Abstract
Many people worldwide suffer from pain and a portion of these sufferers are diagnosed with a chronic pain condition. The management of chronic pain continues to be a challenge, and despite taking prescribed medication for pain, patients continue to have pain of moderate severity. Current pain therapies are often inadequate, with side effects that limit medication adherence. There is a need to identify novel therapeutic targets for the management of chronic pain. One potential candidate for the treatment of chronic pain is therapies aimed at modulating the vasoactive peptide endothelin-1. In addition to vasoactive properties, endothelin-1 has been implicated in pain transmission in both humans and animal models of nociception. Endothelin-1 directly activates nociceptors and potentiates the effect of other algogens, including capsaicin, formalin, and arachidonic acid. In addition, endothelin-1 has been shown to be involved in inflammatory pain, cancer pain, neuropathic pain, diabetic neuropathy, and pain associated with sickle cell disease. Therefore, endothelin-1 may prove a novel therapeutic target for the relief of many types of chronic pain.
Discovery of endothelin-1 as an algogen
Endothelin-1 (ET-1) was discovered by its vasoconstrictive effects on pig arteries.Citation1,Citation2 It was thereafter isolated, cloned, and extensively studied as a new endogenous vasoactive peptide. Since then, the endothelin family has been implicated in several human pathological conditions, including congestive heart failure,Citation3 hypertension, sepsis, asthma, renal failure, cerebral vasospasm, and neoplasia.Citation4 Of great surprise, experimental administration of ET-1 in a human volunteer produced severe and long-lasting pain.Citation5 This discovery created a paradigm shift in the investigation of ET-1 as not solely a vasoactive peptide, but potentially as an endogenous algogen important in the modulation of pain. Many pathological conditions in which pain is a major symptom show an elevation in plasma ET-1. This includes painful vaso-occlusive crisis and acute chest syndrome associated with sickle cell disease, Raynaud’s disease, prostate cancer,Citation6 breast cancer, and complex regional pain syndrome. Thus, there is growing interest in the endothelin family and its receptors as novel therapeutic targets for the treatment of pain.
Cellular and molecular biology of endothelin-1
ET-1 was named after the cells that were first known to produce it: endothelial cells. It is now recognized that ET-1 is produced by a variety of other cells including vascular smooth muscle cells,Citation7 leukocytes, cardiac myocytes,Citation8,Citation9 mesangial cells,Citation10 neurons,Citation11 mast cells,Citation12 and macrophages.Citation13 ET-1 belongs to a family of closely related peptides that include endothelin-2 (ET-2), endothelin-3 (ET-3),endothelin-4 (ET-4), and the sarafotoxins. These are all similar in structure to ET-1 but are separate gene products with tissue-specific expression.Citation3,Citation4
Synthesis and release of ET-1 is modulated by both physiological and pathophysiological factors. Factors that have been shown to inhibit the production of ET-1 include nitric oxide, prostacyclin, and atrial natriuretic hormone.Citation14 Factors that can induce the production of ET-1 include proinflammatory cytokines, growth factors, angiotensin II, norepinephrine, vasopressin, bradykinin, thrombin, mechanical stress, peripheral tissue injury, and hypoxia.Citation15–Citation17 In the vascular system, ET-1 is released from endothelial cells in a polarized fashion toward the smooth muscle interface.Citation18 The elevation of plasma ET-1 levels following vascular injury is likely the result of spill-over from the smooth muscle compartment. ET-1 is thus acting as a local modulator of vascular tone, and not as a circulating hormone. ET-1 has a half-life of 7 minutesCitation19 and is cleared from plasma after degradation by endopeptidases.Citation20,Citation21
The endothelin family signals through the seven transmembrane G protein-coupled endothelin A (ETA) receptorCitation22 and the ETB receptor.Citation2,Citation23,Citation24 The two receptors can be differentiated by agonists and antagonists and in their cellular distributions ().Citation23 The ETA receptor binds ET-1 with the greatest affinity,Citation25 whereas ET-1, ET-2, and ET-3 all have equal affinity for the ETB receptor.Citation26 The ETB receptor has further significance in ET-1 tissue clearance via agonist-dependent receptor internalization and lysosomal degradation.Citation20,Citation21
Table 1 ET receptor agonists and antagonists commonly used in basic research and clinical studies
The ETA receptor couples to Gq/11 and Gq12/13 proteins, whereas the ETB receptor couples to Gi/o and Gq/11 proteins.Citation27 Both receptors mediate their actions through phospholipase C activation, which in turn activates inositol triphosphate and diacylglycerol, causing a mobilization of calciumCitation28 and activation of protein kinase C (PKC),Citation4,Citation29 as well as PKC-independent signaling pathways.Citation4
Clinical significance
ET-1 in the pain pathway
ET-1 and its receptors are found throughout the pain signaling pathway (). In the periphery, ETB receptors are expressed on endothelial cells,Citation30 smooth muscle cells,Citation31 macrophages,Citation32 and keratinocytes within the dermis and epidermis of the skin, where the peripheral endings of nociceptors are located. In contrast, ETA receptors are expressed on the peripheral endings of the nociceptor themselves, as well as on nerve axons and the nociceptor cell bodies located in the dorsal root ganglion (DRG).Citation33 ETA receptors have been found on small, medium, and large-sized nociceptors.Citation34 In contrast, in the DRG, ETB receptors are found exclusively on satellite glial cells and Schwann cells that myelinate nociceptors, but not on the nociceptors themselves.Citation33–Citation35 ETA and ETB receptors are expressed in the DRG in a ratio of 60:40, respectively.Citation36
Figure 1 A schematic of the location of endothelin-1 (ET-1) and endothelin receptors found throughout the pain pathway.
Abbreviation: ET, endothelin-1.
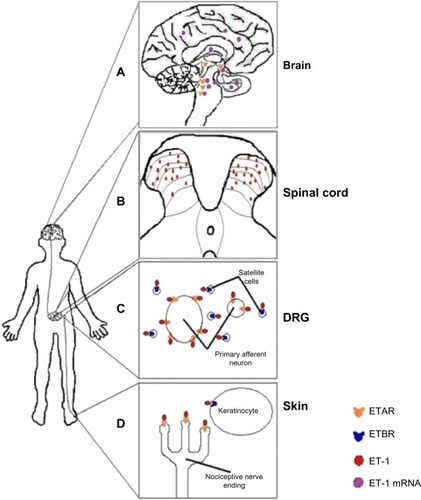
In humans, both small and large nociceptors also express endogenous ET-1 mRNA.Citation37 Brief exposure of nociceptors to ET-1 enhances action potential generation, possibly through decreasing delayed rectifier-type K+ currents.Citation38 In addition, ET-1 evokes hyperpolarizing shifts in tetrodotoxin-resistant sodium channels on nociceptors.Citation28,Citation39 Alteration in these specific channels is known to be involved in pain transmission caused by other algogens.Citation40 The shifts in the tetrodotoxin-resistant sodium currents may be enough to cause depolarization of sensory fibers and reduce the threshold for activation (hyperalgesia) of those fibers.Citation36
ET-1 mRNA is also found in laminae IV–VI in the spinal cordCitation37 and in a variety of brain regions associated with pain signaling, including the hippocampus, striatum, amygdala, hypothalamus, raphe nucleus, locus coeruleus, cerebral cortex, pontine tegmentum, and lateral reticular formation.Citation41,Citation42 Similarly, ET-1 protein is found extensively in laminae I–V,Citation43 and ETA receptors have been found in the hypothalamus, reticular formation, pontine tegmentum, locus coeruleus, and substantia nigra.Citation44,Citation45 Thus, the endothelin system is located across multiple levels of the pain neuroaxis and participates in signaling cascades, the final effectors of which are key players in the physiology of acute pain.
