Abstract
Introduction
Opioid analgesia can be explored with quantitative sensory testing, but most investigations have used models of phasic pain, and such brief stimuli may be limited in the ability to faithfully simulate natural and clinical painful experiences. Therefore, identification of appropriate experimental pain models is critical for our understanding of opioid effects with the potential to improve treatment.
Objectives
The aim was to explore and compare various pain models to morphine analgesia in healthy volunteers.
Methods
The study was a double-blind, randomized, two-way crossover study. Thirty-nine healthy participants were included and received morphine 30 mg (2 mg/mL) as oral solution or placebo. To cover both tonic and phasic stimulations, a comprehensive multi-modal, multi-tissue pain-testing program was performed.
Results
Tonic experimental pain models were sensitive to morphine analgesia compared to placebo: muscle pressure (F=4.87, P=0.03), bone pressure (F=3.98, P=0.05), rectal pressure (F=4.25, P=0.04), and the cold pressor test (F=25.3, P<0.001). Compared to placebo, morphine increased tolerance to muscle stimulation by 14.07%; bone stimulation by 9.72%; rectal mechanical stimulation by 20.40%, and reduced pain reported during the cold pressor test by 9.14%. In contrast, the more phasic experimental pain models were not sensitive to morphine analgesia: skin heat, rectal electrical stimulation, or rectal heat stimulation (all P>0.05).
Conclusion
Pain models with deep tonic stimulation including C fiber activation and and/or endogenous pain modulation were more sensitive to morphine analgesia. To avoid false negative results in future studies, we recommend inclusion of reproducible tonic pain models in deep tissues, mimicking clinical pain to a higher degree.
Keywords:
Introduction
Opioid treatment of clinical pain challenges clinicians due to variable individual responses. This has resulted in continuous research in analgesic effects and underlying drug mechanisms. Confounders such as underlying diseases, comedication, and psychological status provide an inhomogeneous population in clinical studies, and therefore, it can be complicated to demonstrate analgesic effects. In contrast, human experimental pain models offer opportunities to evaluate underlying analgesic mechanisms in standardized laboratories, where reproducible stimuli can be applied and the pain can be assessed quantitatively.Citation1 However, human experimental pain studies have also demonstrated variable results regarding opioid effects, and not all compounds with known analgesic properties are effective in all experimental models.Citation2,Citation3 Therefore, identification of appropriate experimental pain models can be critical for facilitating the rational clinical development of novel analgesic compounds and drug formulations.Citation4
To assess several elementary attributes of the stimuli in the experimental pain model, it has been recommended to use a battery of stimulations (multi-modal) in several tissues (multi-tissue), rather than a single stimulation.Citation5 This approach will complicate the experimental procedure, emphasizing the importance of having consistent, standardized laboratory facilities and experienced research staff available, providing reliable methods.Citation1 Identifying combinations of experimental models and clinical validation seems to be the direction for the development of experimental pain models toward predictive tools in drug development.Citation6
The different methods can be divided into phasic and tonic models based on the nature and duration of the different methods.Citation7,Citation8 Phasic pain models induce fast and sharp pain predominantly transmitted by Aδ fibers; whereas tonic pain models induce slow and dull pain transmitted primarily by C fibers.Citation7,Citation8 Animal studies have demonstrated that opioids preferentially attenuate nociceptive responses produced by C fiber activation.Citation9,Citation10 Additionally, human studies have shown that pain models including C fiber activation and/or characterization of endogenous pain modulation and/or increased affective component are more sensitive to opioids.Citation1 Despite this, most investigations on analgesic effects have been performed using models of phasic pain in superficial tissues.Citation2
Therefore, the aim was to investigate the effect of the gold standard opioid, morphine, on a battery of phasic and tonic pain stimulations in a multi-modal, multi-tissue, human experimental pain study in a group of healthy volunteers.
Methods
The Ethics Committee for the Region of Northern Jutland (reference no N-20100046) and the Danish Medicines Agency (reference no 2612–4319) approved the study, which was carried out in the Research Laboratory at Mech-Sense, Department of Gastroenterology, Aalborg University Hospital, Aalborg, Denmark. The study was conducted in the period November 2010 to April 2012.
