Abstract
Introduction
Spinal cord stimulation (SCS) provides significant relief for lumbosacral radiculopathy refractory to both medical and surgical treatment, but historically only offers limited relief for axial low back pain (LBP). We aim to evaluate the response of chronic axial LBP treated with SCS using a surgically implanted epidural paddle lead.
Materials and methods
This is a retrospective review of a consecutive series of patients with exclusive LBP or predominant LBP associated with lower extremity (LE) pain evaluated and treated with SCS using an implanted paddle lead within the dorsal thoracic epidural space. Baseline LBP, and if present LE pain, were recorded using the visual analogue scale (VAS) at an initial evaluation. At a follow-up visit (a minimum of 12 months later), LBP and LE pain after a spinal cord stimulator implantation were again recorded using the VAS. Patients were also asked to estimate total LBP pain relief achieved.
Results
Patients with either exclusive (n=7) or predominant (n=2) axial LBP were treated with SCS by implantation of a paddle lead at an average spine level of T9. The baseline VAS score for LBP was 7.2; after a follow-up of 20 months, the score decreased to 2.3 (P=0.003). The LE pain VAS score decreased from 7.5 to 0.0 (P=0.103). Patients also reported a subjective 66.4% decrease of their LBP at follow-up. There were no surgical complications.
Conclusions
Axial LBP is refractory to many treatments, including SCS. SCS using a surgically implanted paddle electrode provides significant pain relief for chronic axial LPB, and is a safe treatment modality.
Keywords:
Introduction
Spinal cord stimulation (SCS) provides significant relief for lumbosacral radiculopathy refractory to both medical and surgical treatment. Clinical series report between 50% to 70% successful pain relief in patients treated with SCS based on reduction in pain severity scores, improvement in function, and decreased pain medication dependence.Citation1–Citation7 Until recently, SCS has been considered relatively ineffective for treatment of chronic axial low back pain (LBP).Citation1,Citation2,Citation4,Citation8–Citation12 Most clinical series report better pain control with SCS in patients with lower extremity (LE) radicular pain than in patients with isolated, axial LBP,Citation1,Citation13 an expected finding based on functional neuroanatomy. The representation of the low back within the sensory homunculus of the cerebral cortex and the dorsal columns of the spinal cord is relatively small compared to the representation of the legs.Citation2 From dorsal to ventral, the representation of the low back within the dorsal columns is evenly distributed, and thus low back fibers may not be available to predominantly superficial stimulation of the dorsal columns.Citation2 Improvements in SCS technology including programmable and percutaneously rechargeable implantable generators, increasing capabilities of implantable programmable generators, and paddle design enhancements reducing device migrationCitation14 have resulted in increasingly effective LBP control. Accordingly, some combined low back and LE pain studies have reported satisfactory pain relief with SCS for the chronic LBP component.Citation9
Failed back surgery syndrome (FBSS) is the most common indication for SCS in the United States.Citation15 SCS in non-operated patients (ie, without FBSS) with axial LBP has not been systematically studied. The current study retrospectively analyzes a patient population whose clinical characteristics have generally been considered a poor prognostic indication for SCS. Patients with either exclusive or predominant chronic axial LBP, regardless of previous spinal surgical intervention, are generally expected to respond less favorably to SCS than those patients with predominantly LE radicular pain.Citation1,Citation13 This current study evaluates the direct response of either exclusive or predominant chronic axial LBP to SCS provided with the surgical implantation of paddle leads within the thoracic epidural space.
Materials and methods
Subjects
This is a retrospective review of a consecutive series of patients with either exclusive or predominant chronic axial LBP evaluated and treated at a university health care setting from 2006 to 2011 with surgical implantation of a spinal cord stimulator. A small portion of the patients were also treated for LE radiculopathy that was less severe than the axial LBP, as assessed by a preoperative visual analogue scale (VAS) score for both the axial LBP and LE pain. All patients underwent at least 6 weeks of treatment with physical therapy including heat, ultrasound, and exercises, followed by treatment with lumbar epidural or facet steroid injections as indicated prior to surgical intervention. All patients were found not to be optimal candidates for neural decompressive or fusion procedures after evaluation of their presentation and review of their neuroimaging by a spine surgeon at our institution. The patients were also screened with neuropsychological testing. The study was approved by the university institutional review board.