ET-1 in acute pain
Peripheral administration of ET-1 produces pain and hyperalgesia in humans as well as paw flinching, licking, mechanical hyperalgesia/allodynia, and thermal hyperalgesia/allodynia in dogs, rats, and mice.Citation46–Citation49 ET-1 has been shown to directly activate nociceptors in humans and animals.Citation50,Citation51 In addition, ET-1 potentiates nociception induced by other algogens such as formalin, serotonin, and capsaicin.Citation52 Similar to other algogens, central administration of ET-1 has opposite effects and produces antinociception.Citation53
Human studies
In human volunteers, ET-1 (125 μg) administered into the brachial artery produced a sensation of heat, followed by vomiting and sweating and a deep muscular pain, with maximum severity at 2 hours and resolution at 10 hours; the pain was intensified by touch and muscular contractions.Citation5 Lower doses of ET-1 (12.5–50 μg) administered intradermally into the forearm produced intense itching, erythema, increased sensitivity to pinching, and pressure tenderness that subsided after 2 hours.Citation46 In a similar study, intradermal administration of ET-1 (100 or 10 ng) resulted in spontaneous ongoing pain, with maximal pain score within 1 minute, and returned to normal after 30–60 minutes.Citation49 At these doses, tenderness to mechanical stimulation (von Frey filaments) and cold hyperalgesia developed and outlasted the observation period of 120 minutes; heat hyperalgesia did not develop.Citation49 Similar to intradermal, intracutaneous administration of ET-1 (25 ng–0.25 pg) produced burning and stinging pain in 9 of 34 subjects, itch sensation in 11 of 34, and a mixture of both sensations in 14 of 34 subjects.Citation51 These studies indicate that arterial administration of ET-1 produced long-lasting allodynia and deep muscular pain. Similarly, dermal administration of ET-1 produced itching and mechanical allodynia in low doses, whereas moderate doses produced spontaneous pain, mechanical hyperalgesia, and cold hyperalgesia (summarized in ).Citation46,Citation49,Citation51
Table 2 Effect of endothelin-1 administration in humans
Single-fiber recordings from the peroneal nerve of human participants showed that ET-1 (25 and 2.5 ng) produced activity in 65% of mechanosensitive, but not mechanoinsensitive, fibers with up to a third of the fibers having long-lasting responses (up to 15 minutes).Citation51 Both fiber types showed sensitization to heat after ET-1 injection, with 62% of mechanosensitive fibers responding and 46% of mechano-insensitive fibers.Citation51 It is also interesting that ET-1 produced a different pattern of activity compared with the algogens capsaicin and histamine. In contrast to the long-lasting responses in mechanosensitive fibers after ET-1 administration, capsaicin and histamine cause short bursts of activity in mechanosensitive fibers and longer-lasting activation patterns in mechano-insensitive fibers.Citation54 Thus, these studies suggest that ET-1 directly induces pain via mechanosensitive C-fibers and heat sensitization via both mechanosensitive and mechano-insensitive fibers. The firing pattern suggests that the mechanism of the pain-inducing actions of ET-1 is different from those of capsaicin and histamine.
Rodent studies: spontaneous nociceptive behaviors
As in humans, exogenous administration of ET-1 produces spontaneous pain-associated behaviors, as well as thermal and mechanical hyperalgesia and allodynia in rodents. Intradermal administration of high doses of ET-1 produces C and Aδ fiber activity as well as activity in a subpopulation of Aβ fibers.Citation50 In general, C and Aδ fibers relay nociceptive information, whereas Aβ fibers relay nonpainful tactile information. Flinching behaviors are absent when epinephrine (vasoconstrictor) alone is applied or when ET-1 is applied to surrounding muscle not innervated by the sciatic nerve.Citation55 This finding lends further support to ET-1-induced nociception being independent from its vasoconstrictor effects and is likely through direct activation of nociceptors. In addition to causing spontaneous behaviors after a single subcutaneous administration, a second administration of ET-1 1 day later produces desensitization in the same paw and sensitization in the contralateral paw, dependent on afferent transmission.Citation56
In the majority of studies, spontaneous ET-1-induced abdominal constrictions and ET-1-induced hind paw licking or flinching in the glabrous skin are mediated by the ETA receptor.Citation46,Citation47,Citation50,Citation57–Citation61 In contrast, ET-1 administration into the hairy skin induces transient antinociception mediated by the ETB receptor.Citation62
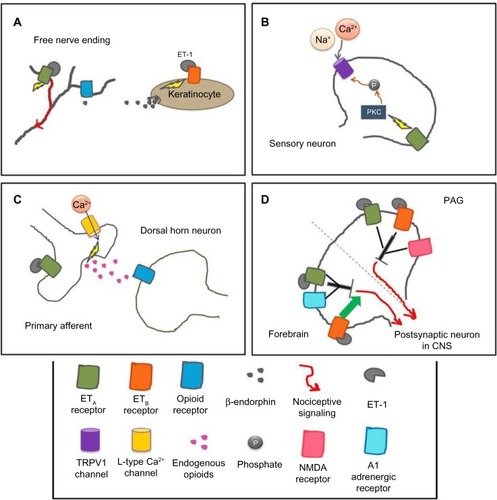
Species differences appear when examining the role of ETB receptors in ET-1-induced nociception. In mice, ETB receptors have no effects in ET-1-induced licking behavior.Citation58,Citation60 In the mouse cheek model, ET-1 causes both pruritic and nociceptive behaviors, and injection of an ETB antagonist causes potentiation of these behaviors, whereas coinjection of an ETA antagonist with the ETB antagonist significantly attenuates both behaviors.Citation63 In contrast, in rats, agonists of the ETB receptor have antinociceptive effects, which are dependent on endogenous opioid release.Citation50,Citation59,Citation61,Citation64 Activation of ETB receptors on keratinocytes induces the release of β-endorphins, which activate μ-opioid receptors on nearby nociceptors, causing hyperpolarization and, ultimately, a decrease in nociception ().Citation64
Figure 2 Mechanisms of the endothelin system in various parts of the pain pathway at the cellular level in rodents.
Abbreviations: CNS, central nervous system; TRPV1, transient receptor potential vanilloid subfamily; NMDA, N-methyl-D-aspartate; PAG, periaqueductal gray; ET, endothelin-1.
Rodent studies: thermal and mechanical hyperalgesia
Similar to studies in humans, low doses of ET-1 produce a localized mechanical hyperalgesia in rodents.Citation46,Citation65,Citation66 At higher doses, ET-1 produces a systemic mechanical hyperalgesia in both the ipsilateral and contralateral hindpaws.Citation56 ETB receptor activation mediates the mechanical hyperalgesia induced by high doses of ET-1, which is in contrast to the analgesic activity of ETB receptor activation in ET-1-induced spontaneous nociception.Citation67,Citation68 In mouse models of inflammatory pain, ETA receptors are involved in both thermal and mechanical hyperalgesia, whereas ETB receptors are only involved in mechanical hyperalgesia.Citation67 It has also been shown that calcitonin gene-related peptide (CGRP) and N-methyl-D-aspartate receptor antagonists can attenuate ET-1-induced mechanical sensitization.Citation69 In addition, the mechanism involved in this mechanical hyperalgesia appears to be independent of ET-1-induced vasoconstriction, sympathetic nervous system activation, and prostaglandins.Citation65
In humans, intradermal ET-1 induces thermal hyperalgesia by enhancing sensitivity of C-fiber nociceptors to heat.Citation51 Similarly in mice and rats, low doses of ET-1 have been reported to produce thermal hyperalgesia.Citation56,Citation60 Studies with ETA and ETB receptor antagonists generally support a prohyperalgesic role of the ETA receptor in thermal hyperalgesia and an unclear role for the ETB receptor.Citation60,Citation70,Citation71 However, ET-1-induced thermal hyperalgesia occurs similarly in both conditional ETA knockout mice and control mice.Citation72 This suggests that thermal hyperalgesia occurs independent of ETA receptors while highlighting the need for further study.
