Abstract
Background
When assessing pain in clinical practice, clinicians often label pain as mild, moderate, and severe. However, these categories are not distinctly defined, and are often used arbitrarily. Instruments for pain assessment use more sophisticated scales, such as a 0–10 numerical rating scale, and apart from pain intensity assess pain-related interference and disability. The aim of the study was to identify cutoff points for mild, moderate, and severe nondental orofacial pain using a numerical rating scale, a pain-related interference scale, and a disability measurement.
Materials and methods
A total of 245 patients referred to the Facial Pain Unit in London were included in the study. Intensity and pain-related interference were assessed by the Brief Pain Inventory. Pain-related disability was assessed by the Chronic Graded Pain Scale. Average pain intensity (0–10) was classified into nine schemes with varying cutoff points of mild, moderate, and severe pain. The scheme with the most significant intergroup difference, expressed by multivariate analysis of variance, provided the cutoffs between mild, moderate, and severe pain.
Results
The combination that showed the greatest intergroup differences for all patients was scheme 47 (mild 1–4, moderate 5–7, severe 8–10). The same combination provided the greatest intergroup differences in subgroups of patients with temporomandibular disorder and chronic idiopathic facial pain, respectively. Among the trigeminal neuralgia patients alone, the combination with the highest intergroup differences was scheme 48 (mild 1–4, moderate 5–8, severe 9–10).
Conclusion
The cutoff points established in this study can discriminate in pain intensity categories reasonably well, and showed a significant difference in most of the outcome measures used.
Introduction
After the correct diagnosis has been established, a vital step for effective treatment of chronic pain is assessment of its intensity and impact. Clinicians most often use categories like mild, moderate, and severe. The categories are not distinctly defined, and are often used arbitrarily. More sophisticated tools like the Brief Pain Inventory (BPI) or the Chronic Graded Pain ScaleCitation1,Citation2 most often use a 0–10 numeric rating scale (NRS). Apart from pain intensity, these instruments assess the degree of pain-related functional interference and its impact on quality of life. In spite of the availability of these instruments, many clinicians prefer the use of the mild, moderate, and severe categories.
The question, therefore, arises if it is possible to define more precisely categories like mild, moderate, and severe using these instruments. An NRS cannot just be divided into three equal parts, because the relationship between pain intensity and functional interference is not linear. The reduction in the same interval of intensity on different parts of an NRS does not produce similar reduction in functional impairment or patient’s general well-being.Citation3 The relationship between pain intensity and pain-related interference would thus be more correctly defined by identifying cutoff points for mild, moderate, and severe pain.
This method, introduced by Serlin et al,Citation3 has been widely applied. Cutoff points have been determined for various chronic pain conditions, such as cancer pain, diabetic neuropathy, osteoarthritis, low-back pain, phantom limb pain, neck pain, and musculoskeletal pain.Citation4–Citation10 In these studies, cutoff points for mild pain ranged from 2 to 5, while cutoff points for severe pain ranged from 6 to 8. The results of these studies suggest that the pain experience and pain-related functional impairment depends on the condition and the affected site. However, cutoff points for nondental orofacial pain have not been identified. Identifying cutoff points for mild, moderate, and severe nondental orofacial pain would aid clinicians and investigators in defining a measurable target range of adequate pain relief. The severity and impact of the different orofacial pains is large, especially if rare conditions, such as trigeminal neuralgia (TN) and the trigeminal autonomic cephalalgias, are included.Citation11–Citation13 The cutoff points could also be used to determine entry into clinical trials.
The aim of this study was to identify cutoff points between mild, moderate, and severe non dental orofacial pain, to assess if the cutoff points can be generally applied to all nondental orofacial pain conditions or if they are specific to each of the disorders, and to determine if the cutoff points discriminate between three intensity categories in several patient-related outcome measures (functional impairment, pain-related disability, depression, and anxiety).
