assessment of subjects’ T1-weighted brain MRI volumes was performed using NeuroQuant software (CorTechs Labs, Inc., La Jolla, CA, USA). This software enables automated analysis of T1-weighted brain volumes and provides a morphology report on the volume of the total cortical gray matter, as well as of the ventricles, brainstem, cerebellum, and some subcortical structures (hippocampus, amygdala, caudate, putamen, pallidum, thalamus, nucleus accumbens, and brainstem), which was corrected for intracranial volume.Citation33 For each structure, the volumes of the structures in the right and left hemisphere were combined. The volumes of the structures of interest (total cortical gray matter, amygdala, hippocampus, brainstem, and nucleus accumbens) were compared between groups and correlated with total IGT scores, pain, and autonomic data.
Statistical analysis
PASW Statistics 18.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses.
Two-tailed, unpaired Student’s t-tests were used to identify the differences between groups on demographic, depression level, pain, and neuropsychological measures, as well as on IGT scores, brain structure volumes, and autonomic activity. Cohen’s d was calculated and classified as small (d=0.15–0.40), medium (d=0.40–0.75), and large (d>0.75).
Paired Student’s t-tests were used for all within-groups analyses of event-related or cardiac autonomic measures. Spearman’s rank–order correlation was performed to assess the relationships between the IGT total score, the IGT total switching, pain level (evaluated using the BPI) before the IGT, and event-related autonomic data, as well as between the IGT total score and brain structure volumes. Possible confounding effects of depression levels on the IGT score were investigated with Spearman’s partial correlation. The significance threshold was set to P≤0.05 (two-tailed) for both Student’s t-tests and Spearman’s correlations.
A mixed-design analysis of variance (ANOVA) (“split-plot ANOVA”) was used to investigate the interactions between the three autonomic measures related to choosing from the advantageous versus disadvantageous decks and subject group (chronic pain patients versus HCs). Outliers were identified as studentized residuals ≥±3 standard deviations. Simple main effects were investigated where significant interactions were found. Effect sizes were calculated as partial eta squared (ηp2), and they were classified as small (>0.01), medium (>0.06), or large (>0.14).
All data are given as the mean ± standard deviation.
Results
Participants
As shown in , there were no differences in age, length of education, or the sex distribution between the patients and control groups. The patient group scored significantly higher on both the average pain for the last 24 hours and average pain at the time of testing than did the controls. ().
Table 1 Demographic, clinical, and working memory measures in patients with chronic pain and matched healthy controls
There were no significant differences on the working memory tests ().
Iowa gambling task behavior
As shown in , the IGT total score was not different between the patients and controls. There were also no differences in the IGT score for the learning or performance phases of the IGT between the groups ().
Table 2 IGT total scores and switching in the learning phase (1–40 cards) and performance phase (41–100 cards), and brain volumes in PCP and matched HCs
During the entire IGT period, there was a trend toward increased switching in the patient group compared to the control group. In the performance phase of the IGT, the chronic pain patients switched significantly more often than did the HCs ().
There were no significant correlations between the different IGT scores and pain level before the test in any of the groups (). These correlations remained nonsignificant after adjusting for depression scores (results not shown).
Table 3 Correlations between pain level, the different IGT scores, and SCR before and during the IGT in PCP and matched HCs
Autonomic activation during decision making
SCR
A mixed-design ANOVA (group [chronic pain patient or control] × deck [advantageous or disadvantageous]) on the SCR before choices showed a weak, nonsignificant main effect for group, no main effect for deck, but a significant group × deck interaction with a large effect size ( and ). There was one outlier in the patient group, but the group × deck interaction remained when excluding the outlier (F[1, 33]=5.227, P=0.029, ηp2=0.137).
Figure 4 Autonomic measures from the anticipatory phase of the Iowa gambling task before drawing from either the advantageous or disadvantageous decks.
Abbreviations: SCR, skin conductance response; RR, R wave to R wave intervals; BP, systolic blood pressure; PCP, patients with chronic pain; HCs, healthy controls.
