Abstract
Background
Fibromyalgia patients from a long-term, open-label study of milnacipran (50–200 mg/day) were eligible to participate in a 12-week, randomized, placebo-controlled withdrawal study. The withdrawal study evaluated loss of therapeutic response in patients who achieved ≥50% pain improvements after receiving up to 3.25 years of milnacipran. This post-hoc analysis investigated whether patients who met lower thresholds of pain improvement also experienced worsening of fibromyalgia symptoms upon treatment withdrawal.
Method
Among patients who received milnacipran ≥100 mg/day during the long-term study, three subgroups were identified based on percentage of pain reduction at randomization: ≥50% (protocol-defined “responders”; n=150); ≥30% to <50% (patients with clinically meaningful pain improvement; n=61); and <30% (n=110). Efficacy assessments included the visual analog scale (VAS) for pain, Fibromyalgia Impact Questionnaire-Revised (FIQR), 36-Item Short-Form Health Survey Physical Component Summary (SF-36 PCS), and Beck Depression Inventory (BDI).
Results
In the ≥30 to <50% subgroup, significant worsening in pain was detected after treatment withdrawal. The difference between placebo and milnacipran in mean VAS score changes for this subgroup (+9.0, P<0.05) was similar to the difference in protocol-defined responders (+9.4, P<0.05). In the <30% subgroup, no worsening in pain was observed in either treatment arm. However, patients in this subgroup experienced significant worsening in FIQR scores after treatment withdrawal (placebo, +6.9; milnacipran, −2.8; P<0.001), as well as worsening in SF-36 PCS and BDI scores.
Conclusion
Patients who experienced ≥30% to <50% pain reduction with long-term milnacipran had significant worsening of fibromyalgia symptoms after treatment withdrawal. These results suggest that the conventional ≥30% pain responder cutoff may be adequate to demonstrate efficacy in randomized withdrawal studies of fibromyalgia. Patients in the <30% pain reduction subgroup had worsening scores on the FIQR and other multidimensional measures after treatment withdrawal, indicating the importance of identifying and managing the multiple symptoms of fibromyalgia.
Introduction
Milnacipran is a serotonin and norepinephrine reuptake inhibitor that is currently approved in the US for the management of fibromyalgia, a chronic pain disorder that affects an estimated 2%–6% of the general population.Citation1,Citation2 The efficacy and safety of milnacipran has been evaluated in several randomized, placebo-controlled trials (3–6 months)Citation3–Citation7 and double-blind extension studies (6–9 months).Citation8,Citation9 Patients from these US studies were then enrolled in a long-term, flexible-dose, follow-up study in which they received open-label milnacipran (50 to 200 mg/day) for up to 3.25 years.Citation10 These studies found improvements with milnacipran in key fibromyalgia symptoms, including widespread pain, fatigue, physical functioning, and cognitive disturbances.
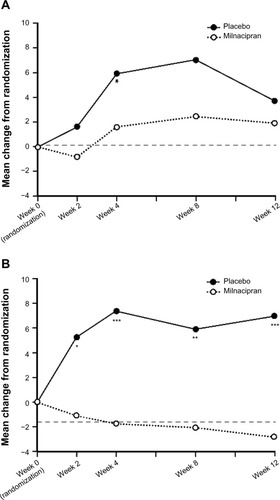
After completing the long-term open-label study, patients were eligible to participate in a 12-week randomized, double-blind withdrawal study that evaluated the effects of discontinuing milnacipran in patients who had experienced substantial improvements in the previous long-term study.Citation11 The pre-specified primary analysis for the milnacipran withdrawal study focused on “responders”, defined as patients at the randomization visit who had ≥50% improvement in visual analog scale (VAS) pain scores from pre-milnacipran exposure (ie, patients’ pain scores prior to entering their first milnacipran study) (). As with another randomized withdrawal study in fibromyalgia patients,Citation12 the 50% pain improvement threshold used to define responders was not selected as a benchmark of clinically significant improvement. Rather, it was chosen in order to enrich the population with responders to increase the probability of detecting a loss of therapeutic response (LTR) after treatment withdrawal. Results from this responder group demonstrated that after having received long-term milnacipran treatment in prior studies, time to LTR was significantly shorter in patients who discontinued treatment as compared with those who continued receiving milnacipran (P<0.001).Citation11
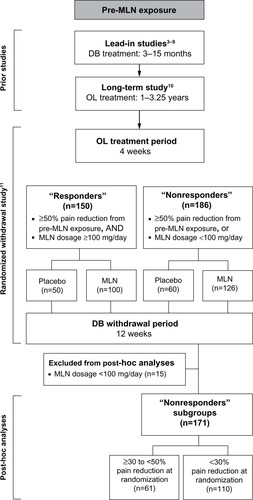
Figure 1 Overview of milnacipran studies.