Materials and methods
The study was reviewed and approved by the European Association of Oral Medicine board. All patients referred to the national Facial Pain Unit in London during a 6-week period in 2009 were asked to participate in the study. The Facial Pain Unit sees 700 new patients and 1,400 follow-up patients per year, and all patients have previously seen at least one primary care provider.
Criteria for referral to the unit are chronic pain, ie, over 3 months and exclusion of dental causes. Therefore, a 6-week period was assumed to provide a representative sample of non dental orofacial pain patients in terms of demographics and diagnosis.
The inclusion criteria were presence of nondental orofacial pain for more than 3 months, age over 18 years, and ability to understand questionnaires (one of the essential requirements for assessment in the clinic is the completion of several questionnaires that assess patients’ treatment expectations, sociodemographic data, pain intensity, pain-related interference, and pain-related disability). Theoretically, patients who did not understand the questionnaires would have been excluded from the study, but this did not occur, as the questionnaires were sent out ahead of the appointment and patients were encouraged to ask for help if they had difficulties completing them. Fifteen however did not complete all the questions, and so were excluded.
Demographic and clinical data (age, sex, duration of pain, and number of specialists seen before referral to the Facial Pain Unit) were registered. Intensity and pain-related functional impairment were assessed by the BPI.Citation1 The BPI uses an eleven-item NRS (0–10), where patients rate their worst, least, average, and current pain intensity, as well as interference with various aspects of everyday life (general activity, mood, walking, work, relationships with other people, sleep, and enjoyment in life). Pain-related disability was assessed by the Chronic Graded Pain Scale.Citation2 This classifies patients into four disability categories (1, low intensity, low disability; 2, high intensity, low disability; 3, high disability, moderately limiting; and 4, high disability, severely limiting) based on interference with everyday activity and period of limited activity because of pain. The Hospital Anxiety and Depression Scale (HADS) was used to assess anxiety and depression.Citation14 According to the HADS score, patients were classified as follows: 0–7, no depression/anxiety; 8–10, borderline depression/anxiety; and 11 and over, depression/anxiety.
Patients were classified into three subgroups according to their diagnosis: all types of TN, myogenic temporomandibular disorder (TMD) and persistent/chronic idiopathic facial pain (CIFP). TN was diagnosed according to the International Classification of Headache Disorders criteria.Citation15 The diagnosis of TMD was made according to the following criteria: 1) pain and tenderness of the muscles of mastication of 3 months’ duration or longer and 2) no clinical and/or radiographic evidence of organic temporomandibular joint disorder.Citation16 The third group was heterogeneous, and consisted of patients who did not fit into the previous two groups (patients with CIFP, burning mouth syndrome, and posttraumatic neuropathic pain). The diagnoses of these were made according to the International Classification of Headache Disorders criteria.Citation15
Statistical analysis
SPSS software (version 20 for Windows; IBM, USA) was used for data analysis. Depending on the distribution of the data, mean and standard deviation or median and range were used to summarize the data. For categorical variables, differences between groups were tested by the χ2 test. For numerical variables, group differences were assessed by one-way analysis of variance (ANOVA), followed by post hoc Bonferroni test or the Kruskal–Wallis test followed by Mann–Whitney U tests, with P-values adjusted for multiple testing if the assumptions of the ANOVA were not satisfied.
Determination of cutoff points was performed as described by Serlin et al.Citation3 Average pain intensity was classified into nine schemes with different cutoff points of mild, moderate and severe pain: 1, scheme 35 (mild 1–3, moderate 4–5, severe 6–10); 2, scheme 36 (mild 1–3, moderate 4–6, severe 7–10); 3, scheme 37 (mild 1–3, moderate 4–7, severe 8–10); 4, scheme 38 (mild 1–3, moderate 4–8, severe 9–10); 5, scheme 46 (mild 1–4, moderate 5–6, severe 7–10); 6, scheme 47 (mild 1–4, moderate 5–7, severe 8–10); 7, scheme 48 (mild 1–4, moderate 5–8, severe 9–10); 8, scheme 57 (mild 1–5, moderate 6–7, severe 8–10); and 9, scheme 58 (mild 1–5, moderate 6–8, severe 9–10). Nine multivariate one-way ANOVAs were performed, with the intensity group (mild, moderate, and severe) as the independent variable and seven pain-interference domains from the BPI as the dependent variable. The scheme with the most significant intergroup difference, expressed by the smallest P-value determined from Wilks’ lambda, was considered to indicate the maximum difference between the groups, and thus provided the cutoffs between mild, moderate, and severe pain.