The trial was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01245244, EUDRACT no 2010-020894-17). The study was monitored according to the rules of Good Clinical Practice (GCP) by the GCP unit Aarhus University Hospital, Aarhus, Denmark.
Participants
Forty participants were enrolled by a medical doctor, gave their informed consent and were compensated for participation. Inclusion criteria were: 1) age between 20 and 65 years; 2) opioid naïve, ie, has not recently taken an opioid medication regularly; 3) no known allergy to study medication; 4) no ongoing participation in other drug studies; 5) not pregnant; 6) no previous addictive behavior; 7) no previous pain causing diseases or psychiatric disorders. All included woman were on safe contraceptive medication during the study. Before inclusion, a medical doctor conducted a routine health screening for each participant, ruling out any pain-related conditions.
Study protocol
A double-blind, randomized, two-way crossover, single-dose study was conducted with at least 1-week washout intervals. A pharmacist not involved in the study generated a randomization list by http://www.randomization.com; all participants were randomized into four blocks to receive morphine or placebo on day 1 or day 2. The same pharmacist handled the morphine oral mixture and secured and documented that all participants received the correct medication for specific periods. Thus, the experimenters and the participants were fully blinded for randomization. One-week washout intervals were chosen to ensure that the morphine was fully excreted before the second study period. Each participant fasted at least 6 hours prior to the study. Prior to the first dosing day, a training session, including all experimental pain procedures was conducted, in order to familiarize the participants with the laboratory environment and to verify that the participants could tolerate the comprehensive experimental pain-testing procedure. The study was controlled for menstrual cycle so that each woman was investigated in the same phase of her individual menstrual cycle. All testing was performed by well-trained experimenters in a quiet room. The same experimenter tested each participant at the same time of the day on both study days.
Medication
Each participant received morphine 30 mg (2 mg/mL) as an oral solution or placebo. The color and taste of the solution was masked by adding 5 mL of orange juice concentrate to the solution; hence, the total amount of one dose was 20 mL (15 mL morphine solution or pure water [placebo] and 5 mL of orange juice). Oral solution was used instead of tablets to avoid variability in dissolution of the tablets.
Pain assessment
Somatic stimulations were interrupted when the participant reported “pain tolerance threshold” (PTT). A modified numerical/categorical analog scale for assessment of nonpainful and painful sensations was used for the rectal stimulations and for the cold pressor stimulation. The scale is a continuous scale from 0 to 10, where 5= the pain detection threshold (PDT). This scale has been validated for reliability and robustness, and is described in detail elsewhere.Citation11
Pain-testing procedures
A comprehensive multi-modal, multi-tissue, pain-testing program was performed, including pain thresholds and pain intensity ratings. A variety of pain models were selected to cover both superficial and deep pain as well as phasic and tonic stimulations ( and ). Hereby, sufficient resting periods were included in the protocol and the risk for sensitization was diminished.
Figure 1 Graphical overview of the experimental procedure.
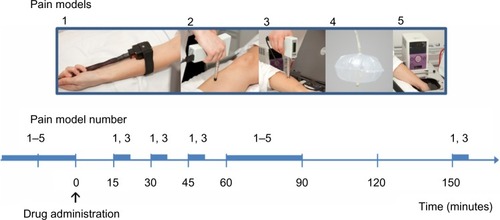
Table 1 Overview of included pain models
Skin stimulation
Thermal
Thermal heat stimuli were applied by a contact heat-evoked potential stimulator (CHEPS; Medoc Ltd, Ramat Yishai, Israel), placed 10 cm distal to the elbow on the right forearm. The start temperature was set to 32°C and the temperature increase was set at 1°C/s. Participants were asked to press a button at PTT, and immediately afterward, the thermode cooled down to 32°C, with a cooling rate of 10°C/s. This was repeated three times, and the average stimulus intensity (°C) was calculated and used for further data analysis.