Spinal cord stimulation implantation technique
Under intravenous sedation and local anesthesia, SCS paddles were implanted in the dorsal thoracic epidural space at an average T9 level through a laminotomy. The patients were positioned prone over chest rolls on the operating table and were fully conscious after the paddle implantation for an intraoperative epidural SCS trial of approximately 15 minutes, performed as previously described.Citation16 After repositioning adjustments of the paddle as needed, the acute intraoperative SCS screening trials ultimately successfully provided at least 50% pain relief in the habitual axial LBP distribution for all patients. Among the two patients with LE radiculopathy, acute intraoperative SCS screening also provided at least 50% pain relief along the LE pain distribution. The epidural SCS paddles used were a three-column Tripole lead (St Jude Medical, Inc., Saint Paul, MN, USA) or a five-column Penta lead (St Jude Medical). The paddle leads were anchored into position after the intraoperative screening trials and the connecting cables were subcutaneously tunneled to a buttock area. The cables were then attached to an implanted programmable generator within a pocket created in the subcutaneous tissue (). Implanted programmable generators used were the Eon rechargeable generator (St Jude Medical).
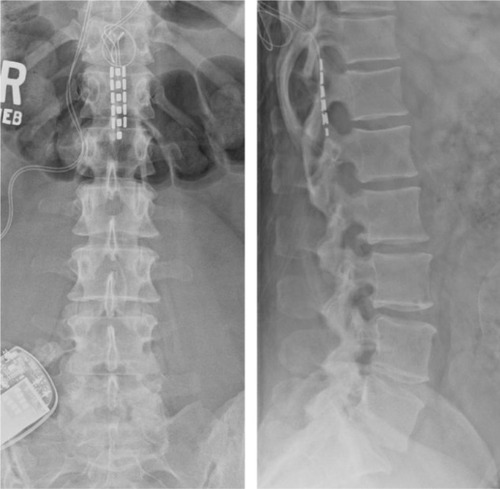
Figure 1 Plain posterior–anterior and lateral radiographs of Case 5.
Abbreviation: SCS, spinal cord stimulation.
Pre- and postspinal cord stimulation pain severity
Preoperative pain severity of axial LBP and LE pain were recorded during an initial evaluation using the VAS scale. Following the placement of an implanted spinal cord stimulator, patients were followed for a minimum of 12 months, at which time the VAS scores for axial LBP and LE pain were again recorded. In addition, each patient at their follow-up visit was asked to rate the estimated decrease in axial LBP as a subjective measure of pain relief.
Statistical analysis
Quantitative data were reported as means ± standard error of the mean. All statistical analyses were conducted using Stata 11 statistical software package (StataCorp, College Station, TX, USA). The Wilcoxon rank-sum test was used to test for statistically significant differences of the nonparametric means. P values were calculated as two-tailed, and P<0.05 was considered statistically significant.
Results
Clinical and demographic data
A total of nine patients with either exclusive (n=7) or predominant (n=2) axial LBP were treated with spinal cord stimulation using a surgically implanted paddle lead within the thoracic epidural space (). In this series, there were three males and six females with a mean age of 54.2 years (range: 33 to 75 years). The etiology of the LBP was FBSS (n=4), lumbar-sacral degenerative joint disease (n=3), lumbar-sacral degenerative disc disease (n=4), and lumbar spine fracture (n=1). Degenerative joint disease was defined as facet arthropathy evident on a magnetic resonance imaging scan.
Table 1 Demographic and clinical data on nine patients with exclusive or predominant low back pain treated with paddle spinal cord stimulation
Spinal cord stimulation data
summarizes the findings of this current case series. The average thoracic spine level of the epidural spinal cord stimulator paddle lead implant was approximately the T9 level (range, T6–T12). Patients were followed for response to the SCS treatment for a minimum duration of 12 months (range, 12–41 months). The distribution of LBP VAS scores for the initial evaluation and the postspinal cord stimulator implantation is depicted in . The mean VAS score for axial LBP prior to implantation of a spinal cord stimulator was 7.2±0.8 and 2.3±0.9 after the spinal cord stimulator implant (P=0.003, ). Of the two patients with concurrent LE pain, the mean VAS score for the LE pain was 7.5±1.5 prior to SCS and 0.0±0.0 after SCS (P=0.103). The subjective estimated relief of LBP provided by the surgically implanted spinal cord stimulator was 66%±9.3%. There were no surgical complications in this study.
Figure 2 Distributions of LBP VAS scores recorded from patients pre- and post-SCS paddle lead implantation.
Abbreviations: LBP, low back pain; SCS, spinal cord stimulation; VAS, visual analogue scale.
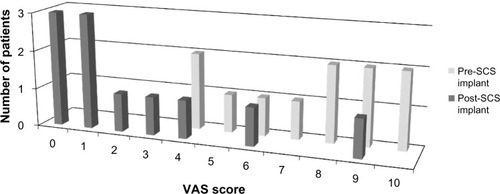
Figure 3 Mean LBP VAS scores recorded for pre- and post-SCS with paddle lead implantation.
Abbreviations: LBP, low back pain; SCS, spinal cord stimulation; VAS, visual analogue scale.