To assess if the determined cutoff points discriminated adequately between pain-intensity categories, patients were compared on various outcome measures using one-way ANOVAs, followed by Bonferroni post hoc comparisons where appropriate (for pain-related interference), or χ2 tests (for pain-related disability, depression, and anxiety). In all analyses, P<0.01 was considered statistically significant. This significance level was chosen rather than the conventional 0.05 level to avoid spuriously significant results arising from multiple testing. Effect size was expressed by η2 or φ-coefficient where appropriate.
Results
Demographic and clinical characteristics of the participants
A total of 245 patients were included in the study. There were 186 (76%) female patients and 59 (24%) male patients. A total of 112 patients had TMD, 85 patients had CIFP, and 48 patients had TN. The median age of the participants was 47 (range 18–84) years. The demographic and clinical data of the patients are shown in .
Table 1 Demographic and clinical data of the patients
No significant difference in sex was observed between the three groups of patients. A significant difference in median age was observed between the groups: TN patients were significantly older than CIFP and TMD patients (P=0.002 and P<0.001, respectively). CIFP patients were on average significantly older than patients with TMD (P<0.001). Median duration of pain was significantly longer in TN patients compared to TMD and CIFP patients (P<0.001 and P=0.027, respectively). TN and CIFP patients visited significantly more pain specialists before referral to the Facial Pain Unit than TMD patients (P=0.004 and P<0.001, respectively). No significant difference in the proportion of patients with anxiety and depression was found between the three groups of participants.
The mean value of the average pain intensity was significantly higher in CIFP patients than in TMD patients (P=0.003). No significant differences in the mean values of average pain intensity were observed between TMD and TN patients or CIFP and TN patients (P=0.999 and P=0.186, respectively). No significant differences were observed in the mean worst, least, and current pain-intensity scores between three groups of participants (P=0.081, P=0.025, and P=0.097, respectively). No significant differences in pain-related interference were observed between the three groups of participants (P=0.058). No significant differences in pain-related disability were observed between the three groups of participants (P=0.206).
Determination of cutoff points
Cut-off points were determined as described in the Materials and methods section. The combination that showed the greatest intergroup differences for all patients was scheme 47 (mild 1–4, moderate 5–7, severe 8–10). The same combination provided the greatest intergroup differences in TMD and CIFP patients. Among the TN patients alone, the combination with the highest intergroup differences was scheme 48 (mild 1–4, moderate 5–8, severe 9–10) (). No significant difference in cutoff points between males and females was observed. (η2 ranged from 0.35 – 0.41, indicating strong effect).
Table 2 Combination of cutoff points with associated Wilks’ lambda statistics
Assessment of cutoff points
As explained in the Materials and methods section, in order to assess if the optimal cutoff points discriminated between the pain-intensity categories, the three intensity groups were compared on various outcome measures using univariate one-way ANOVAs, followed by Bonferroni post hoc comparisons where appropriate (pain-related interference), or χ2 tests (pain-related disability, depression, and anxiety).