Muscle stimulation
Mechanical
Pressure was applied to the supinator muscle on the left forearm, 15 cm distal to the elbow by a handheld electronic pressure algometer (Somedic AB, Stockholm, Sweden) with a standard probe of 1 cm2. The pressure increase rate was 30 kPa/s and the algometer was set with a safety maximum of 2,000 kPa. The stimulation was interrupted when the participants reported PTT and the maximum stimulus intensity (kPa) was noted and used for further analysis.
Bone stimulation
Mechanical
The mechanical stimulation was conducted on the right tibia bone, 10 cm distal to the patella by a handheld electronic pressure algometer (Somedic AB) with a specially designed probe of 3.1 mm2 (Aalborg University, Aalborg, Denmark). This probe has been validated in previous studies.Citation12,Citation13 The pressure increase rate was 999 kPa/s. Stimulation was interrupted when PTT was reported. The maximum stimulus intensity (kPa) was noted and used for further analysis.
Visceral stimulation
For rectal stimulation, a custom-designed probe (Ditens, Egaa, Denmark) with a polyester urethane bag for mechanical and thermal stimulation, and stainless steel electrodes for electrical stimulation was used. The rectal probe has been validated and is described in detail elsewhere.Citation14 Prior to the experiment, a 5 mL enema with 2 mg/mL bisacodyl (Toilax®; Orion Pharma, Espoo, Finland) was administered. A lubricated anoscope (Cat No E-03. 19. 925; Heine Optotechnik, Herrsching, Germany) was placed in the anal canal and through this, the probe was placed in the rectum 20 cm from the anal sphincter. All data were displayed online (Openlab; GMC, Hornslet, Denmark) and stored for later analysis.
Mechanical
For mechanical stimulation of the rectum, the bag was inflated with 37°C water from a water bath (Julabo VWR 5; Julabo, Labortechnik GMBH, Seelbach, Germany) controlling the temperature. A peristaltic pump (Type 111; Ole Dich Instrumentmakers, Hvidovre, Denmark) was used to inflate the bag at a rate of 200 mL/minute. Three distensions to the PDT were performed with a 1-minute interval to precondition the tissue. Hereafter, a single distension to “moderate pain” was performed. The bag was emptied at the same rate as it was inflated. The volumes in the bag (mL) at PDT and at moderate pain were noted and used for further analysis.
Electrical
For the electrical stimulation, two stainless steel bipolar electrodes mounted on the tip of the rectal probe with an inter-electrode distance of 2 mm were connected to a computer-controlled constant current stimulator (Digitimer Ltd, Welwyn Garden City, UK). Impedance was kept below 3 kΩ to ensure sufficient mucosal contact. The electrical stimulation intensity slowly increased in increments of 1.0 mA increments. To blind the participant, sham stimulations with same or lower intensities were included. Stimulus intensities (mA) at PDT and at moderate pain were noted and used for further analysis.
Thermal
Before thermal stimulation, 60 mL of 37°C water was added to the polyester urethane bag of the probe. The temperature elevation was reached by recirculation of 68°C water through the bag. A water bath (VWR 5; Julabo) controlled the temperature in the closed circuit. A peristaltic pump (Type 111; Ole Dich Instrumentmakers) circulated the water at a flow rate of 150 mL/minute, and a temperature sensor (Buhl & Bønsøe AS, Virum, Denmark) was located in the inflated bag. During thermal stimulation, the anal canal was shielded with the anoscope in order to minimize stimulation of somatic tissue. The temperature increased until the participants reported moderate pain, and immediately hereafter, the heated water was withdrawn to minimize discomfort for the participant. Thermal stimulus was computed as area under the time–temperature curve.
Cold pressor test
For the cold pressor stimulation, a cold pressor test apparatus (Grant instruments; Fischer Scientific, Slangerup, Denmark) was used. The participants immersed their left hand into the 2°C cooled water up to the wrist for 2 minutes. The participants rated the perceived pain continuously on the electronic handheld device.
Statistical analysis
All data were baseline corrected before statistical analysis. Thus, data for statistical analysis represent individual relative changes from baseline values expressed as percentages. Coefficients of difference between treatments and associated confidence intervals were provided for time point 60 minutes.