The transient receptor potential vanilloid subfamily (TRPV1) channel may play a role in ET-1-induced thermal hyperalgesia. In mice with conditional deletion of the ETA receptor on sensory neurons, thermal and mechanical sensitivities were unaffected; however, capsaicin-induced nocifensive behaviors were significantly reduced.Citation72 TRPV1 channels are colocalized with ETA receptors on sensory neurons in the DRG, and both endothelin receptors are co-localized with TRPV1 channels on neurons in the rat trigeminal ganglia.Citation34,Citation73 ET-1 potentiates capsaicin-induced TRPV1 currents via activation of PKCε.Citation73,Citation74 Therefore, activation and sensitization of nociceptors by ET-1 may be mediated by ETA receptors activating PKCε, which targets TRPV1 channels (). In support of this mechanism, TRPV1 knockout mice show reduced ET-1-induced thermal hyperalgesia.Citation70 In addition, in HEK293 cells expressing both ETA and TRPV1 channels, ET-1 evokes inward current responses, which are not seen in cells expressing only ETA receptors. In HEK293 cells and in the skin, ET-1 leads to the phosphorylation of TRPV1.Citation70 Together, these results suggest there is an interaction between ETA and TRPV1 channels that may have pronociceptive effects.
Rodent studies: potentiation of algogens
Formalin, capsaicin, and serotonin are algogens that induce spontaneous nociceptive behaviors when administered subcutaneously in rodents. In rats and mice, ET-1, through ETA and ETB receptors, potentiates formalin-induced nociception; this effect is also seen contralaterally in rats.Citation52,Citation56,Citation71 In addition, ET-1 potentiates capsaicin-induced nociception via the ETA receptorCitation75 and enhances capsaicin-stimulated release of CGRP in sensory neurons and c-Fos expression in the dorsal horn of the spinal cord.Citation69,Citation76 Serotonin-primed capsaicin-induced nociception is abolished by administration of high doses of ET-1 or an ETB receptor agonist.Citation75 The general hypothesis is that at lower ET-1 doses, the ETA receptor potentiates capsaicin-induced hyperalgesia, but at higher ET-1 doses, the ETB receptor mediates an antinociceptive effect. This is similar to the working model, in which a high dose of ET-1 induces algesia via ETA receptors and a concurrent analgesic effect mediated via ETB receptors.Citation55
Rodent studies: acute postsurgical pain
The endothelins have been implicated in postsurgical pain, using a rat model of postincisional pain in which both primary and secondary mechanical allodynia and hyperalgesia develop.Citation77 The ETA receptor mediates primary and secondary mechanical allodynia. In contrast, the ETA receptor mediates secondary hyperalgesia, but not primary hyperalgesia.Citation77 These findings suggest that local blockade of ETA receptors before incision may be a viable therapeutic approach for reducing postincisional pain.
ET-1 in the central nervous system
In contrast to peripheral studies showing ET-1 as an algogen, administration of ET-1 into the central nervous system is often antinociceptive. Intrathecal ET-1 produces dose-dependent thermal analgesia in mice that is reduced by the nonspecific opioid receptor antagonist naloxone or a δ-opioid receptor antagonist.Citation78 The analgesic actions of intrathecal ET-1 administration occur through activation of L-type Ca2+ channels and the release of endogenous opioids ().Citation78 Although ET-1 administered peripherally enhances formalin-induced nociception, intrathecal ET-1 decreases formalin-induced nociception via the ETA receptor.Citation79 Similarly, transgenic mice that overexpress ET-1 in astrocytes have a significant decrease in nociceptive behaviors during the second phase of the formalin test compared with nontransgenic mice, further supporting an antinociceptive role for ET-1 in the spinal cord.Citation80
Administration of ET-1 into the periaqueductal gray (PAG) of mice produces thermal analgesia mediated by both the ETA and ETB receptors and dependent on N-methyl-D-aspartate activation ().Citation53,Citation81 Intracerebroventricular ET-1 dose-dependently produces analgesia to thermal stimuli,Citation82 which is mediated by the ETA receptor and the α1 adrenergic receptor and antagonized by the ETB receptor ().Citation82 Thus, ET-1’s analgesic actions in the brain may be mediated via ETA and ETB receptors through a noradrenergic pathway that activates a descending inhibitory pathway to the spinal cord, possibly through the release of endogenous opioids.
Age and sex differences
Cardiovascular studies suggest that the biological activity of ET-1 in humans varies depending on age, sex, and concentration of ET-1. Some of this variability results from sex- and age-dependent expression of ETA and ETB receptors. In the saphenous vein, postmenopausal women demonstrate a 1:1 ratio of ETA:ETB expression compared with age-matched male ratios of 3:1 expression.Citation83 Age-specific expression patterns have also been demonstrated, with infants having higher peripheral levels of ET-1Citation84 and a higher density of endothelin receptors in the heart compared with older children and adults,Citation85 suggesting a decrease in ET-1 activity after infancy.
Similarly, adult rodents show sex differences in the onset of ET-1-induced mechanical hyperalgesia, with a more rapid onset of hyperalgesia in adult males and ovariectomized females when compared with intact females.Citation86 In addition, male and ovariectomized female rats, but not intact females, show progressively increased hyperalgesia after repeated mechanical stimulation. In neonatal rats, ET-1-induced spontaneous licking and flinching behaviors are age- and sex-dependent.Citation61 ET-1-induced nociceptive behaviors are significantly greater in younger animals and decrease with age. At early ages, males show increased nociception compared with females. The sex difference in ET-1-induced spontaneous nociception decreases with maturation. Similarly, an age- and sex-dependence was observed for the priming effect of ET-1 on subsequent ET-1 exposures, which is important in certain disease states in which there are repeated exposures to ET-1, such as sickle cell disease. Exposure to ET-1 during the neonatal period produced an increase in nociceptive behaviors in response to a second exposure to ET-1 4 days later in male rats.Citation87 In contrast, a priming exposure to ET-1 in neonatal female rats decreased nociceptive behaviors when exposed to a second administration of ET-1.Citation87 Furthermore, sensitization to a second ET-1 exposure was also observed in 2-month-old male rats, but the desensitization to a second ET-1 exposure was not observed in older females. This suggests a long-lasting sensitization in male rats exposed to ET-1 in the neonatal period and a short-lasting desensitization in female rats. A subsequent study showed that a first exposure to ET-1 at 3 weeks after birth did not show the same priming to a second exposure to ET-1 as found in the neonatal rats, suggesting a window of vulnerability for ET-1-induced priming of nociceptive responses.
Mechanistically, ET-1-induced priming in neonatal males decreased ETB receptor expression in the skin and sensitized the rats to subsequent ET-1 administration. In neonatal females, ET-1-induced priming increased ETB receptor expression in the skin and desensitized the rats to subsequent ET-1 administration. No changes in ETA or μ-opioid receptor expression were found.Citation87 Furthermore, ET-1-induced sensitization in neonatal males could be prevented with morphine administration at the time of the priming dose of ET-1, which prevented the downregulation of ETB receptor expression. These studies highlight the importance of the ETA-to-ETB receptor ratio in the modulation of ET-1-induced pain.
ET-1 in inflammatory pain
Endogenous ET-1 is implicated as a mediator in inflammatory pain. ET-1 is released during the inflammatory process, and its production is stimulated by the inflammatory mediators interleukin 1β, tumor necrosis factor α, and tumor necrosis factor β.Citation88,Citation89 In addition, human neutrophils synthesize ET-1, providing an additional source of ET-1 in inflammatory states.Citation77 Both acute (carrageenan) and chronic (complete Freund’s adjuvant) inflammatory rodent models produce thermal and mechanical hyperalgesia that is modulated by endogenous ET-1 signaling. Thermal hyperalgesia in these mouse models appear to be primarily modulated by ETA receptors.Citation67 However, in a rat complete Freund’s adjuvant model, both endothelin receptors appear to be involved in modulating thermal hyperalgesia with a more pronounced role for the ETB receptor.Citation90 Both endothelin receptors are also involved in modulating carrageenan- and complete Freund’s adjuvant-induced mechanical hyperalgesia.Citation67,Citation90 One theory for the role of the ETB receptor in inflammatory pain is that activation of the ETB receptor causes the release of tumor necrosis factor α and interleukin 6, which activate nociceptors to produce mechanical hyperalgesia. Nociception induced by ET-1 in inflammatory pain is mediated via different receptors, depending on the type of inflammation.Citation91,Citation92 In the naïve rat joint, ETA receptors mediate inflammation; in the carrageenan-primed joint, both endothelin receptors are involved; and in the carrageenan-primed joint challenged with lipopolysaccharide, the ETB receptor mediates the effects. These studies show that the interaction between inflammation and the endothelin system is highly complex and depends on the specifics of the inflammation.