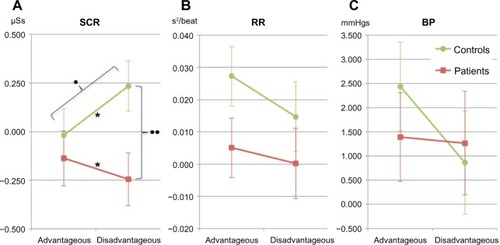
Table 4 Results of the mixed ANOVA (group × choice type) analyses of the autonomic SCR, heart rate (RR), and BP before choosing from the disadvantageous or the advantageous decks in PCP and matched HCs
The SCRs before choosing from advantageous and disadvantageous decks were significantly different, with a large effect size observed within the control group ( and ). In the patient group, there was no difference in SCRs before choosing from advantageous and disadvantageous decks ( and ). For disadvantageous decks, the SCR was significantly higher in controls than in patients with a large effect size (F[1, 34]=6.581, P=0.015, ηp2=0.162). There was no such group difference in SCR before the advantageous deck choices ().
Figure 5 SCR during the Iowa gambling task for pain patients and controls.
Abbreviations: SCL, skin conductance level; SCR, skin conductance response; PCP, patients with chronic pain; HCs, healthy controls; ANOVA, analysis of variance.
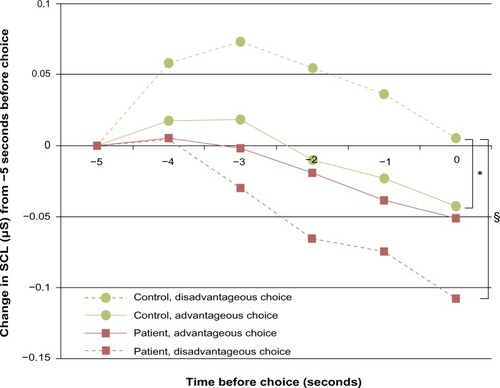
Table 5 SCR before disadvantageous and advantageous choices, and after receiving reward and punishment cards in the IGT in PCP and matched HCs
For HCs, there was a significant correlation between the SCR before the disadvantageous deck choices and the IGT total score (Spearman’s rho =0.568, P=0.011). This was not found in the patient group. Rather, the SCRs before the disadvantageous and advantageous deck choices were made were correlated with each other in the chronic pain group (Spearman’s rho =0.539, P=0.026). Except for the aforementioned results, no correlations were found in the chronic pain group between SCR and IGT behavior, or between SCR and pain level (). None of these correlations changed in significance after adjusting for depression level (results not shown).
There were no differences in the postchoice SCRs between receiving a punishment and a no-punishment card within the patient or control groups, or between the groups ().
RR intervals
A mixed-design ANOVA on the RR integral before choices (group [chronic pain patient or control] × deck [advantageous or disadvantageous]) showed no main effect for group, deck, or the group × deck interaction (). There were no outliers in any group.
Blood pressure
The mixed-design ANOVA on the blood pressure integral before choices (group [chronic pain patient or control] × deck [advantageous or disadvantageous]) showed no main effect for group, deck, or the group × deck interaction (). There was one outlier in the control group, but removing this did not alter the results.
Cardiac autonomic regulation
The Student’s t-tests showed no significant group differences for the Baseline HR or Baseline HR variability measures (LF/HF, LF, or HF), or the Baseline blood pressure between the patient and the control groups, and all of the effect sizes were small. There was also no significant group difference for the change from Baseline to Activity on any of the cardiac autonomic measures. However, there were medium effect sizes for the group differences on ΔHR, ΔLF/HF, and ΔHF ().
Table 6 Cardiac autonomic regulation during the IGT and rest in PCP and matched HCs
Brain structure volumes
As shown in , the nucleus accumbens volume was significantly reduced in the chronic pain group. For the other four brain structures, no significant group differences were found, but a medium effect size was present for the reduced total cortical gray matter volume in the chronic pain patients ().
The chronic pain group had a significantly positive correlation between total cortical gray matter volume and IGT total score (). Moreover, there was a negative correlation between IGT total switching and amygdala volume in the chronic pain patients (). No such correlations with IGT behavior were found in the control group. All correlations remained significant after adjusting for depression levels (results not shown).