In addition to analyzing LTR in the responder population, the milnacipran withdrawal study was designed to randomize and follow those patients who did not have ≥50% pain improvement from pre-milnacipran exposure at the randomization visit. Although labeled as “nonresponders” for the purpose of the primary analysis, this group included a subset of patients who did have ≥30% pain improvements at randomization. This definition, which has been anchored to patient-reported global improvements (“much improved” or “very much improved”),Citation13 is an accepted threshold for clinically meaningful pain response in fibromyalgia clinical trials.Citation14,Citation15 Post-hoc analyses were conducted in this ≥30% to <50% pain reduction subgroup to explore whether a ≥30% pain responder criterion could be used to evaluate the effects of discontinuing treatment in randomized withdrawal studies of fibromyalgia, as has been done in withdrawal studies of neuropathic pain.Citation16–Citation18 Post-hoc analyses were also conducted to determine the effect of treatment withdrawal in the subgroup of patients who had <30% pain reduction at randomization.
Methods
Male and female outpatients with fibromyalgia who had participated in a long-term, flexible-dose, open-label trial of milnacipranCitation10 were eligible to participate in a randomized, double-blind, placebo-controlled, withdrawal study (). Upon entering the withdrawal study, patients received 4 weeks of open-label milnacipran at the same dosage (50–200 mg/day) that they had been taking during the preceding long-term trial.
At the randomization visit of the withdrawal study, patients were classified as responders or nonresponders based on mean change in VAS 24-hour recall pain score from pre-milnacipran exposure (ie, baseline score of the placebo-controlled study in which the patient was first enrolled). Responders were defined as patients who had ≥50% decrease in VAS pain scores from pre-milnacipran exposure to the randomization; the remaining patients were defined as nonresponders. Both the responders and nonresponders were then randomized (1:2) separately to placebo or milnacipran to evaluate the effects of treatment withdrawal. The pre-specified primary analyses for the milnacipran withdrawal study focused on the responders, and results for this group have been previously published.Citation11 Some of the data from the responders are also included in this report as a reference point for comparison with the two nonresponder subgroups that were identified for post-hoc analyses.
The post-hoc analyses were conducted using data from nonresponders who received ≥1 dose of study medication during the randomized withdrawal period and had ≥1 post-randomization efficacy assessment. For consistency with dosing recommendations,Citation19 the post-hoc analyses excluded 15 patients who were classified as nonresponders because they were treated with milnacipran <100 mg/day during the prior long-term study. The remaining nonresponders were categorized into two subgroups: those with clinically meaningful pain response (≥30% to <50% pain reduction in VAS pain score from pre-milnacipran exposure at randomization) and those with <30% pain reduction.
Since the primary analyses in responders were defined by worsening in VAS pain scores, the post-hoc analyses included mean changes in VAS pain from pre-milnacipran exposure and from randomization at each study visit in the withdrawal period (weeks 2, 4, 8, and 12). Mean changes from randomization to the end of withdrawal (week 12) in the Fibromyalgia Impact Questionnaire-Revised (FIQR)Citation20 were also analyzed. The FIQR is a multidimensional instrument specifically developed to evaluate multiple fibromyalgia symptoms (ie, pain, energy/fatigue, stiffness, tenderness, sleep disturbances, depressed mood, anxiousness, difficulties with memory, problems with balance, and sensitivity to non-painful stimuli), as well as a patient’s ability to function and perform daily tasks. As an overall indicator of fibromyalgia severity, the FIQR was chosen to evaluate whether study participants might have experienced worsening in symptoms other than pain. Additional analyses included changes from randomization to the end of withdrawal in the 36-Item Short-Form Health Survey Physical Component Summary (SF-36 PCS)Citation21 and Beck Depression Inventory (BDI)Citation22 total scores. Missing data were imputed using a last observation carried forward (LOCF) approach for all measures except the BDI, which was analyzed based on observed cases. Statistical analyses were conducted using 2-sided hypothesis tests with a significance level of 0.05.