For statistical comparison of placebo versus morphine effects on thermal skin stimulation and mechanical muscle stimulation, data were analyzed by two-way repeated measures analysis of variance (ANOVA), as these assessments were performed at several time points (15, 30, 45, 60, and 150 minutes after drug administration) in each study arm. Factors for these two-way ANOVAs were: 1) treatment and 2) time. For rectal electrical and mechanical stimulations, analyses were performed at two pain levels (PDT and moderate pain). These data were analyzed by two-way ANOVA, where factors were: 1) treatment and 2) pain level. When outcome measures were only assessed at one time point (60 minutes after drug administration), one-way ANOVA was used for statistical analysis (mechanical bone stimulation, rectal thermal stimulation, and the cold pressor test). Stata software (v12.1) was used for analysis. P-values of <0.05 were considered significant.
As this study investigated the effects of morphine on multiple endpoints, a precise calculation of sample size for each endpoint was not possible. A sample size was estimated based on data from a previous study, from our group,Citation27 of opioid effect assessed by heat stimulation of the skin in healthy volunteers, as skin heat stimulation was hypothesized to be the least sensitive outcome. To detect a difference of 4% in analgesic effect between placebo and morphine, 40 subjects should be included (alpha =0.05, power =0.90).
Results
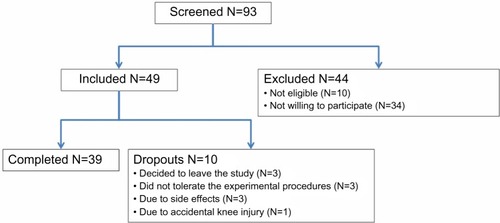
The study was conducted over 18 months over the period October 2010 to April 2012, during which 93 healthy volunteers were screened for participation and 39 (18 females and 21 males; average age: 26.9±6.5 years) completed the study (). Forty-nine participants were initially included; ten of these were dropouts due to different reasons. Nine dropouts took place at the training session, before randomization. Thus, only one participant dropped out after randomization, and the balance of the study was reserved. Thirty-nine participants finalized the study and were included in further analyses.
Figure 2 Flow diagram of the study.
Side effects
Three participants experienced morphine side effects to such a degree that the experiment was interrupted. They reported dizziness, tiredness, and intense pain in the epigastrium radiating to both sides. In total, 26 of 39 participants experienced side effects due to the morphine treatment (nine reported nausea, 23 reported dizziness, four reported itching, and two reported sweating). Eight out of 39 participants experienced side effects in the placebo arm (five reported nausea, four dizziness, and one itching).
Dynamic effects
Thirty-nine participants completed both experimental sessions. However, not all of them completed all tests on both days, due to technical challenges such as equipment failure or balloon leakage on the rectal probe. For the cold pressor test, only participants who tolerated 2 minutes conditioning stimuli were included in the analysis and those who withdrew their hand before 2 minutes were excluded. Total numbers of participants included in each analysis are provided in , which provides an overview of the results. Subanalyses of sex-related differences in morphine responsiveness were performed for each dynamic endpoint; however, no differences were found (all P>0.05).
Table 2 Results for all investigated pain stimulations given as mean values and confidence intervals
For all significant outcomes, coefficients of significant differences between placebo and morphine and associated confidence intervals for effects at the 60-minute time point are provided in .
Table 3 Significant outcomes; coefficients of differences in percentage at the 60-minute time point
Skin stimulation
Thermal
Thirty-seven participants completed the skin heat stimulation test at both experimental sessions. No difference was found between placebo and morphine (F=0.21, P=0.65).