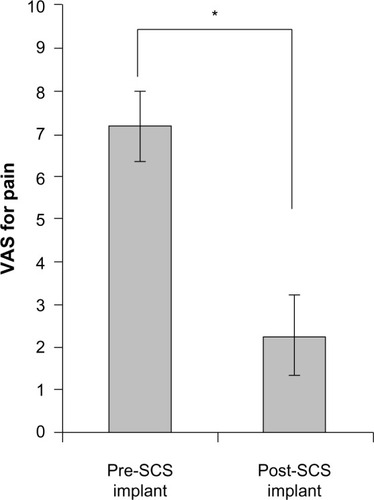
Table 2 Summary of demographic and clinical data on nine patients with exclusive or predominant low back pain treated with paddle spinal cord stimulation
Discussion
SCS has been traditionally recognized as an effective treatment for chronic pain of neuropathic origin, including LE radiculopathy.Citation1–Citation7 Axial LBP pain, on the other hand, has been difficult to treat with SCS alone,Citation1,Citation2,Citation4,Citation8–Citation12 possibly due to the nociceptive origin of the axial spine pain. Vascularized granulation tissue, for example, has been found to be associated with chronic lumbar disc disease and histologically contains nociceptors that may be the cause of the nociceptive nature of isolated LBP in this population.Citation17 FBSS and chronic, unhealed fractures likely contain granulation tissue which may contribute to chronic LBP. This study demonstrates that SCS with surgical implantation of an epidural paddle lead can provide effective long-term pain control in patients with exclusive or predominant axial LBP. The etiology of axial LBP in this series was diverse including FBSS, degenerative joint the diverse utility of SCS as a treatment of axial LBP.
Of the nine patients enrolled in this series, eight patients (89%) reported ≥50% relief of chronic axial LBP at a mean follow-up evaluation at 19.9 months after a spinal cord stimulator implant. The 33-year-old patient that did not report benefit from the implanted spinal cord stimulator, Case 5, was the youngest patient of the series (). She reported that her LBP increased from 8 to 9 at a 24-month follow-up visit. The paddle lead was initially inserted into the epidural space at approximately the T9 level through a laminotomy at T10, but she did not receive pain relief in the distribution of her chronic LBP. Ultimately, the paddle lead was passed inferiorly through the T10 laminotomy and provided at least 50% pain relief during the intraoperative SCS trial (). The failure of the SCS benefit at follow-up emphasizes the point that the T9 level may be the optimal level to place SCS leads for adequate coverage of the low back.Citation2
SCS epidural leads can be generally classified into percutaneously placed linear leads or paddle leads surgically implanted through a laminotomy. SCS for relief of axial LBP has been demonstrated to be superior to conservative medical management alone in a recent multicenter, randomized trial,Citation18 but SCS for axial LBP had been primarily evaluated with percutaneously placed epidural electrodes. Placement of three individual percutaneously placed linear leads creating a tripolar configuration, for instance, was shown to benefit a single case of FBSS utilizing a guarded cathode arrangement.Citation19 Several larger case series studies demonstrate improved axial LBP with SCS using various configurations of percutaneously placed leads.Citation12,Citation20–Citation22
Laminotomy for paddle lead placement is a more invasive procedure relative to placement of percutaneous leads, but laminotomy placed paddle leads have been shown to provide more durable pain coverage with less tendency to migrate.Citation23–Citation25 Newer hybrid lead designs that mimic the benefits of paddle leads can be placed percutaneously into the epidural space. A few recent studies have reported encouraging results using hybrid leads for treatment of axial LBP.Citation14,Citation26,Citation27 Another study showed that SCS using a paddle lead with external radio-frequency stimulation benefited patients with predominantly axial LBP.Citation28 Our current study shows that SCS with paddle leads connected to an implanted pulse generator provides sustained relief of axial LBP.
There are clear limitations to this current study. This was a small case series at a single institution, limiting the generalization of the findings. As with other studies evaluating SCS, this series was not blinded and did not include a control group to evaluate treatment relative to the natural progression of axial LBP, though in one study SCS has been shown superior to medical treatment alone.Citation18 This current study also does not include standardized functional outcome scores for the patients at follow-up. The study does, however, add to the growing amount of evidence found in the literature that axial LBP can be successfully treated by SCS using paddle leads in a carefully selected population of patients.
Conclusion
SCS using a surgically implanted paddle electrode within the thoracic epidural space provides significant pain relief for chronic axial LBP and is a safe treatment modality. These findings support the development of a multicenter, prospective trial of SCS for axial LBP.