Pain interference in pain-intensity groups
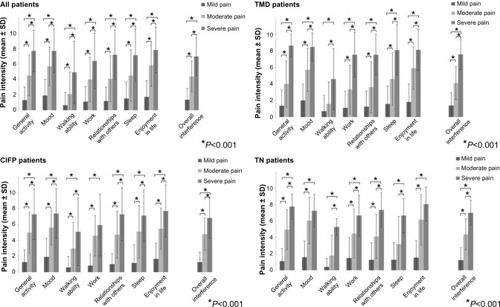
In all patients, a significant difference in the means between mild-, moderate-, and severe-pain categories was found for all BPI interference scales, as well as for overall interference (); η2 ranged from 0.26 to 0.57, indicating a strong effect. In the subgroup of TMD patients, a significant difference between mild, moderate, and severe pain was found for all BPI interference scales, as well as for overall interference. A post hoc test did not reveal significant difference in interference with walking ability among patients with mild and moderate pain (P=0.286); η2 ranged from 0.22 to 0.61, indicating a moderate-to-strong effect. In the subgroup of CIFP patients, a significant difference between mild, moderate, and severe pain was found for all BPI interference scales, as well as for overall interference. A post hoc test did not reveal a significant difference in interference with work among patients with moderate and severe pain (P=0.228); η2 ranged from 0.24 to 0.48, indicating moderate-to-strong effect. In the subgroup of TN patients, a significant difference between mild, moderate, and severe pain was observed for all BPI interference scales, as well as for overall interference. Post hoc tests did not reveal significant differences in interference with mood or enjoyment of life among patients with moderate and severe pain (P=0.786 and P=0.270, respectively). Furthermore, in the TN subgroup, nonsignificant differences in interference with walking ability and sleep were found between patients with mild and moderate pain (P=0.366 and P=0.318, respectively); η2 ranged from 0.3 to 0.56, indicating a strong effect.
Pain-related disability in pain-intensity groups
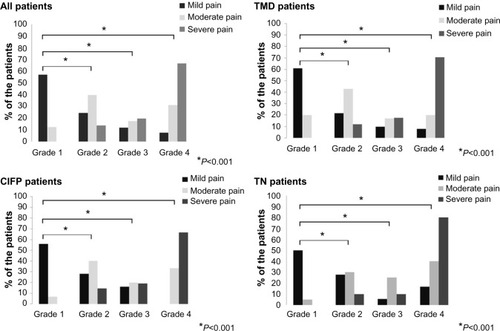
A significant association was found between intensity of pain (mild, moderate and severe pain) and pain-related disability. Statistical significance was observed in all patients, as well as in all three subgroups of patients (P<0.001, P<0.001, P<0.001, and P=0.005, respectively); φ-values ranged from 0.66 to 0.73, indicating a strong effect ().
Depression and anxiety in pain-intensity groups
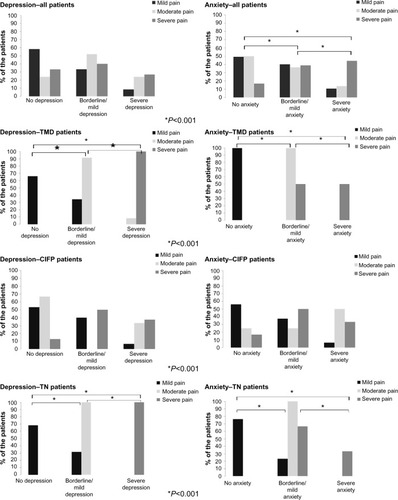
A significant association was observed between pain intensity (mild, moderate, and severe pain) and anxiety (P=0.008) in all patients. No significant association was observed between pain intensity and depression and P=0.014 in any patients. Significant differences in the percentage of anxiety and depression among patients with mild, moderate, and severe pain were found in the TMD (P<0.001 and P<0.001, respectively) and TN subgroups (P<0.001 and P<0.001, respectively). No significant difference in the percentages of either anxiety or depression between patients with mild, moderate, and severe pain was observed in the CIFP subgroup (P=0.141 and P=0.175, respectively) (); φ-values ranged from 0.34 to 1 indicating a moderate-to-strong effect.