Muscle stimulation
Mechanical
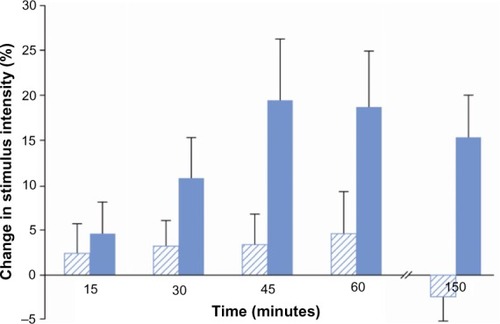
Thirty-nine participants completed the muscle pressure stimulation test at both experimental sessions. In comparison to placebo, morphine increased pain thresholds to muscle pressure over time (F=4.87, P=0.03). The effect started at 30 minutes and reached the maximum at 45 minutes, remaining effective until the end of the examination period (150 minutes; ).
Figure 3 Analgesic effect on mechanical muscle stimulation.
Bone stimulation
Mechanical
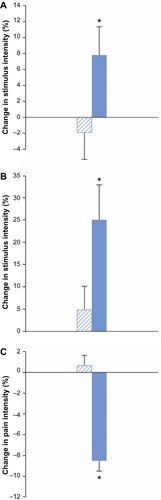
Thirty-nine participants completed the bone pressure stimulation at both experimental sessions. Morphine increased pain thresholds compared to placebo. However, this effect was at the border of significance (F=3.98, P=0.05; ).
Figure 4 Analgesic effects.
Visceral stimulation
Electrical
Thirty participants completed the rectal electrical stimulation at both experimental sessions. No difference was found between placebo and morphine effects (F=0.02, P=0.88).
Mechanical
Thirty-six participants completed the rectal mechanical stimulation at both experimental sessions. Morphine increased pain thresholds to rectal mechanical stimulation compared to placebo (F=4.25, P=0.04; ).
Thermal
Thirty-eight participants completed the rectal heat stimulation at both experimental sessions. No difference was found between placebo and morphine (F=0.33, P=0.57).
Cold pressor test
Thirty-four participants completed the 2-minute cold pressor test. Morphine decreased pain ratings compared to placebo (F=25.3, P<0.001; ).
Discussion
This is the first study that compares multi-modal and multi-tissue stimulations in the same experiment in a fairly large group of well-characterized healthy volunteers. We demonstrated that models including deep structure stimulations (muscle, bone, cold pressor, and rectum) with relatively long (tonic) duration were more sensitive to morphine analgesia than phasic and superficial models.
Comparison between modalities
Skin pain
The method used for skin heat stimulation in the present study fulfilled previous recommendations.Citation15 Despite this, it was not sensitive to morphine analgesia in the present study, as no difference was found between placebo and morphine. Opioid effects on heat pain have been tested through various stimulation paradigms and conflicting results exist.Citation1,Citation2 In the present study, the heat stimulation was stopped at moderate pain, meaning that it was more intense than a stimulus stopped at pain detection. It has been assumed that an A delta-mediated nociceptive component will show increasing dominance at high-intensity heatingCitation16 and it has previously been demonstrated that for heat stimulation, pricking pain by A delta fibers was felt at the end of the stimulation at high intensities.Citation17 Therefore, in the present study, it could be speculated that A delta fibers were activated in the noxious range. Thus, the fact that no morphine analgesia was demonstrated may reflect the limited effectiveness of attenuating the A delta-mediated nociceptive component. Thus, in future experimental pain studies of morphine effects, it is not recommended to use high-intensity stimulations for heat stimulation of the skin by the method of limits. Instead, the heat PDT should be used. Furthermore, it is known that morphine will inhibit longer duration stimulation at lower doses than short duration stimulation,Citation5 and it could be speculated that a higher dose of oral-administered morphine or an intravenous approach may be effective on skin heat pain. Finally, different thermodes were used in different studies, and even when the skin surface temperature is controlled, stimuli applied with different methods are not necessarily comparable.Citation1,Citation18
Muscle pain
Morphine reduced deep muscle pain in the present study. The majority of A delta and C fibers in muscles serve as polymodal nociceptors,Citation19 and findings may be caused by a combined effect. This finding contradicted a previous study by Schulte et al,Citation20 in which no morphine effect was seen in a deep muscle pain model. However, this study only included 15 healthy volunteers, and methods for pain induction were different as they used repeated intramuscular electrical stimulation and muscular infusion of hypertonic saline for induction of tonic muscle pain. In the present study, the maximum effect on muscle pressure pain was found 45 minutes after oral administration. Hence, the findings correspond well with the time it takes for morphine to reach the central nervous system, where the majority of opioid receptors in supraspinal components of the pain-modulating circuit are responsible for analgesic effect.Citation21
Bone pain
Opioids are not the first choice of treatment for bone pain, but are often used when pain is intense and severe.Citation22 Bone is innervated differently from the skin by A delta and C fibers.Citation23 The terminals of these fibers mostly contain polymodal receptors.Citation24 The posterium is therefore very sensitive to a variety of stimuli, where mechanical activation is the most clinically relevant. Thus, bone pressure was included in the present study as a model of pain stimulation in deep tissue. As the results were only marginally significant, bone pain stimulation may be less sensitive to opioid effects, and results should be more cautiously interpreted.