One common inflammatory pain condition is arthritis, and it has been shown that ET-1 plasma levels are increased in patients with rheumatoid arthritis and osteoarthritis compared with control patients.Citation89 ET-1 levels are also higher in patients with active rheumatoid arthritis compared with those with inactive rheumatoid arthritis.Citation89 In a mouse model of antigen-induced arthritis, both the genes encoding for ET-1 and the ETA receptor were upregulated in lumbar DRGs.Citation93 A mixed ETA/ETB receptor antagonist inhibited acute and chronic inflammation during antigen-induced arthritis flare-up reactions. Similarly, in an ovalbumin-induced mouse model of chronic arthritis, a mixed ETA/ETB receptor antagonist or a selective ETA receptor antagonist reduced nociceptive behavior, whereas a selective ETB receptor antagonist enhanced nociception.Citation94 These studies suggest that the pain caused by arthritis may be alleviated via ET receptor antagonists; however, the dichotomy of ETB receptor actions in these models remains to be elucidated.
Specific diseases
Cancer
ET-1 protein or mRNA is hypersecreted by many cancer types, such as prostate, breast, pancreatic, colon cancer, and human oral squamous carcinoma cell lines.Citation4,Citation95,Citation96 ET-1 is believed to be involved in many aspects of cancer progression, such as tumor growth and cell proliferation.Citation6 Pain is prevalent in about 70% of people with metastatic cancers such as prostate and breast cancer.Citation97 Exogenous application of ET-1 in animal models of cancer has been found to potentiate cancer-induced nociception and sensitization of C-fibers to heat via activation of ETA receptors, a similar pathway as that seen in ET-1-induced potentiation of formalin- and capsaicin-induced nociception.Citation98,Citation99 It has been shown that the concentration of ET-1 has a more direct correlation to pain levels than the size of the tumor in mice,Citation100 illustrating the importance of evaluating algogens released by cancerous tumors, such as ET-1, to identify novel therapeutic targets to treat cancer pain.
Broadly speaking, there are two categories of endothelin-derived tumors: those that hypersecrete ET-1, upregulate ETA receptors, and moderately downregulate ETB receptors (colon, ovarian, pancreatic, prostate, and renal cell carcinoma), and those that hypersecrete ET-1, upregulate ETB receptors, and moderately downregulate ETA receptors (lung and breast).Citation6 Several types of cancer cells are known to produce ET-1 in culture or after injection into animal models, as indicated by increased ET-1 protein and/or mRNA levels.Citation96,Citation101–Citation104 These high levels of ET-1 seem to correlate with increases in nociceptive behaviors, mechanical hyperalgesia, and mechanical allodynia, which are mediated by ETA receptors.Citation72,Citation101–Citation104 In murine models of cancer pain, thermal hyperalgesia is significantly increased, which may be mediated by ETB receptors in the early stage and the ETA receptor in long-lasting hyperalgesia.Citation96,Citation105 Clinical studies using the ETA receptor antagonist atrasentan have already shown some promise in improving cancer-related pain.Citation4 These results suggest that targeting the endothelin receptors may prove a novel pain-reducing therapy in cancer pain.
Diabetes
Patients with diabetes often develop sensory neuropathy characterized by alterations in their ability to sense the environment. Diabetic neuropathy can manifest as a painless syndrome, in which there is a loss of touch, temperature, and pain sensation, or as a painful syndrome, in which thermal, mechanical, and tactile hyperalgesia and/or allodynia are present.Citation35 The ET-1 system has been affected in diabetic neuropathy in experimental animals. In a model of type 1 diabetes using streptozotocin (STZ), plasma ET-1 immunoreactivity is significantly increased and ETB receptors have an abnormal appearance on satellite cells, in addition to a decrease in expression.Citation35,Citation106 STZ-treated animals and transgenic rats (DBH-ETB:ETBsl/sl) exhibit significant increases in both mechanical hyperalgesia and tactile allodynia.Citation35 Tactile allodynia of STZ-treated rats can be reduced with acute or chronic inhibition of ETA receptors, but not ETB receptors alone or effectively with inhibition of both receptors.Citation107 Intrathecal administration of ET-1 produces antinociception in control but not STZ-treated mice, unless the dose of ET-1 is reduced, which produces dose-dependent antinociception in STZ-treated but not control mice.Citation108 This suggests there may be an upregulation of endothelin receptors in the STZ model of diabetic mice. ET-1 and its receptors are implicated in diabetic neuropathies, as suggested in the aforementioned animal models. The dichotomy of endothelin receptors still remains to be fully characterized, and further investigation is needed. Use of endothelin receptor antagonists in the treatment of diabetic pain is yet to be researched in humans.
Neuropathic pain
Neuropathic pain is a type of chronic pain in which nerve damage has occurred, and it is often difficult to diagnose and treat effectively. The endothelin system has been implicated in being directly involved in the development of neuropathic-like pain in animal models. In a model of trigeminal neuralgia, inducing injury to the trigeminal nerve results in mechanical allodynia that is only reduced by ETB receptor antagonism, as opposed to an ETA or mixed receptor antagonist.Citation109 Cold hyperalgesia also develops in this model, and this effect is suppressed by both ETB and ETA receptor antagonists.Citation110 In a mouse model of complex regional pain syndrome type 1, ET-1 administration causes hypersensitivity and an ETA receptor antagonist reduces ET-1-induced sustained nociceptive behaviors, whereas an ETB receptor antagonist enhances those behaviors.Citation111 In the chronic constriction injury and spinal nerve ligation (SNL) peripheral nerve injury models, the ETA and both ETA and ETB receptors, respectively, play important roles in the development of nociceptive-associated behaviors.Citation112,Citation113 In the chronic constriction injury model, ET-1 mRNA and ETA receptor mRNA and protein are increased in the nerve and at the site of injury.Citation112 In the SNL model, both ETA and ETB receptor proteins are increased in the injured nerve.Citation113 In rats with SNL, injection of ET-1 into the hind paw causes significantly greater nociceptive behaviors compared with sham animals, which are attenuated by an ETA receptor antagonist. SNL also causes an enhancement of ET-1-induced increases in intracellular calcium in neuronal cells, which may help explain the increase in nociceptive behaviors that develops after ET-1 administration in those animals.Citation113 These studies suggest the endothelin system may provide an effective target in pain therapy for neuropathic pain conditions.
Gastrointestinal disorders
The endothelin system has been implicated in playing a role in some forms of visceral pain. In mice, an intraperitoneal injection of ET-1 causes rapid onset of abdominal constrictions.Citation114 Mice lacking either one or both copies of the ETB receptor gene have significantly reduced or absent abdominal constrictions in response to phenylbenzoquinone, which is used to elicit overt nociception in the abdomen.Citation115 The endothelin system is also known to play an important role in the development of the enteric nervous system. When the ET-3 or ETB receptor gene is deleted, mice develop a condition similar to Hirschsprung’s disease in humans, in which the enteric nervous system fails to innervate the colorectum, leading to distension of the bowel.Citation116 These mice also display a lack of nociceptive response to distension of the rectum compared with wild-type mice, which is thought to be a result of an impairment in the signaling from low-threshold, wide-dynamic range afferents from the rectum.Citation116
In humans, ET-1 has been found to be elevated in patients with active inflammatory bowel disease,Citation117 Crohn’s disease, and ulcerative colitis,Citation118,Citation119 which are gastrointestinal conditions known to have pain as a major symptom, compared with controls. Although the endothelin system has not been directly linked to the pain associated with these conditions, it may be worth exploring, as ET-1 has been implicated in other inflammatory pain conditions.