Table 7 Correlations between IGT behavior and autonomic measures and brain volumes in PCP and matched HCs
Discussion
To our knowledge, this is the first study to show that patients with chronic pain lack SCR before making disadvantageous decisions. In line with our hypothesis, the patient group was impaired at generating SCRs before choosing from disadvantageous card decks in the IGT. The other main finding in this study was that the decision-making ability in the chronic pain patient group correlated positively with total cortical gray matter volume.
As predicted by the somatic marker hypothesis, a specific and significant increase in anticipatory SCR appeared when controls chose from the disadvantageous decks, and this correlated positively with the total IGT scores. This finding is in line with those from previous studies that showed a positive relationship between IGT performance and strength of the anticipatory SCR in healthy subjects.Citation34–Citation36 Similar findings were not present in the patient group.
The lack of the anticipatory SCRs in the patients with pain was not caused by a general impairment in SCR generation. There were no group differences in terms of the autonomic measures that indicated the presence of autonomic dysfunction in the patient group. For instance, despite abnormal anticipatory SCRs before the choices were made, the patient group exhibited similar SCRs after the choices were made as the controls when receiving punishment or reward. These results suggest that the chronic pain group was able to trigger somatic responses due to innate or learned stimuli, but they were impaired in the somatic marker structures necessary for sensing (sensory brain stem nuclei), processing (insula, somatosensory cortices, posterior cingulate cortex, and precuneus), or triggering (ventromedial prefrontal cortex, hippocampus, and dorsolateral prefrontal cortex) the somatic states during the pondering of choices ().Citation37 illustrates the possible abnormal pathways, shown as dotted lines, in the chronic pain group that could lead to the observed lack of SCR generation before making disadvantageous choices. The figure is based on the model by Bechara,Citation7 as described in the Introduction.
Patients and controls also displayed different behavior during the IGT. The patient group switched significantly more between advantageous and disadvantageous decks compared to the controls in the performance phase of the test. This is in line with results from previous studies that reported a difference in switching measures among patients.Citation2,Citation4 The current study did not find a significant group difference in the IGT total score between patients and controls. While this is in line with the findings from the largest study conducted thus far on the IGT in chronic pain patients,Citation3 the opposite has been reported in two other studies.Citation2,Citation4 Compared with the reports on the IGT studies that exhibited group differences,Citation2,Citation4 our control group appears to have performed subpar, but based on a review of previous IGT studies, the HCs scored within the range of the control groups.Citation38 Furthermore, the mean score of the current study’s chronic pain group lies between the two chronic pain subgroups of the only past chronic pain study that reported mean scores of the IGT, albeit graphically.Citation4 The current study thus suggests that increased switching and SCR deficits are more sensitive to chronic pain-induced impairments in decision making than in the total IGT score. The lack of standardized scores for the IGT and the general lack of mean score reporting complicate the interpretation and comparison between publications.
Moreover, normal IGT-scores have been seen in subjects without SCRs,Citation39 as other body signals can help construct somatic markers in the brain.Citation7 Thus, it is possible that other bodily signals guided the patient group’s decisions. However, we failed to find any signs of increased reliance on other somatic states (ie, cardiac autonomic measures) in the patient group. Still, decision making in chronic pain patients can be supported by signaling pathways that were not monitored (for example, proprioceptive, vagal, or humoral pathways),Citation7 or by the as–if loop between the effector structures and sensory structures that bypass the body altogether (). Another possibility is that the chronic pain group draws on cognitive resources for decision making, as suggested by the association between cortical volumes and IGT scores in the chronic pain patients.