Visceral pain
In this study, a comprehensive visceral model, stimulating the rectum offered possibilities of multi-modal stimulations. The model has previously been shown to be reproducibleCitation14 and suitable for studying pharmacological interventions in healthy controls.Citation14 External validity of the model of rectal distention has previously been demonstrated, as patients have reported that discomfort of pain induced by rectal distension was similar to their irritable bowel syndrome symptoms.Citation25 In contrast, the model for electrical stimulation of the rectum is less relevant to a definable group of patients. Electrical pain is phasic, and intense electrical stimuli excites all peripheral fibers in a nondifferential fashion.Citation8 Thus, analgesic effects of opioids on this dynamic endpoint have previously only been demonstrated in patients with chronic pain, suggesting that an upregulated pain system is needed for demonstration of analgesic effect.Citation1 The most reliable proxy of thermal energy load to the tissue is assessment of the area under the temperature curve,Citation26 which was used in the present study. However, no morphine analgesia could be demonstrated. This finding was supported by two previous visceral studies from our group, in which no effects of morphine were demonstrated when stimulating the esophagus with heat.Citation27,Citation28 Thus, the results may be explained by the phasic nature of this stimulation.
Cold pressor pain
Activation of descending inhibitory pathways preferentially attenuates C fiber activity, and exogenous opioids affect descending pain modulation.Citation29 Opioid sensitivity to the cold pressor model (affecting descending inhibitory pathways) has also previously been reported.Citation3,Citation30 Thus, the temporal configurations of the stimulus in addition to the different types of primary afferents being stimulated could contribute to the higher sensitivity of the cold pressor test to detect the analgesic effect of opioids.Citation3
Sensory versus affective pain
Different modalities of experimental noxious stimuli can also be described according to their characteristic contributions to the sensory and affective components of the evoked pain.Citation31 For example, the unpleasantness evoked by heat and electrical stimulation of the skin was less than that evoked by the cold pressor test,Citation31 suggesting that tonic pain models affect these psychophysical parameters (eg, anxiety and catastrophizing) to a higher degree and consequently mimic clinical pain to a higher degree. It has been suggested that morphine more potently attenuates the affective component as compared to the sensory component.Citation32,Citation33 Hence, the cold pressor test, which is very sensitive to morphine, is known to involve strong affective and autonomic components.Citation34
Methodological considerations
In the present study, all primary endpoints (tonic pain stimulations) provided significant results and no adjustment for multiplicity was made.Citation35 It is well-known that different opioids exert different mechanisms in both experimental and clinical settings.Citation36 Additionally, different administrations and dosing intervals will affect the outcome. Hence, it cannot be excluded that other findings would be seen if different opioids or doses were administered. It should therefore be emphasized that this discussion was based exclusively on a single oral dose of morphine if nothing else was stated.