Sickle cell disease
Children and adults with sickle cell disease (SCD) experience recurrent, unpredictable painful vasoocclusive episodes. The endothelin pathway has been implicated in this type of painful episode, both as a trigger in the onset of vasoocclusive episodes and as a direct activator of nociceptors.Citation57,Citation114 Red blood cells and sickled red blood cells from homogenous sickle cell patients cause an increase in the release of ET-1 and the induction of ET-1 mRNA, respectively, from endothelial cells, suggesting ET-1 may be an important factor in facilitating vasoocclusive episodes.Citation120,Citation121 In endothelial cells exposed to plasma from SCD patients at different disease stages, there is an increased production of ET-1 during acute chest syndrome, with the highest levels of ET-1 production occurring with samples that were taken 4 days before hospital admittance for vasoocclusive episodes.Citation122 Increased circulating ET-1 levels have been demonstrated clinically in sickle cell patients during vasoocclusive episodes.Citation122–Citation126 Hydroxyurea is used in the treatment of painful vasoocclusive episodes, and it has been shown to downregulate ET-1 gene expression in endothelial cells.Citation127,Citation128 Children with SCD treated with hydroxyurea have been shown to have levels of circulating ET-1 that were two times lower than those of untreated SCD children or controls.Citation129 Current research is being conducted to determine the relationship of the endothelin pathway and painful vasoocclusive episodes.
Vasoocclusive pain has been modeled in rats by injecting endothelin in the hind paw. One hypothesis is that prior exposure to ET-1 will alter behavioral responses to subsequent ET-1 administration in a sex-specific manner. Young male rats become sensitized to pain after being “primed” with ET-1, whereas females appear to become desensitized to pain after being primed with ET-1.Citation61 Additional results suggest that these differences between males and females may be a result of changes in the ETB receptor.Citation87 In a mouse model of SCD, the ET-1 gene has been found to be upregulated after chronic exposure to hypoxia, and a mixed ETA/ETB receptor antagonist can prevent hypoxia-induced vasoocclusive episodes.Citation130,Citation131 Clinical studies have also been done to examine correlations between ET-1 levels and pain levels. Elevated plasma levels of ET-1 have been found in symptomatic SCD patients, and these levels increase with increased pain ratings.Citation124 In these patients, ET-1 levels decreased with a decrease in pain levels and a subsiding of symptoms but did not reach levels seen in healthy controls. Similarly, high plasma levels of ET-1 and Big Endothelin, the precursor of ET-1, in children with SCD were positively correlated with higher baseline pain ratings and greater baseline pain responses, respectively, before venipuncture.Citation132
In addition to contributing to painful episodes in SCD, the endothelin system may also be involved in the sickling of red blood cells and other complications of SCD. ET-1 has been shown to play a role in modulating the activity of the Gardos channel, which is important in sickle erythrocyte dehydration, in erythrocytes via the ETB receptor.Citation133–Citation135 A polymorphism in the gene encoding for ET-1 is associated with the occurrence of vasoocclusive crisis and acute chest syndrome, a common complication of SCD, in children with SCD.Citation136 A disruption of nitric oxide homeostasis has been implicated in SCD; one of its many consequences is an increase in ET-1.Citation137–Citation139 Nitric oxide that is derived from endothelial cells regulates ET-1 expression, which contributes to the modulation of vessel tone.Citation139
These studies expose the need for a better understanding of the role of the endothelin system in SCD and the pain associated with it, so that alternative treatments for SCD can be explored.
Morphine analgesia/tolerance
Endothelin antagonists have not only been found to be analgesic themselves but have also been shown to enhance the analgesia of morphine. Centrally administered ETA receptor antagonists have been shown to increase morphine analgesia.Citation140 Similarly, centrally administrated ETA receptor antagonists may reverse opioid tolerance and rescue opioid analgesia.Citation140 In contrast, an ETB receptor agonist does not increase morphine analgesia.Citation141 These studies suggest that centrally administered ETA receptor antagonists may enhance and extend the time of morphine analgesia, in addition to decreasing morphine tolerance. Thus, ETA receptor antagonists may not only reduce pain experienced but may also aid other analgesics in combination therapies.
Implications to human disease and conclusions
The current research investigating the nociceptive effects of ET-1 in rodent models all concur that ET-1 receptors may prove a novel target for pain-relieving therapies. Studies using antagonists have shown that activation of ETA receptors in the periphery is pronociceptive (); therefore, blocking this receptor would provide pain relief. Identifying the actions of the ETB receptor presents a more challenging feat (). The effects of the receptor appear to be pronociceptive in mediating mechanical hyperalgesia in low and high doses.Citation46,Citation65,Citation68 Conversely, this receptor appears to be analgesic when high doses of ET-1 are administered in the vicinity of keratinocytes.Citation59 In other animal models, it appears that activation of the ETB receptor has no effect on nociception ().Citation58,Citation60 These seemingly contradictory findings may be the result of differences in the species used, sex differences, ET-1 doses, nociceptive testing procedures, or other such caveats. Therefore, further studies are needed to elucidate the value of selective ETB receptor antagonists and agonists in pain-reduction therapies.
Table 3 Participation of ETA and ETB receptors in different models
Several human disease states are candidates for ETA receptor antagonist pain reduction therapies. Diseases implicated include diabetic neuropathies, trigeminal neuralgia, crushed nerve syndrome, chronic arthritis, and many types of cancer pain (bone, oral, prostate, and breast). In addition to these diseases, several others have been implicated as ET-1 receptor antagonist clinical trial candidates on the basis of their overproduction of ET-1. Research on ET-1’s involvement in several painful conditions such as sickle cell crisis, acute chest syndrome, complex regional pain syndrome, and Raynaud’s syndrome are currently being investigated, with pain-relieving results being reported. For example, three patients with secondary Raynaud’s phenomenon received the mixed ETA/ETB receptor antagonist bosentan twice daily for 4 weeks, followed by an increased dose twice daily for 12 weeks. Patient questionnaires using the visual analog scale revealed that pain severity decreased significantly in all patients during the treatment period.Citation142 ET-1 was investigated as a potential marker for patients admitted to the emergency room for a sickle cell pain crisis and in patients with complex regional pain syndrome.Citation143,Citation144
At this time, the only licensed ET receptor antagonist medications available in the United States and Europe are bosentan, a dual ETA/ETB receptor antagonist, and ambrisentan, a selective ETA receptor antagonist, for the treatment of pulmonary hypertension.Citation145 The ETA receptor antagonists or combined ETA/ETB receptor antagonists are currently in clinical trials for the treatment of diseases, including prostate, kidney, ovarian, fallopian, and peritoneal cancer, as well as diabetic neuropathy, diastolic heart failure, pulmonary arterial hypertension, pulmonary fibrosis, scleroderma, and subarachnoid hemorrhage.Citation145 Many of the clinical trials are not aimed at pain relief but are targeting other actions of ET-1 in other systems. However, to gain the maximum benefit from these trials, the pain-producing (or pain-relieving) effects of ET-1 need to be better understood and evaluated. Pain reduction has already been reported in trials of bone and prostate cancer pain.Citation4
Therefore, it is imperative that the current trials index changes in pain to elucidate all the possible benefits of endothelin receptor antagonists. This review of the literature on ET-1 beckons to clinical researchers to include a pain analysis within the ongoing studies. Possible pain-relieving adverse effects may be completely overlooked without the needed attention from clinical studies. Significant pain relief may be an additional benefit in patients taking endothelin receptor antagonists for a variety of pathologies.