Acknowledgments
Dr Weinand has received research grant support from St Jude Medical. Dr Weinand developed the hypothesis for this study. Dr Stidd performed clinical study coordination and IRB administration. All authors participated in drafting the manuscript and data analysis. All authors have approved the submitted version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- StojanovicMPAbdiSSpinal cord stimulationPain Physician20025215616616902666
- OakleyJCSpinal cord stimulation in axial low back pain: solving the dilemmaPain Medicine20067S1S58S63
- KumarKNathRWyantGMTreatment of chronic pain by epidural spinal cord stimulation: a 10-year experienceJ Neurosurg19917534024071869942
- BurchielKJAndersonVCBrownFDProspective, multicenter study of spinal cord stimulation for relief of chronic back and extremity painSpine (Phila Pa 1976)19962123278627948979327
- CahanaAMavrocordatosPGeurtsJWGroenGJDo minimally invasive procedures have a place in the treatment of chronic low back pain?Expert Rev Neurother20044347949015853544
- RainovNGDemmelWHeideckeVDual electrode spinal cord stimulation in chronic leg and back painActa Neurochir Suppl200797Pt 1858917691361
- TaylorRJTaylorRSSpinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysisInt J Technol Assess Health Care200521335135816110715
- SlavinKVBurchielKJAndersonVCCookeBEfficacy of transverse tripolar stimulation for relief of chronic low back pain: results of a single centerStereotact Funct Neurosurg1999731–412613010853117
- NorthRBEwendMGLawtonMTKiddDHPiantadosiSFailed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantationNeurosurgery19912856926991831547
- NorthRBEwendMGLawtonMTPiantadosiSSpinal cord stimulation for chronic, intractable pain: superiority of “multi-channel” devicesPain19914421191302052378
- DarioAFortiniGBertolloDBacuzziAGrizzettiCCuffariSTreatment of failed back surgery syndromeNeuromodulation20014310511022151654
- NorthRBKiddDHOlinJSpinal cord stimulation for axial low back pain: a prospective, controlled trial comparing dual with single percutaneous electrodesSpine (Phila Pa 1976)200530121412141815959371
- KramesESpinal Cord Stimulation: Indications, Mechanism of Action, and EfficacyCurr Rev Pain19993641942610998699
- KinfeTMSchuSQuackFJWilleCVesperJPercutaneous implanted paddle lead for spinal cord stimulation: technical considerations and long-term follow-upNeuromodulation201215440240722672364
- NorthRBNigrinDJFowlerKRSzymanskiREPiantadosiSAutomated ‘pain drawing’ analysis by computer-controlled, patient-interactive neurological stimulation systemPain199250151571381071
- WeinandMEMadhusudanHDavisBMelgarMAcute vs prolonged screening for spinal cord stimulation in chronic painNeuromodulation200361151922150909
- PengBWuWHouSLiPZhangCYangYThe pathogenesis of discogenic low back painJ Bone Joint Surg Br2005871626715686239
- KumarKTaylorRSJacquesLSpinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndromePain20071321–217918817845835
- BuvanendranALubenowTJEfficacy of transverse tripolar spinal cord stimulator for the relief of chronic low back pain from failed back surgeryPain Physician200811333333818523504
- OhnmeissDDRashbaumRFPatient satisfaction with spinal cord stimulation for predominant complaints of chronic, intractable low back painSpine J20011535836314588316
- MironerYESatterthwaiteJRLewisEMEfficacy of a Single, Percutaneous, Across Midline, Octrode® Lead Using a “Midline Anchoring” Technique in the Treatment of Chronic Low Back and/or Lower Extremity Pain: A Retrospective StudyNeuromodulation200811428629522151143
- Van BuytenJPVan ZundertJMilbouwGTreatment of failed back surgery syndrome patients with low back and leg pain: a pilot study of a new dual lead spinal cord stimulation systemNeuromodulation19992425826522151259
- VillavicencioATLevequeJCRubinLBulsaraKGoreckiJPLaminectomy versus percutaneous electrode placement for spinal cord stimulationNeurosurgery2000462399405 discussion 405–40610690729
- NorthRBKiddDHOlinJCSierackiJMSpinal cord stimulation electrode design: prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I: technical outcomesNeurosurgery2002512381389 discussion 389–39012182776
- NorthRBKiddDHPetrucciLDorsiMJSpinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: part II-clinical outcomesNeurosurgery2005575990996 discussion 990–99616284568
- VonhögenLHVancampTVannesteSPercutaneously implanted plates in failed back surgery syndrome (FBSS)Neuromodulation2011144319324 discussion 324–32521992425
- de VosCCDijkstraCLendersMWHolsheimerJSpinal cord stimulation with hybrid lead relieves pain in low back and legsNeuromodulation2012152118123 discussion 123–22074412
- BarolatGOakleyJCLawJDNorthRBKetcikBSharanAEpidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back painNeuromodulation200142596622151612