Dropouts in quantitative sensory testing studies are not unusual. For example, a high rate of dropouts (64%) in a 60-second long cold pressor test has been reported in an aged matched group of participants.Citation37 However, in the present study, 87% did not withdraw their hand. Finally, it should be noted that analgesic effects of a drug may be assessed in several ways: effect on latency to pain onset; effect on peak pain intensity; effect on area under the pain intensity curve; and effect on time to threshold.Citation30,Citation38 Different assessments could also affect model sensitivity.Citation4 The use of the modified rating scale was recently supported by a study with a rating scale ranging from “no sensation” to “unbearable pain”, allowing participants to rate stimulus intensities that are perceived but are not painful, and may reduce bias and be more reliable for experimental pain assessment.Citation39
The fact that 34 of 93 screened healthy volunteers were not willing to participate after further information and consideration indicates a study selection bias, and hence, results may not reflect the Danish population. However, as participation is voluntary, experimental pain studies will always be affected by such study selection bias.
Recommendations
Several factors should be considered when planning an experimental human pain study for assessment of analgesic effects.Citation1 Using multi-modal tests and multi-tissue stimulations, the present study confirmed that: 1) deep stimuli should be included to mimic the clinical situation; 2) tonic stimulations should be used rather than phasic stimulations to evaluate pain intensity before and after drug administration; and 3) models involving activation predominantly of C fibers should be included.
Conclusion
Pain models with deep tonic stimulation including C fiber activation and/or endogenous pain modulation were more sensitive to morphine analgesia. To avoid false negative results in future studies, we recommend inclusion of reproducible tonic pain models in deep tissues, mimicking clinical pain to a higher degree.
Acknowledgments
The study was supported by the Danish Council for Strategic Research and Det Obelske Familiefond.
Disclosure
The funding sources for this work were not involved in the conduct of the study or in the development of the submission. The authors report no conflicts of interest in this work.
References
- OlesenAEAndresenTStaahlCDrewesAMHuman experimental pain models for assessing the therapeutic efficacy of analgesic drugsPharmacol Rev201264372277922722894
- StaahlCOlesenAEAndresenTArendt-NielsenLDrewesAMAssessing analgesic actions of opioids by experimental pain models in healthy volunteers – an updated reviewBr J Clin Pharmacol200968214916819694733
- KoltzenburgMPokornyRGasserUERicharzUDifferential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphinePain20061261–316517416901645
- AngstMSClarkJDComment on Koltzenburg et al. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphinePain20061261657416901645 Pain2007128329229417276008
- PetersenKLExperimental cutaneous hyperalgesia in humansBerdeCBRowbothamMCTechnical Corner from IASP NewsletterWashington, DCInternational Association for the Study of Pain1997
- OertelBGLotschJClinical pharmacology of analgesics assessed with human experimental pain models: Bridging basic and clinical researchBr J Pharmacol2012168353455323082949
- KandelERSchwartzJHJessellTMPart V perceptionKandelERSchwartzJHJessellTMPrinciples of Neural Science United States of AmericaThe McGraw-Hill Publishing Company, Inc2000411624
- Le BarsDGozariuMCaddenSWAnimal models of nociceptionPharmacol Rev200153459765211734620
- LuYPirecVYeomansDCDifferential antinociceptive effects of spinal opioids on foot withdrawal responses evoked by C fibre or A delta nociceptor activationBr J Pharmacol19971216121012169249259
- Le BarsDGuilbaudGJurnaIBessonJMDifferential effects of morphine on responses of dorsal horn lamina V type cells elicited by A and C fibre stimulation in the spinal catBrain Res19761153518524974761
- DrewesAMGregersenHArendt-NielsenLExperimental pain in gastroenterology: a reappraisal of human studiesScand J Gastroenterol200338111115113014686714
- AndresenTPfeiffer-JensenMBrockCDrewesAMArendt-NielsenLA human experimental bone pain modelBasic Clin Pharmacol Toxicol2013112211612322925354