Acknowledgments
This work was funded by National Institutes of Health Grants R01 DA023593 and NS26363; a South Carolina Post-Baccalaureate Research Education Programs for Minorities, funded by grants R25 GM066526 and R25 GM076277 from the National Institutes of Health; and the Alfred P Sloan Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- YanagisawaMKuriharaHKimuraSGotoKMasakiTA novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channelsJ Hypertens Suppl198864S188S1912853725
- RubanyiGMPolokoffMAEndothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiologyPharmacol Rev19944633254157831383
- GiannessiDDel RySVitaleRLThe role of endothelins and their receptors in heart failurePharmacol Res200143211112611243712
- LalichMMcNeelDGWildingGLiuGEndothelin receptor antagonists in cancer therapyCancer Invest200725878579418058475
- DahlöfBGustafssonDHednerTJernSHanssonLRegional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reactionJ Hypertens1990898118172172370
- NelsonJBHedicanSPGeorgeDJIdentification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostateNat Med1995199449497585222
- LermanAEdwardsBSHallettJWHeubleinDMSandbergSMBurnettJCJrCirculating and tissue endothelin immunoreactivity in advanced atherosclerosisN Engl J Med19913251499710011886637
- SuzukiTKumazakiTMitsuiYEndothelin-1 is produced and secreted by neonatal rat cardiac myocytes in vitroBiochem Biophys Res Commun199319138238308466520
- HaradaMItohHNakagawaOSignificance of ventricular myocytes and nonmyocytes interaction during cardiocyte hypertrophy: evidence for endothelin-1 as a paracrine hypertrophic factor from cardiac nonmyocytesCirculation19979610373737449396478
- HansGDeseureKAdriaensenHEndothelin-1-induced pain and hyperalgesia: a review of pathophysiology, clinical manifestations and future therapeutic optionsNeuropeptides200842211913218194815
- HasueFKuwakiTKisanukiYYIncreased sensitivity to acute and persistent pain in neuron-specific endothelin-1 knockout miceNeuroscience2005130234935815664691
- LiuYYamadaHOchiJImmunocytochemical studies on endothelin in mast cells and macrophages in the rat gastrointestinal tractHistochem Cell Biol199810943013079562379
- EhrenreichHAndersonRWFoxCHEndothelins, peptides with potent vasoactive properties, are produced by human macrophagesJ Exp Med19901726174117481701822
- CarducciMAJimenoATargeting bone metastasis in prostate cancer with endothelin receptor antagonistsClinical Cancer Research: An Official Journal of the American Association for Cancer Research101520061220 Pt 26296s6300s17062717
- AhnGYButtKIJindoTYaguchiHTsuboiROgawaHThe expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alphaJ Dermatol199825278849563273
- KopetzESNelsonJBCarducciMAEndothelin-1 as a target for therapeutic intervention in prostate cancerInvest New Drugs200220217318212099577
- MoreauPd’UscioLVShawSTakaseHBartonMLüscherTFAngiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET(A)-receptor antagonistCirculation1997965159315979315552
- WagnerOFChristGWojtaJPolar secretion of endothelin-1 by cultured endothelial cellsJ Biol Chem19922672316066160681644793
- RubinSALevinERClinical review 53: The endocrinology of vasoactive peptides: synthesis to functionJ Clin Endocrinol Metab19947816108288715
- BremnesTPaascheJDMehlumASandbergCBremnesBAttramadalHRegulation and intracellular trafficking pathways of the endothelin receptorsJ Biol Chem200027523175961760410747877
- BurkhardtMBartonMShawSGReceptor- and non-receptor-mediated clearance of big-endothelin and endothelin-1: differential effects of acute and chronic ETA receptor blockadeJ Hypertens200018327327910726713
- GohlaASchultzGOffermannsSRole for G(12)/G(13) in agonist-induced vascular smooth muscle cell contractionCirc Res200087322122710926873
- MasakiTEndothelin in vascular biologyAnn N Y Acad Sci199471411011088017758
- NicolGDETET – phone the pain clinicTrends Neurosci2004274177180 discussion 18015108686
- AraiHHoriSAramoriIOhkuboHNakanishiSCloning and expression of a cDNA encoding an endothelin receptorNature199034863037307322175396
- SakuraiTYanagisawaMTakuwaYCloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptorNature199034863037327352175397
- RashidAJO’DowdBFGeorgeSRMinireview: Diversity and complexity of signaling through peptidergic G protein-coupled receptorsEndocrinology200414562645265215044354
- ZhouZDavarGStrichartzGEndothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1J Neurosci200222156325633012151509
- MasakiTMiwaSSawamuraTNinomiyaHOkamotoYSubcellular mechanisms of endothelin action in vascular systemEur J Pharmacol19993751–313313810443571
- GhoneimMAYamamotoTHiroseSNagasawaTHagiwaraHEndothelium localization of ETB receptor revealed by immunohistochemistryJ Cardiovasc Pharmacol199322Suppl 8S111S1127509918
- ShettySSOkadaTWebbRLDelGrandeDLappeRWFunctionally distinct endothelin B receptors in vascular endothelium and smooth muscleBiochem Biophys Res Commun199319124594648461004
- Sakurai-YamashitaYYamashitaKYoshidaARat peritoneal macrophages express endothelin ET(B) but not endothelin ET(A) receptorsEur J Pharmacol199733821992039456003
- PomonisJDRogersSDPetersCMGhilardiJRMantyhPWExpression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociceptionJ Neurosci2001213999100611157085
- ChichorroJGFiuzaCRBressanEClaudinoRFLeiteDFRaeGAEndothelins as pronociceptive mediators of the rat trigeminal system: role of ETA and ETB receptorsBrain Res20101345738320450894
- Berti-MatteraLNGariepyCEBurkeRMHallAKReduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetesExp Neurol2006201239940616806184
- PlantTDZöllnerCKepuraFEndothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase CMol Pain2007313518001466
- GiaidAGibsonSJIbrahimBNEndothelin-1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root gangliaProc Natl Acad Sci U S A19898619763476382678110
- FengBStrichartzGEndothelin-1 raises excitability and reduces potassium currents in sensory neuronsBrain Res Bull200979634535019409452
- HouckCSKhodorovaARealeAMStrichartzGRDavarGSensory fibers resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in ratsPain2004110371972615288413
- CardenasLMCardenasCGScroggsRS5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na(+) channelsJ Neurophysiol200186124124811431505
- LeeMEde la MonteSMNgSCBlochKDQuertermousTExpression of the potent vasoconstrictor endothelin in the human central nervous systemJ Clin Invest19908611411472195059
- GiaidAGibsonSJHerreroMTTopographical localisation of endothelin mRNA and peptide immunoreactivity in neurones of the human brainHistochemistry19919533033142050550
- YoshizawaTKimuraSKanazawaIUchiyamaYYanagisawaMMasakiTEndothelin localizes in the dorsal horn and acts on the spinal neurones: possible involvement of dihydropyridine-sensitive calcium channels and substance P releaseNeurosci Lett19891022–31791842478929
- KurokawaKYamadaHOchiJTopographical distribution of neurons containing endothelin type A receptor in the rat brainJ Comp Neurol199738923483609416926
- YamadaHKurokawaKHistochemical studies on endothelin and the endothelin-A receptor in the hypothalamusJ Cardiovasc Pharmacol199831Suppl 1S215S2189595441
- FerreiraSHRomitelliMde NucciGEndothelin-1 participation in overt and inflammatory painJ Cardiovasc Pharmacol198913Suppl 5S220S2222473319
- RaffaRBJacobyHIEndothelin-1, -2 and -3 directly and big-endothelin-1 indirectly elicit an abdominal constriction response in miceLife Sci19914817PL85PL901826753
- RaffaRBSchupskyJJMartinezRPJacobyHIEndothelin-1-induced nociceptionLife Sci19914911PL61PL651875787
- HansGDeseureKRobertDDe HertSNeurosensory changes in a human model of endothelin-1 induced pain: a behavioral studyNeurosci Lett2007418211712117403578
- GokinAPFareedMUPanHLHansGStrichartzGRDavarGLocal injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in ratsJ Neurosci200121145358536611438612