Discussion
The results of this study indicate that in nondental orofacial pain conditions, the reduction in equal interval of pain intensity on an NRS will not produce the same level of reduction in functional impairment. This finding confirms the nonlinear relationship between pain intensity and functional impairment, as suggested by Serlin et al.Citation3 Using the eleven-item NRS, intensity of nondental orofacial pain can be defined as follows: mild pain 0–4, moderate pain >4–7, and severe pain >7–10. Cutoff points established in this way provide significant differences for almost all outcome measures used in this study, which is especially emphasized in the overall pain-related interference and pain-related disability. This combination of cutoff points was found to be optimal also for phantom limb pain, peripheral diabetic neuropathy, knee osteoarthritis, and cancer pain.Citation5–Citation8 Chronic pain conditions like back pain, general pain, neck pain, and headache had different combinations of optimal cutoff points.Citation4,Citation6,Citation17 These findings indicate that the impact of chronic pain on daily activities does not depend solely on the pain intensity, but is also dependent on the nature of the condition.
Demographic factors, such as sex and age, did not affect the established cutoff points. It is known that chronic orofacial pain conditions, such as TN, TMD, and CIFP, are more prevalent among women. Women tend to report higher pain intensity and duration of pain.Citation18,Citation19 However, no difference between men and women was found in any of the studies that determined cutoff points for mild, moderate, and severe pain in different chronic pain conditions.Citation4–Citation10 It seems that the impact of pain on everyday function diminishes sex-related differences in pain perception. There are no studies that compare pain intensity in different age-groups for CIFP and TN. The exception is TMD, where pain intensity appears to decrease with age of 60 years and over.Citation20–Citation22
Even though anxiety and depression are often found in chronic pain, their relationship is not linear.Citation23–Citation25 Anxiety and depression depend not only on pain intensity but also on other factors, such as pain acceptance, individual coping strategies, and other risk factors.Citation26–Citation28 A significant difference between the three pain-intensity groups was found only for TMD and TN patients. The difference was not statistically significant in the CIFP group, which could be due to heterogeneity of the group or the aforementioned mentioned pain acceptance and individual coping strategies. Anxiety and depression were used for the comparison of intensity groups in only two studies that used the same method for the identification of cutoff points in chronic pain.Citation5,Citation8 Paul et al did not find significant difference in either depression or anxiety between cancer patients classified as having mild, moderate, and severe pain.Citation8 On the other hand, in a study of Hoffman et al, anxiety and depression subscales discriminated significantly between pain-intensity subgroups in patients with diabetic neuropathy.Citation5 The difference between the studies could have been due to the use of different scales for the assessment of depression and anxiety: Paul et al used the shortened version of the Profile of Mood States, while Hoffmann et al used the HADS.Citation5,Citation8 In spite of the differences, it remains important to monitor patients’ psychological health, as chronic pain is a risk factor for the onset of anxiety and/or depressive disorders.Citation23–Citation25
Cutoff points are not characteristic for an individual condition, but can be used in almost all nondental chronic orofacial pain conditions. The exception was TN patients, where the highest intergroup difference was obtained in scheme 48 (mild pain 1–4, moderate >4–8, severe >8–10). There can be several reasons for this. First of all, the group of TN patients was very small, and the statistical analysis could have been affected by the subtle changes in the number of patients in intensity-level subgroups. On the other hand, these results could reflect the character of TN. Despite high pain intensity, the pain in TN is episodic, and higher pain intensity and longer duration may be needed to result in a meaningful interference with daily activities. Furthermore, in the majority of TN patients, pain can be adequately controlled with medications that can have a positive impact on pain-related interference with daily activities.Citation30 Having experienced very severe episodes of pain, patients with TN may be more discriminating about their pain severity, ie, they often distinguish between what they term “twinges” compared to “electric shocks”. Unlike TN, in TMD and CIFP the pain is more or less constant and often not successfully controlled with medications that can affect patients’ daily activities and quality of life. Furthermore, patients with TMD and CIFP are more likely to have other chronic pain and more psychosocial predisposing factors than TN patients, and so require a holistic treatment approach.Citation31–Citation36 Only 17% of the TN patients in this cohort compared to 90% of patients in the TMD and CIFP subgroups had other chronic pain including headache. This might explain why lower pain intensity can result in higher disability/functional impairment.