To our knowledge, this is the first study to show that performance on the IGT correlates with changes in the cerebral morphology of chronic pain patients. The present study demonstrated a strong positive correlation between the IGT total score and cortical gray matter volume in the patient group. Such a correlation was not found for the subcortical structures or the brain stem. Previous clinical studies have showed a relationship between IGT performance and cortical thickness of the ventromedial prefrontal cortex in patient groups with Parkinson’s disease and alcoholism,Citation40,Citation41 but not in controls.Citation42 The current results could suggest that the IGT is sensitive to cortical thinning in chronic pain patients. The location of such thinning cannot be derived from the current results, but all the cortical areas involved in decision making are known to be affected in chronic pain patients.Citation10
The present findings indicate that decision-making deficits in chronic pain patients are dependent on cortical volume rather than on reductions in subcortical structures, including the nucleus accumbens. The latter structure is suggested in the somatic marker hypothesis to be involved both in registering the somatic states and as an effector structure ().Citation37,Citation43 Although this study demonstrated a reduction in nucleus accumbens volume in the patient group, which was in line with previous research,Citation44 no correlation between the size of the nucleus accumbens and the IGT total score in the patient group was found. Furthermore, the amygdala, brainstem, and hippocampus are important for the generation of anticipatory SCRs and decision making (). Although size alone does not determine function, their normal volume in the patient group suggests the observed anticipatory SCR impairment in this group has its neurophysiological correlates elsewhere.
Different mechanisms within the framework of the somatic marker hypothesis could explain the neurophysiology behind the altered decision making in chronic pain patients (see and ). One possibility is that the processing structures (for example, the insula, cingulate cortex, or somatosensory cortices) interpret pain as part of the somatic state. Chronic pain may create a backdrop of noise that increases the time necessary for a somatic marker to form. There is some support for this speculation in the current data, as there is a weak, nonsignificant correlation between IGT score and pain rating immediately before testing (). A not mutually exclusive possibility is that the sensory structures or the aforementioned processing structures are affected by the abnormal amount of pain signals in chronic pain patients, making them less sensitive to normal interoceptive signals that contribute to the formation of the somatic markers. This explanation draws some support from the correlation between the IGT total score and cortical gray matter volume, assuming reduced cortical volume is indicative of reduced sensory functions.
Limitations
Unlike other decision-making tests such as the Cambridge Gambling Task, the IGT is reliant on working memory.Citation45 Differences in cognitive abilities did not seem to contribute to the observed difference in IGT behavior between the chronic pain patients and controls since there were no correlations (data not shown) between the working memory and the different IGT scores in any of the groups.
The IGT procedure in the current study closely resembles that of the original computerized IGT,Citation16 with a few exceptions previously described. Notably, the positions of all four decks were randomized on screen from subject to subject, as opposed to the original computerized IGT, where placements of the advantageous and disadvantageous decks are standardized.Citation16 Studies have shown that decision making is affected by the placement of the options.Citation17,Citation46,Citation47 Randomization of placement is a simple tool that can be used to eliminate any possible confounding effect of placement in the original IGT.Citation18
The number of participants was relatively low, but still higher than in the other studies conducted assessing the IGT in chronic pain groups.Citation2,Citation4 Another limitation is the lack of a common pain etiology in the patient group. In general, the use of heterogeneous groups makes a study more vulnerable to type 2 errors, and there is indication that patients with different pain etiologies may have different changes in brain morphology.Citation48 A recent meta-analysis of studies of changes in brain morphology in chronic pain patients did, however, find significant changes compared to controls.Citation10 This indicates that, although pain etiology-specific differences may be found in brain morphology, different etiologies do have important common findings. The effect sizes of the reported significant findings in the current study do not indicate a type 2 error. Findings in a heterogeneous pain group have stronger external validity than do more homogeneous studies, as chronic pain patients are a mixed-etiology group in real-life settings. A finding in a mixed-etiology pain group is also less likely to be dependent on a cause underlying the pain per se, and increases the probability that the findings are related to chronic pain.
Two-tailed statistical tests were chosen due to the low number of participants in the current study to avoid a type 1 error. While this method increases the chance of a type 2 error, it ensures that any results from the current study are worth further investigation. To avoid too high a risk of a type 2 error, correction for multiple comparisons was not applied.