- NamerBHilligesMOrstavikKEndothelin-1 activates and sensitizes human C-nociceptorsPain20081371414917884295
- PiovezanAPD’Orléans-JustePTonussiCRRaeGAEndothelins potentiate formalin-induced nociception and paw edema in miceCan J Physiol Pharmacol19977565966009276135
- D’AmicoMDi FilippoCRossiFSelective and non-selective ET antagonists reveal an ET(A)/ET(B) receptor mediated ET-1-induced antinociceptive effect in PAG area of miceLife Sci19976125PL 397PL 401
- SchmelzMSchmidtRWeidnerCHilligesMTorebjorkHEHandwerkerHOChemical response pattern of different classes of C-nociceptors to pruritogens and algogensJ Neurophysiol20038952441244812611975
- DavarGHansGFareedMUSinnottCStrichartzGBehavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerveNeuroreport1998910227922839694215
- KhodorovaAStrichartzGRContralateral paw sensitization following injection of endothelin-1: effects of local anesthetics differentiate peripheral and central processesNeuroscience2010165255356019874873
- RaffaRBSchupskyJJJacobyHIEndothelin-induced nociception in mice: mediation by ETA and ETB receptorsJ Pharmacol Exp Ther199627626476518632332
- PiovezanAPD’Orléans-JustePSouzaGERaeGAEndothelin-1-induced ET(A) receptor-mediated nociception, hyperalgesia and oedema in the mouse hind-paw: modulation by simultaneous ET(B) receptor activationBr J Pharmacol2000129596196810696096
- KhodorovaAFareedMUGokinAStrichartzGRDavarGLocal injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive mannerJ Neurosci200222177788779612196602
- MenéndezLLastraAHidalgoABaamondeANociceptive reaction and thermal hyperalgesia induced by local ET-1 in mice: a behavioral and Fos studyNaunyn Schmiedebergs Arch Pharmacol20033671283412616338
- McKelvyADMarkTRSweitzerSMAge- and sex-specific nociceptive response to endothelin-1J Pain20078865766617553749
- ShresthaSGraciasNGMujendaFKhodorovaAVaskoMRStrichartzGRLocal antinociception induced by endothelin-1 in the hairy skin of the rat’s backJ Pain200910770271419559389
- GomesLOHaraDBRaeGAEndothelin-1 induces itch and pain in the mouse cheek modelLife Sci Mar20129113–14628633
- KhodorovaANavarroBJouavilleLSEndothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injuryNat Med2003981055106112847519
- da CunhaJMRaeGAFerreiraSHCunhaFQEndothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase CEur J Pharmacol20045011–3879415464066
- MottaEMCalixtoJBRaeGAMechanical hyperalgesia induced by endothelin-1 in rats is mediated via phospholipase C, protein kinase C, and MAP kinasesExp Biol Med (Maywood)200623161141114516741065
- BaamondeALastraAVillazónMBordalloJHidalgoAMenéndezLInvolvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in miceNaunyn Schmiedebergs Arch Pharmacol2004369224525114661069
- VerriWAJrSchivoIRCunhaTMLiewFYFerreiraSHCunhaFQInterleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive mannerJ Pharmacol Exp Ther2004310271071715075358
- KhodorovaARichterJVaskoMRStrichartzGEarly and late contributions of glutamate and CGRP to mechanical sensitization by endothelin-1J Pain200910774074919559390
- KawamataTJiWYamamotoJNiiyamaYFuruseSNamikiAContribution of transient receptor potential vanilloid subfamily 1 to endothelin-1-induced thermal hyperalgesiaNeuroscience200815431067107618495351
- MottaEMChichorroJGD’Orléans-JustePRaeGARoles of endothelin ETA and ETB receptors in nociception and chemical, thermal and mechanical hyperalgesia induced by endothelin-1 in the rat hindpawPeptides200930591892519428770
- StösserSAgarwalNTappe-TheodorAYanagisawaMKunerRDissecting the functional significance of endothelin A receptors in peripheral nociceptors in vivo via conditional gene deletionPain2010148220621419879049
- PlantTDZöllnerCMousaSAOkscheAEndothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptorExp Biol Med (Maywood)200623161161116416741069
- YamamotoHKawamataTNinomiyaTOmoteKNamikiAEndothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neuronsNeuroscience2006137394996016298080
- PiovezanAPD’Orléans-JustePTonussiCRRaeGAEffects of endothelin-1 on capsaicin-induced nociception in miceEur J Pharmacol1998351115229698200
- SmithTBeasleySSmithSMarkISweitzerSMEndothelin-1-induced priming to capsaicin in young animalsNeurosci Lett2014567151824674771
- MujendaFHDuarteAMReillyEKStrichartzGRCutaneous endothelin-A receptors elevate post-incisional painPain20071331–316117317467172
- KameiJHitosugiHKawashimaNMisawaMKasuyaYAntinociceptive effects of intrathecally administered endothelin-1 in miceNeurosci Lett1993153169728510826
- YamamotoTShimoyamaNAsanoHMizuguchiTAnalysis of the role of endothelin-A and endothelin-B receptors on nociceptive information transmission in the spinal cord with FR139317, an endothelin-A receptor antagonist, and sarafotoxin S6c, an endothelin-B receptor agonistJ Pharmacol Exp Ther199427111561637965709
- HungVKChenSMTaiLWChenAYChungSKCheungCWOver-expression of endothelin-1 in astrocytes, but not endothelial cells, ameliorates inflammatory pain response after formalin injectionLife Sci20129113–1461862222820168
- D’AmicoMBerrinoLMaioneSFilippelliAde NovellisVRossiFEndothelin-1 in periaqueductal gray area of mice induces analgesia via glutamatergic receptorsPain1996652–32052098826508
- HasueFKuwakiTYamadaHFukudaYShimoyamaMInhibitory actions of endothelin-1 on pain processingJ Cardiovasc Pharmacol200444Suppl 1S318S32015838310
- ErgulAShoemakerKPuettDTackettRLGender differences in the expression of endothelin receptors in human saphenous veins in vitroJ Pharmacol Exp Ther199828525115179580591
- YoshibayashiMNishiokaKNakaoKPlasma endothelin levels in healthy children: high values in early infancyJ Cardiovasc Pharmacol199117Suppl 7S404S4051725392
- GiannessiDDel RySAndreassiMGHigh density of endothelin binding sites in the hearts of infants and childrenLife Sci199964869770510069532
- JosephEKLevineJDSexual dimorphism in endothelin-1 induced mechanical hyperalgesia in the ratExp Neurol2012233150551222155617
- McKelvyADSweitzerSMEndothelin-1 exposure on postnatal day 7 alters expression of the endothelin B receptor and behavioral sensitivity to endothelin-1 on postnatal day 11Neurosci Lett20094511899319111903
- CuiPTaniKKitamuraHA novel bioactive 31-amino acid endothelin-1 is a potent chemotactic peptide for human neutrophils and monocytesJ Leukoc Biol200170230631211493624
- MiyasakaNHirataYAndoKIncreased production of endothelin-1 in patients with inflammatory arthritidesArthritis Rheum19923543974001567488
- KhodorovaAZouSRenKDubnerRDavarGStrichartzGDual Roles for Endothelin-B Receptors in Modulating Adjuvant-Induced Inflammatory Hyperalgesia in RatsOpen Pain J200921304020559459
- De-MeloJDTonussiCRD’Orléans-JustePRaeGAArticular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonistsPain19987732612699808351
- De-MeloJDTonussiCRD’Orléans-JustePRaeGAEffects of endothelin-1 on inflammatory incapacitation of the rat knee jointJ Cardiovasc Pharmacol199831Suppl 1S518S5209595530
- ImhofAKGlückLGajdaMBräuerRSchaibleHGSchulzSPotent anti-inflammatory and antinociceptive activity of the endothelin receptor antagonist bosentan in monoarthritic miceArthritis Res Ther2011133R9721689431
- PiovezanAPD’Orléans-JustePFrighettoMSouzaGEHenriquesMGRaeGAEndothelins contribute towards nociception induced by antigen in ovalbumin-sensitised miceBr J Pharmacol2004141475576314744803
- PirtskhalaishviliGNelsonJBEndothelium-derived factors as paracrine mediators of prostate cancer progressionProstate2000441778710861760
- QuangPNSchmidtBLPeripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in micePain2010149225426220206445
- DavarGEndothelin-1 and metastatic cancer painPain Med200121242715102314
- YuyamaHKoakutsuAFujiyasuNInhibitory effects of a selective endothelin-A receptor antagonist YM598 on endothelin-1-induced potentiation of nociception in formalin-induced and prostate cancer-induced pain models in miceJ Cardiovasc Pharmacol200444Suppl 1S479S48215838353