Results
Patient characteristics
Of the 336 patients in the intent-to-treat population, 186 were classified as nonresponders (). Similar to the demographics of the responder population,Citation11 the majority of these patients were female (96.2%) and white (90.3%), with a mean age of 54.8 years. The post-hoc analyses included 61 patients in the ≥30% to <50% pain reduction subgroup and 110 patients in the <30% pain reduction subgroup.
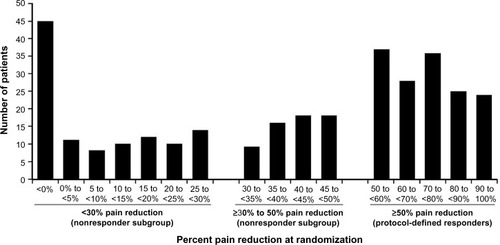
When analyzed by 5% increments in pain improvement, the number of patients at randomization in each increment was distributed evenly across the ≥30% to <50% subgroup (range, n=16 to 18), with fewer patients having ≥30% to <35% pain reduction (n=9) (). In the <30% subgroup, the number of patients in each 5% increment was distributed fairly evenly from 0% to <30% (range, n=8 to 14). However, this subgroup also included 45 patients with <0% pain reduction at randomization, indicating no change or worsening in VAS pain scores from pre-milnacipran exposure to randomization.
Figure 2 Distribution of pain response at randomization in patients who received milnacipran ≥100 mg/day during the prior long-term, open-label study.
Prior to milnacipran exposure, mean baseline VAS pain scores were generally similar across subgroups (), with scores >60 indicating moderate-to-severe pain levels.Citation23 After long-term treatment with milnacipran, mean VAS pain scores at randomization in the ≥30% to <50% subgroup were approximately 40% lower than the pre-milnacipran scores. In the <30% subgroup, mean pain severity decreased by approximately 5% from pre-milnacipran exposure to randomization. In both subgroups, mean VAS pain scores at randomization were similar between patients who were subsequently switched to placebo (ie, withdrawn from treatment) and those who continued receiving milnacipran.
Table 1 Mean scores prior to milnacipran exposure and at study randomizationTable Footnotea
Pre-milnacipran exposure data were not available for the FIQR since the previous milnacipran studies had used an earlier version of the scale (FIQCitation24). However, in both the ≥30% to <50% and <30% subgroups, mean FIQR total scores at randomization were lower than the mean total score (56.6) of fibromyalgia patients in the validation study of the FIQR,Citation20 suggesting that these patients experienced improvements in overall fibromyalgia severity after long-term milnacipran treatment. In both subgroups, mean FIQR total scores at randomization were generally similar between treatment arms. For SF-36 PCS, mean scores increased (ie, improved) by approximately 10% from pre-milnacipran exposure to randomization in the ≥30% to <50% pain subgroup; no change was detected in the <30% subgroup.
Due to the exclusion of patients with major depressive episodes in the previous milnacipran studies, all patients in the withdrawal study generally had, at most, only mild or moderate depressive symptoms prior to any milnacipran exposure. However, mean BDI scores did decrease by approximately 40% and 20% from pre-milnacipran exposure to randomization in the ≥30% to <50% and <30% subgroups, respectively.
Efficacy analyses
Pain
Patients in the ≥30% to <50% pain reduction subgroup who were switched to placebo experienced significant mean increases in pain severity by the end of the 12-week randomized withdrawal period, as compared with patients who remained on milnacipran ( and ). Mean changes from randomization in VAS pain score were +8.5 and −0.5 for placebo and milnacipran, respectively (P<0.05). In the <30% subgroup, no significant increase in pain severity was found in patients who were withdrawn from treatment.
Figure 3 Mean changes from pre-milnacipran exposure in VAS pain scores.
Abbreviations: MLN, milnacipran; VAS, visual analog scale.
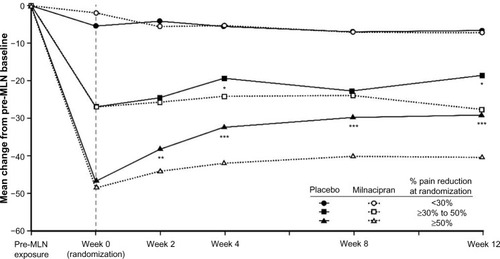
Table 2 Mean changes from randomization to end of the 12-week withdrawal period
FIQR
In the ≥30% to <50% subgroup, patients switched from milnacipran to placebo showed greater worsening in fibromyalgia severity (ie, FIQR total scores) than those who continued treatment, with statistical differences seen at week 4 (). Patients in the <30% subgroup who were withdrawn from milnacipran had significant mean worsening in overall fibromyalgia severity at every study visit during the randomized withdrawal period () compared with patients who remained on milnacipran. At the end of withdrawal, the difference between placebo and milnacipran for mean change in FIQR total score was statistically significant in the <30% pain reduction subgroup (+9.7, P<0.001); the difference was smaller in the ≥30% to <50% subgroup and not statistically significant (+1.8, P>0.05; ).