- AndresenTStaahlCOkscheAMansikkaHArendt-NielsenLDrewesAMEffect of transdermal opioids in experimentally induced superficial, deep and hyperalgesic painBr J Pharmacol2011164393494521182491
- BrockCNissenTDGravesenFHMultimodal sensory testing of the rectum and rectosigmoid: development and reproducibility of a new methodNeurogastroenterol Motil200820890891818482255
- RosierEMIadarolaMJCoghillRCReproducibility of pain measurement and pain perceptionPain2002981–220521612098633
- McCormackKPratherPChapleoCSome new insights into the effects of opioids in phasic and tonic nociceptive testsPain199878279989839818
- NielsenJArendt-NielsenLThe influence of rate of temperature change and peak stimulus duration on pain intensity and qualitySomatosens Mot Res19981532202299874521
- HandwerkerHOKobalGPsychophysiology of experimentally induced painPhysiol Rev19937336396718332641
- CairnsBEPhysiological properties of thin-fiber muscle afferents: excitation and modulatory effectsGraven-NielsenTArendt-NielsenLMenseSFundamentals of Musculoskeletal Pain1st edSeattle, WAIASP Press20081932
- SchulteHGraven-NielsenTSolleviAJanssonYArendt-NielsenLSegerdahlMPharmacological modulation of experimental phasic and tonic muscle pain by morphine, alfentanil and ketamine in healthy volunteersActa Anaesthesiol Scand20034781020103012904196
- FieldsHState-dependent opioid control of painNat Rev Neurosci20045756557515208698
- MattiaCDi BussoloEColuzziFNon-analgesic effects of opioids: the interaction of opioids with bone and jointsCurr Pharm Des201218376005600922747537
- FinocchiettiSAndresenTArendt-NielsenLGraven-NielsenTPain evoked by pressure stimulation on the tibia bone – influence of probe diameter on tissue stress and strainEur J Pain201216453454222396082
- ByersMRBonicaJJPeripheral pain mechanisms and nociceptor plasticityLoeserLDButlerSHChapmanCRTurkDCBonica’s Management of PainPhiladelphia, PALippincott Williams & Wilkins2001
- MorganVPickensDGautamSKesslerRMertzHAmitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndromeGut200554560160715831901
- BrockCArendt-NielsenLWilder-SmithODrewesAMSensory testing of the human gastrointestinal tractWorld J Gastroenterol200915215115919132764
- StaahlCChristrupLLAndersenSDArendt-NielsenLDrewesAMA comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain modelPain20061231–2283616600508
- OlesenAEStaahlCArendt-NielsenLDrewesAMDifferent effects of morphine and oxycodone in experimentally evoked hyperalgesia: a human translational studyBr J Clin Pharmacol201070218920020653672
- Arendt-NielsenLAndresenTMalverLPOkscheAMansikkaHDrewesAMA double-blind, placebo-controlled study on the effect of buprenorphine and fentanyl on descending pain modulation: a human experimental studyClin J Pain201228762362722156892
- JonesSFMcQuayHJMooreRAHandCWMorphine and ibuprofen compared using the cold pressor testPain19883421171223174150
- RainvillePFeineJSBushnellMCDuncanGHA psychophysical comparison of sensory and affective responses to four modalities of experimental painSomatosens Mot Res1992942652771492527
- van der KamELVryJDSchieneKTzschentkeTMDifferential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the ratPain2008136337337917825490
- KupersRCKoningsHAdriaensenHGybelsJMMorphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of painPain19914715121663226
- CleelandCSNakamuraYHowlandEWMorganNREdwardsKRBackonjaMEffects of oral morphine on cold pressor tolerance time and neuropsychological performanceNeuropsychopharmacology19961532522628873108
- TurkDCDworkinRHMcDermottMPAnalyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on methods, measurement, and pain assessment in clinical trialsPain2008139348549318706763
- DrewesAMJensenRDNielsenLMDifferences between opioids: pharmacological, experimental, clinical and economical perspectivesBr J Clin Pharmacol2013751607822554450
- RuscheweyhRStumpenhorstFKnechtSMarziniakMComparison of the cold pressor test and contact thermode-delivered cold stimuli for the assessment of cold pain sensitivityJ Pain201011872873620338822
- GrachMMassalhaWPudDAdlerREisenbergECan coadministration of oxycodone and morphine produce analgesic synergy in humans? An experimental cold pain studyBr J Clin Pharmacol200458323524215327582
- KempJDespresODufourAUnreliability of the visual analog scale in experimental pain assessment: a sensitivity and evoked potentials studyPain Physician2012155e693e69922996863