- HamamotoDTKhasabovSGCainDMSimoneDATumor-evoked sensitization of C nociceptors: a role for endothelinJ Neurophysiol200810042300231118684911
- PickeringVJay GuptaRQuangPJordanRCSchmidtBLEffect of peripheral endothelin-1 concentration on carcinoma-induced pain in miceEur J Pain200812329330017664075
- WacnikPWEikmeierLJRugglesTRFunctional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer painJ Neurosci200121239355936611717369
- PetersCMLindsayTHPomonisJDEndothelin and the tumorigenic component of bone cancer painNeuroscience200412641043105215207337
- SchmidtBLPickeringVLiuSPeripheral endothelin A receptor antagonism attenuates carcinoma-induced painEur J Pain200711440641416807013
- FujitaMAndohTSaikiIKuraishiYInvolvement of endothelin and ET(A) endothelin receptor in mechanical allodynia in mice given orthotopic melanoma inoculationJ Pharmacol Sci2008106225726318270470
- BaamondeALastraAFresnoMFImplantation of tumoral XC cells induces chronic, endothelin-dependent, thermal hyperalgesia in miceCell Mol Neurobiol200424226928115176440
- TadaHMuramatsuINakaiTKigoshiSMiyaboSEffects of chronic diabetes on the responsiveness to endothelin-1 and other agents of rat atria and thoracic aortaGen Pharmacol1994256122112287875548
- JarvisMFWessaleJLZhuCZABT-627, an endothelin ET(A) receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic painEur J Pharmacol20003881293510657544
- KameiJHitosugiHKawashimaNMisawaMKasuyaYEffects of diabetes on the antinociceptive effects of intrathecally administered endothelin-1Res Commun Chem Pathol Pharmacol19937922092178451542
- ChichorroJGZampronioARRaeGAEndothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic painExp Biol Med (Maywood)200623161136114016741064
- ChichorroJGZampronioARSouzaGERaeGAOrofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugsPain20061231–2647416563629
- MillecampsMLaferrièreARagavendranJVStoneLSCoderreTJRole of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I)Pain2010151117418320675053
- KlassMHordAWilcoxMDensonDCseteMA role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerveAnesth Analg200510161757176216301255
- WernerMFTrevisaniMCampiBAndréEGeppettiPRaeGAContribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in ratsEur J Pain201014991191720350830
- RaffaRBSchupskyJJLeeDKJacobyHICharacterization of endothelin-induced nociception in mice: evidence for a mechanistically distinct analgesic modelJ Pharmacol Exp Ther19962781178764328
- GriswoldDEDouglasSAMartinLDEndothelin B receptor modulates inflammatory pain and cutaneous inflammationMol Pharmacol199956480781210496965
- ZagorodnyukVPKylohMNicholasSLoss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung’s diseaseJ Physiol2011589Pt 71691170621320883
- KanazawaSTsunodaTOnumaEMajimaTKagiyamaMKikuchiKVEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammationAm J Gastroenterol200196382282811280558
- MurchSHBraeggerCPSessaWCMacDonaldTTHigh endothelin-1 immunoreactivity in Crohn’s disease and ulcerative colitisLancet199233987903813851346658
- LetiziaCBoirivantMDe TomaGPlasma levels of endothelin-1 in patients with Crohn’s disease and ulcerative colitisItal J Gastroenterol Hepatol19983032662699759593
- ShiuYTMcIntireLVUddenMMSickle erythrocytes increase prostacyclin and endothelin-1 production by cultured human endothelial cells under flow conditionsEur J Haematol200268316316912068797
- PhelanMPerrineSPBrauerMFallerDVSickle erythrocytes, after sickling, regulate the expression of the endothelin-1 gene and protein in human endothelial cells in cultureJ Clin Invest1995962114511517635951
- HammermanSIKourembanasSConcaTJTucciMBrauerMFarberHWEndothelin-1 production during the acute chest syndrome in sickle cell diseaseAm J Respir Crit Care Med199715612802859230761
- TharauxPLHagègeIPlacierSUrinary endothelin-1 as a marker of renal damage in sickle cell diseaseNephrol Dial Transplant200520112408241316144850
- Graido-GonzalezEDohertyJCBergreenEWOrganGTelferMMcMillenMAPlasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisisBlood1998927255125559746797
- ErgulSBrunsonCYHutchinsonJVasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstrictionAm J Hematol200476324525115224360
- RybickiACBenjaminLJIncreased levels of endothelin-1 in plasma of sickle cell anemia patientsBlood1998927259425969746804
- BrunMBourdoulousSCouraudPOElionJKrishnamoorthyRLapoumeroulieCHydroxyurea downregulates endothelin-1 gene expression and upregulates ICAM-1 gene expression in cultured human endothelial cellsPharmacogenomics J20033421522612931135
- OdièvreMHBrunMKrishnamoorthyRLapouméroulieCElionJSodium phenyl butyrate downregulates endothelin-1 expression in cultured human endothelial cells: relevance to sickle-cell diseaseAm J Hematol200782535736217373676
- LapouméroulieCBenkerrouMOdièvreMHDucrocqRBrunMElionJDecreased plasma endothelin-1 levels in children with sickle cell disease treated with hydroxyureaHaematologica200590340140315749673
- De FranceschiLPlattOSMalpeliGProtective effects of phosphodiesterase-4 (PDE-4) inhibition in the early phase of pulmonary arterial hypertension in transgenic sickle cell miceFASEB J20082261849186018245171
- SabaaNde FranceschiLBonninPEndothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell diseaseJ Clin Invest200811851924193318382768
- SchlenzAMMcClellanCBMarkTRSensitization to acute procedural pain in pediatric sickle cell disease: modulation by painful vaso-occlusive episodes, age, and endothelin-1J Pain201213765666522633685
- RiveraAJarolimPBrugnaraCModulation of Gardos channel activity by cytokines in sickle erythrocytesBlood200299135760311756192
- BenzaRLPulmonary hypertension associated with sickle cell disease: pathophysiology and rationale for treatmentLung2008186424725418470553
- RiveraAReduced sickle erythrocyte dehydration in vivo by endothelin-1 receptor antagonistsAm J Physiol Cell Physiol20072933C960C96617494628
- ChaarVTarerVEtienne-JulanMDiaraJPElionJRomanaMET-1 and ecNOS gene polymorphisms andsusceptibility to acute chest syndrome and painful vaso-occlusive crises in children with sickle cell anemiaHaematologica20069191277127816956834
- GladwinMTCrawfordJHPatelRPThe biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulationFree Radic Biol Med200436670771714990351
- MorrisCRGladwinMTKatoGJNitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disordersCurr Mol Med20088762063218991648
- WoodKCHsuLLGladwinMTSickle cell disease vasculopathy: a state of nitric oxide resistanceFree Radic Biol Med20084481506152818261470
- BhallaSMatwyshynGGulatiAMorphine tolerance does not develop in mice treated with endothelin-A receptor antagonistsBrain Res200510641–212613516289404
- MatwyshynGABhallaSGulatiAEndothelin ETA receptor blockade potentiates morphine analgesia but does not affect gastrointestinal transit in miceEur J Pharmacol20065431–3485316814278
- Selenko-GebauerNDuschekNMinimairGStinglGKarlhoferFSuccessful treatment of patients with severe secondary Raynaud’s phenomenon with the endothelin receptor antagonist bosentanRheumatology (Oxford)200645Suppl 3iii45iii4816987835
- NaprawaJTBonsuBKGoodmanDGRanalliMASerum biomarkers for identifying acute chest syndrome among patients who have sickle cell disease and present to the emergency departmentPediatrics20051163e420e42516099851
- MuntsAGZijlstraFJNibberingPHAnalysis of cerebrospinal fluid inflammatory mediators in chronic complex regional pain syndrome related dystoniaClin J Pain2008241303418180633
- DhaunNWebbDJEndothelin-receptor antagonism: the future is brightLancet200837196302061206218572063
- GriswoldDEDouglasSAMartinLDTargeted disruption of the endothelin-B-receptor gene attenuates inflammatory nociception and cutaneous inflammation in miceJ Cardiovasc Pharmacol2000365Suppl 1S78S8111078342
- CainDMWacnikPWEikmeierLBeitzAWilcoxGLSimoneDAFunctional interactions between tumor and peripheral nerve in a model of cancer pain in the mousePain Med200121152315102313