Physical function and depression
No significant mean worsening in physical functioning or depressive symptoms was detected in either of the nonresponder subgroups (). However, differences between placebo and milnacipran for mean changes in SF-36 PCS and BDI total scores were greater in the <30% subgroup than in the ≥30% to <50% subgroup.
Discussion
Fibromyalgia patients who received open-label milnacipran for up to 3.25 yearsCitation10 were enrolled in a 12-week, randomized, double-blind, placebo-controlled withdrawal studyCitation11 in which they were classified and stratified at randomization as responders or nonresponders based on their pain improvement after long-term treatment with milnacipran. Patients achieving a ≥50% decrease from pre-milnacipran exposure in VAS pain scores were considered responders and patients not meeting this pain improvement threshold were classified as nonresponders. Previously published results showed that patients in the responder group experienced significant worsening in pain and other symptoms when active treatment was discontinued in the withdrawal study, confirming that these patients had experienced substantial pain relief during long-term treatment with milnacipran.Citation11 However, data were also collected for patients who did not meet the responder criteria. Post-hoc analyses were conducted on two subgroups that were characterized by pain response: patients with clinically meaningful pain improvements after long-term milnacipran treatment (ie, ≥30% to <50% decrease in VAS pain score from pre-milnacipran exposure to randomization in the withdrawal study) and those who had <30% pain improvement at randomization. These analyses were conducted to further evaluate the effects of discontinuing milnacipran in patients who met varying thresholds of pain response after long-term treatment.
Mean VAS pain scores at pre-milnacipran exposure indicated that all patients in the withdrawal study (nonresponders as well as responders) had severe pain prior to entering their first milnacipran study. As expected, the protocol-defined responders experienced the greatest mean improvements after long-term treatment, with more than half of these patients (85/150) having ≥70% pain reduction at randomization. Patients in the subgroup with ≥30% to <50% pain reduction at randomization had approximately 40% improvements in VAS pain scores after long-term milnacipran treatment. However, this group was relatively small (n=61), which may have limited the ability to detect statistical significance between placebo and milnacipran in some of the outcome measures that were analyzed for this report. The overall mean reduction in VAS pain scores from pre-milnacipran exposure to randomization was relatively low in the <30% subgroup (approximately 5%), partly due to the inclusion of 45 patients who had no change or worsening of pain severity after long-term milnacipran treatment. However, this group also included 46 patients who had ≥10% to <30% pain reduction at randomization. It may be important to consider this wide range of pain responses when interpreting the results for the entire <30% subgroup that are presented in this report.
Since loss of pain improvement had been demonstrated in the pre-specified primary analysis for the responders, the main purpose of analyzing the data from the nonresponder group post-hoc was to assess whether these patients also had worsening in pain after withdrawal of long-term milnacipran treatment. Results from this analysis indicate that patients in the ≥30% to <50% subgroup did experience significant worsening of pain after treatment withdrawal, although the results should be interpreted with some caution. Given that the pain improvement at randomization (week 0), by definition, was smaller in the ≥30% to <50% subgroup than in the ≥50% subgroup (ie, protocol-defined responders), statistical significance during the randomized withdrawal period (weeks 2–12) was expected to be less marked and less consistent in the ≥30% to <50% subgroup than in the responders. Significance was seen at two of the three last study visits (), and the week 2 visit may have been too early to demonstrate a loss of effect. At endpoint (week 12), the difference between placebo and milnacipran in pain score change from randomization was similar between the ≥30% to <50% group (+9.0, P<0.05) and the ≥50% responders (+9.4, P<0.01; ). These results suggest that a threshold of ≥30% improvement in pain may be an adequate criterion to identify treatment responders in randomized withdrawal studies of fibromyalgia, and that this conventional definition of pain response can be used to monitor the long-term effectiveness of milnacipran on fibromyalgia pain.