Conclusion
The current study shows that chronic pain patients have impaired generation of anticipatory SCRs before making disadvantageous decisions, possibly caused by the interpretation of pain as part of the somatic state, or by a reduced ability to process the somatic state due to chronic pain. It can be hypothesized that the patient group compensated for the reduction in anticipatory signals by becoming more dependent on cortical resources in their decision making, and they demonstrated increased switching between advantageous and disadvantageous decks during the IGT.
In summary, the presence of chronic pain was found to affect fundamental aspects of decision making, which may have significant implications for everyday functioning and choices in this patient group.
Acknowledgments
We would like to thank Professor Trond Sand, Department of Neurophysiology, St Olav’s University Hospital, for letting us use his facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
- LandrøNIForsEAVåpenstadLLHoltheØStilesTCBorchgrevinkPCThe extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning?Pain2013154797297723473784
- WalterosCSánchez-NavarroJPMuñozMAMartínez-SelvaJMChialvoDMontoyaPAltered associative learning and emotional decision making in fibromyalgiaJ Psychosom Res201170329430121334501
- Verdejo-GarcíaALópez-TorrecillasFCalandreEPDelgado-RodríguezABecharaAExecutive function and decision-making in women with fibromyalgiaArch Clin Neuropsychol200924111312219395361
- ApkarianAVSosaYKraussBRChronic pain patients are impaired on an emotional decision-making taskPain20041081–212913615109516
- BecharaADamasioARDamasioHAndersonSWInsensitivity to future consequences following damage to human prefrontal cortexCognition1994501–37158039375
- DamasioARThe somatic marker hypothesis and the possible functions of the prefrontal cortexPhilos Trans R Soc Lond B Biol Sci19963511346141314208941953
- ReimannMBecharaAThe somatic marker framework as a neurological theory of decision-making: review, conceptual comparisons, and future neuroeconomics researchJ Econ Psychol2010315767776
- BecharaATranelDDamasioHDamasioARFailure to respond autonomically to anticipated future outcomes following damage to prefrontal cortexCereb Cortex1996622152258670652
- HarlowJMRecovery from the passage of an iron bar through the headBulletin of the Massachusetts Medical Society18682327347
- SmallwoodRFLairdARRamageAEStructural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volumeJ Pain201314766367523685185
- Rodriguez-RaeckeRNiemeierAIhleKRuetherWMayAStructural brain changes in chronic pain reflect probably neither damage nor atrophyPLoS One201382e5447523405082
- MayAChronic pain may change the structure of the brainPain2008137171518410991
- WilliamsonAHoggartBPain: a review of three commonly used pain rating scalesJ Clin Nurs200514779880416000093
- ApkarianAVBalikiMNGehaPYTowards a theory of chronic painProg Neurobiol2009872819718952143
- OldfieldRCThe assessment and analysis of handedness: the Edinburgh inventoryNeuropsychologia197191971135146491
- BecharaATranelDDamasioHCharacterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesionsBrain2000123Pt 112189220211050020
- RodwayPSchepmanALambertJPreferring the one in the middle: further evidence for the centre-stage effectAppl Cogn Psychol2012262215222
- DunnBDDalgleishTLawrenceADThe somatic marker hypothesis: a critical evaluationNeurosci Biobehav Rev200630223927116197997
- Task Force of the European Society of Cardiology and the North American Society of Pacing and ElectrophysiologyHeart rate variability: standards of measurement, physiological interpretation and clinical useCirculation1996935104310658598068
- CleelandCSPain assessment in cancerOsobaDEffect of Cancer on Quality of LifeBoca Raton, FLTaylor and Francis1991293305
- KlepstadPLogeJHBorchgrevnikPCMendozaTRKaasaSThe Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patientsJ Pain Symptom Manage200224551752512547051
- FurmanDJWaughCEBhattacharjeeKThompsonRJGotlibIHInteroceptive awareness, positive affect, and decision making in major depressive disorderJ Affect Disord2013151278078523972662