One question that arose during the randomized withdrawal study was why patients with <30% pain improvement chose to complete the prior long-term milnacipran study despite having relatively lower levels of pain response (or even worsening) by the end of treatment. It was postulated that these patients may have been experiencing relief in other symptoms besides pain, and subgroup analyses were conducted to assess whether or not they had worsening of other symptoms when switched to placebo. The FIQR was used to address this question because it is a multidimensional instrument that was specifically developed to assess multiple symptom domains (eg, fatigue, tenderness, memory problems, depressed mood) and daily functioning in patients with fibromyalgia.Citation20 The difference between placebo and milnacipran in the FIQR analyses showed that patients in the <30% pain reduction subgroup experienced a worsening after treatment withdrawal (+9.7, P<0.001) that was similar to the difference observed in the responders (+7.2, P<0.001). Moreover, significantly greater worsening in FIQR was observed at each study visit in patients who were switched to placebo versus milnacipran (), suggesting that patients who do not experience meaningful improvements in pain with milnacipran still may have improvement in other symptoms domains due to milnacipran and then experience worsening in these other fibromyalgia symptoms when treatment is removed. The nonsignificant difference between placebo and milnacipran in FIQR at the end of the study in the ≥30% to <50% subgroup was somewhat surprising, since worsening of overall disease severity after treatment withdrawal was expected in patients who had experienced clinically meaningful improvements in pain. Although the reasons for this outcome are unknown, the relatively small size of the ≥30% to <50% subgroup may have been a contributing factor.
No significant mean worsening in SF-36 PCS or BDI total score was found in patients who discontinued milnacipran in either nonresponder subgroup. However, the numerical difference between placebo and milnacipran for change in SF-36 PCS score in the <30% subgroup (−3.0) was slightly greater than the significant difference found in the protocol-defined responders (−2.4, P<0.01). For BDI total score, depressive symptoms worsened to a greater extent when milnacipran was withdrawn than when treatment was continued. Although such worsening was observed in both nonresponder subgroups, the difference between milnacipran and placebo was somewhat greater in the <30% subgroup (+1.9) than in the ≥30% to <50% subgroup (+1.0) or responders (+0.1). It should be noted, however, that since patients only had mild-to-moderate depressive symptoms prior to enrolling in their first milnacipran study (due to study exclusion criteria), none of these results reflect a clinically meaningful change in depressive symptoms. Taken together, results from the FIQR, SF-36 PCS, and BDI analyses in the <30% subgroup suggest that pain should not be the only standard used to measure clinical efficacy in patients with fibromyalgia. There may be a subset of patients who experience improvements in other key fibromyalgia symptoms and benefit from treatment despite their lower levels of pain improvement.
The general limitations of the withdrawal study have been previously reported.Citation11 For the post-hoc analyses presented here, several limitations should also be noted. First, although the withdrawal study was designed to randomize and follow patients who did not achieve the ≥50% pain responder criterion, the decision to further analyze this group using the ≥30% cutoff was made post-hoc in order to differentiate “nonresponders” with clinically meaningful pain improvements after long-term milnacipran treatment (≥30% to <50%) from those with less pain improvement (<30%). Second, this choice of analyses resulted in relatively small subgroups, which may have limited the ability to detect statistically significant differences between treatment arms. Finally, for consistency with prescribing guidelines for milnacipran,Citation19 the post-hoc analyses were limited to patients who had received a minimum dosage of 100 mg/day during the prior long-term study. It seems unlikely that including patients who received milnacipran <100 mg/day would have altered the outcomes presented in this report.
Conclusion
Similar to previous findings in patients with ≥50% pain improvement after long-term milnacipran treatment, discontinuation of treatment in patients who had clinically meaningful (≥30%) pain improvements but did not meet the 50% threshold resulted in significant worsening of pain. These results suggest that the more conventional ≥30% pain responder definition may be adequate to define responders for enrichment in randomized withdrawal studies of fibromyalgia. Despite only modest (<30%) pain improvements after receiving long-term milnacipran, some patients experienced significant worsening in nonpain measures of fibromyalgia severity upon withdrawal of treatment, under-scoring the clinical importance of identifying and managing the multiple symptoms of fibromyalgia.
Acknowledgments
This work was supported by Forest Research Institute, Inc. (Jersey City, NJ, USA), a subsidiary of Forest Laboratories, Inc. Writing and editorial assistance was provided by Mildred Bahn of Prescott Medical Communications Group (Chicago, IL, USA) with support from Forest Research Institute, Inc.
Disclosure
Dr Mease has received research/grant funding and consultation fees from Forest Laboratories, Inc., Cypress Bioscience, Inc. (now Royalty Pharma), Eli Lilly and Company, Pfizer Inc., Allergan, Inc., Wyeth Pharmaceuticals, Jazz Pharmaceuticals, and Fralex Therapeutics. Dr Clauw has received grants/research support from Pfizer Inc. and Forest Laboratories. He has been a consultant for and served on advisory boards for Pfizer Inc., Eli Lilly and Company, Forest Laboratories, Inc., Cypress Bioscience, Inc, Pierre Fabre Pharmaceuticals, UCB, and AstraZeneca. Drs Trugman and Wang are full-time employees of Forest Research Institute, Inc. Dr Palmer was a full-time employee of Forest Research Institute, Inc. at the time of the study and during the development and approval of this manuscript.
References
- WolfeFRossKAndersonJRussellIJHebertLThe prevalence and characteristics of fibromyalgia in the general populationArthritis Rheum199538119287818567
- VincentALahrBDWolfeFPrevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology ProjectArthritis Care Res (Hoboken)201365578679223203795
- GendreauRMThornMDGendreauJFEfficacy of milnacipran in patients with fibromyalgiaJ Rheumatol200532101975198516206355
- MeasePJClauwDJGendreauRMThe efficacy and safety of milnacipran for treatment of fibromyalgia. A randomized, double-blind, placebo-controlled trialJ Rheumatol200936239840919132781
- ClauwDJMeasePPalmerRHGendreauRMWangYMilnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trialClin Ther200830111988200419108787
- ArnoldLMGendreauRMPalmerRHGendreauJFWangYEfficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trialArthritis Rheum20106292745275620496365
- TrugmanJMPalmerRHMaYMilnacipran effects on 24-hour ambulatory blood pressure and heart rate in fibromyalgia patients: a randomized, placebo-controlled, dose-escalation studyCurr Med Res Opin201430458959724188161
- GoldenbergDLClauwDJPalmerRHDurability of therapeutic response to milnacipran for fibromyalgia. Results of a randomized, double-blind, monotherapy 6-month extension studyPain Med201011218019420002596
- FerreraRPalmerRWangYGendreauRImprovements in fibromyalgia symptoms are sustained for 1 year with milnacipran treatment: results from 2 double-blind, dose-controlled extension studies [abstract]J Pain2009104 Suppl 1S60
- ArnoldLMPalmerRHMaYA 3-year, open-label, flexible-dosing study of milnacipran for the treatment of fibromyalgiaClin J Pain201329121021102823446068
- ClauwDJMeasePJPalmerRHTrugmanJMWangYContinuing efficacy of milnacipran following long-term treatment in fibromyalgia: a randomized trialArthritis Res Ther2013154R8823953493
- CroffordLJMeasePJSimpsonSLFibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalinPain2008136341943118400400
- FarrarJTYoungJPJrLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain200194214915811690728
- MeasePJClauwDJChristensenRToward development of a fibromyalgia responder index and disease activity score: OMERACT module updateJ Rheumatol20113871487149521724721
- ArnoldLMWilliamsDAHudsonJIDevelopment of responder definitions for fibromyalgia clinical trialsArthritis Rheum201264388589421953205
- GilronIWajsbrotDTherrienFLemayJPregabalin for peripheral neuropathic pain: a multicenter, enriched enrollment randomized withdrawal placebo-controlled trialClin J Pain201127318519321178603
- TothCMawaniSBradySAn enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic painPain2012153102073208222921260
- RaskinPHuffmanCTothCPregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trialClin J Pain201430537939023887339
- Savella (milnacipran HCl) [prescribing information]St Louis, MOForest Pharmaceuticals, Inc2013
- BennettRMFriendRJonesKDWardRHanBKRossRLThe Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties [Erratum, Arthritis Res Ther 2009;11:415]Arthritis Res Ther2009114R12019664287
- WareJEJrSherbourneCDThe MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selectionMed Care19923064734831593914
- BeckATSteerRABrownGKManual for the Beck Depression Inventory-IISan Antonio, TXPsychological Corporation1996
- BennettRMKaminMKarimRRosenthalNTramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled studyAm J Med2003114753754512753877
- BurckhardtCSClarkSRBennettRMThe fibromyalgia impact questionnaire: development and validationJ Rheumatol19911857287331865419