- BeckATSteerRABrownGManual for the Beck Depression Inventory-II2nd edSan Antonio, TXPsychological Corporation1996
- BecharaADamasioHTranelDAndersonSWDissociation Of working memory from decision making within the human prefrontal cortexJ Neurosci19981814284379412519
- BerrymanCStantonTRJane BoweringKTaborAMcFarlaneALorimer MoseleyGEvidence for working memory deficits in chronic pain: a systematic review and meta-analysisPain201315481181119623707355
- FosLori AGreveKevin WSouthMarne BMathiasCharlesBenefieldHopePaced Visual Serial Addition Test: an alternative measure of information processing speedApplied Neuropsychology20007314014611125707
- BrandMHeinzeKLabuddaKMarkowitschHJThe role of strategies in deciding advantageously in ambiguous and risky situationsCogn Process20089315917318231817
- CavediniPaoloZorziClaudiaBaraldiClementinaPatriniSaraSalomoniGiulianaBellodiLauraFreireRafael CPernaGiampaoloThe somatic marker affecting decisional processes in obsessive-compulsive disorderCognitive Neuropsychiatry201217217719021991936
- CroneEveline AVan Der MolenMaurits WDevelopment of Decision Making in School Aged Children and Adolescents: Evidence From Heart Rate and Skin Conductance AnalysisChild Development20077841288130117650139
- LawrenceNatalia SWoodersonSarahMataix-ColsDavidDavidRhodriSpeckensAnnePhillipsMary LDecision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorderNeuropsychology200610440941916846259
- WernerNatalie SDuschekStefanSchandryRainerRelationships between affective states and decision-makingInternational Journal of Psychophysiology200974325926519808059
- BecharaADamasioHDamasioARLeeGPDifferent contributions of the human amygdala and ventromedial prefrontal cortex to decision-makingJ Neurosci199919135473548110377356
- BrewerJBFully-automated volumetric MRI with normative ranges: translation to clinical practiceBehav Neurol2009211212819847042
- CroneEASomsenRJVan BeekBVan Der MolenMWHeart rate and skin conductance analysis of antecedents and consequences of decision makingPsychophysiology200441453154015189476
- GuillaumeSJollantFJaussentILawrenceNMalafosseACourtetPSomatic markers and explicit knowledge are both involved in decision-makingNeuropsychologia200947102120212419427005
- CarterSSmith PasqualiniMStronger autonomic response accompanies better learning: a test of Damasio’s somatic marker hypothesisCogn Emot2004187901911
- BecharaAThe somatic marker framework and the neurological basis of decision makingEbsteinRShamay-TsoorySChewSHFrom DNA to Social CognitionHoboken, NJJohn Wiley & Sons, Inc.2011157183
- SteingroeverHWetzelsRHorstmannANeumannJWagenmakersEJPerformance of healthy participants on the Iowa Gambling TaskPsychol Assess201325118019322984804
- HeimsHCCritchleyHDDolanRMathiasCJCipolottiLSocial and motivational functioning is not critically dependent on feedback of autonomic responses: neuropsychological evidence from patients with pure autonomic failureNeuropsychologia200442141979198815381028
- Ibarretxe-BilbaoNJunqueCTolosaENeuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s diseaseEur J Neurosci20093061162117119735293
- Le BerreAPRauchsGLa JoieRImpaired decision-making and brain shrinkage in alcoholismEur Psychiatry201429312513323182846
- GanslerDAJerramMWVannorsdallTDSchretlenDJHierarchical organization of cortical morphology of decision-making when deconstructing Iowa Gambling Task performance in healthy adultsJ Int Neuropsychol Soc201218358559422394607
- SimonNWMontgomeryKSBeasBSDopaminergic modulation of risky decision-makingJ Neurosci20113148174601747022131407
- BalikiMNPetreBTorbeySCorticostriatal functional connectivity predicts transition to chronic back painNat Neurosci20121581117111922751038
- RogersRDOwenAMMiddletonHCChoosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortexJ Neurosci199919209029903810516320
- RaghubirPValenzuelaACenter-of-inattention: position biases in decision-makingOrgan Behav Hum Decis Process20069916680
- ValenzuelaARaghubirPPosition-based beliefs: the center-stage effectJ Consum Psychol2009192185196
- BalikiMNSchnitzerTJBauerWRApkarianAVBrain morphological signatures for chronic painPLoS One2011610e2601022022493