Classification of mild, moderate, and severe pain as defined in this study is in line with patients’ definition of acceptable outcome. According to the studies of Thorne and MorleyCitation37 and Farrar et alCitation38 on more than 2,000 patients with various chronic pain conditions, patients’ definition of “much improvement” implies reduction of 2–3 raw points or 30% on an NRS. In TMD patients, clinically important change was defined as an intensity visual analog scale score reduction of 19.5 mm and percentage change of 37.9% from baseline.Citation39 Percentage change showed higher sensitivity, since raw visual analog scale score reduction was significantly affected by the baseline pain levels.Citation39 Furthermore, the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT) recommends reporting on the percentages of patients achieving ≥30% reduction in the NRS, since this reduction appears to reflect at least moderate clinically important difference.Citation40 Reduction of ≥50%, on the other hand, reflects substantial improvement and should also be reported. This is especially relevant in TN, where unlike other types of nondental orofacial pain, nearly 100% pain reduction can be achieved; if not by medication, then by surgery.Citation41
This study has several limitations that need to be addressed. According to Hirschfeld and Zernikow, the statistical method for determination of cutoff points applied in this study does not take into account the variability of the sample.Citation17 The authors state that the differences between the groups were as a result of a chance variation rather than true differences in pain-related interference. Bootstrapping was performed, the rank ordering of the cut-off points was not affected, and the combination of 47 and 48 was still identified as the most appropriate for TMD, CIFP, and TN. The cutoff points could not represent the optimal relationship between pain intensity and functional interference in every individual patient, but for the majority of nondental orofacial pain patients, these measures are probably valid. Furthermore, one of the pain characteristics not assessed in the BPI that could be of importance is interference with eating. This characteristic would probably be emphasized in nondental chronic orofacial pain and might influence the results. An extended BPI, called BPI facial, which includes seven additional oral/facial parameters, has recently been validated in patients with TN.Citation42 However, no data are yet available on its validity in other facial pain conditions. Therefore, we decided to use a validated instrument, as was used in all similar studies.Citation3–Citation10 Another limitation of this study is the small number of TN patients. Further studies with larger numbers of patients are therefore required.
In spite of the study limitations, we believe that the cutoff points determined in this study discriminate pain-intensity categories reasonably well and provide significant difference in most of the outcome measures used. These cutoff points would help clinicians and researchers to define more precisely satisfactory levels of pain relief in nondental orofacial pain patients. They would be of use in clinical trials and for providers of pain services when assessing pain-related outcome measures.
Author contributions
Both authors collected the data and contributed to the manuscript equally. Both authors discussed the results and commented on the manuscript.
Acknowledgments
We are grateful to Aviva Petrie and David Boniface, who advised on the statistical analysis. VB undertook this work as part of his grant from the European Association of Oral Medicine. JMZ undertook this work at UCL/UCLHT, who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre funding.
Disclosure
The authors report no conflicts of interest in this work.
References
- CleelandCSRyanKMPain assessment: global use of the Brief Pain InventoryAnn Acad Med Singapore19942321291388080219
- Von KorffMOrmelJKeefeFJDworkinSFGrading the severity of chronic painPain19925021331491408309
- SerlinRCMendozaTRNakamuraYEdwardsKRCleelandCSWhen is cancer pain mild, moderate or severe? Grading pain severity by its interference with functionPain19956122772847659438
- FejerRJordanAHartvigsenJCategorising the severity of neck pain: establishment of cut–points for use in clinical and epidemiological researchPain20051191–317618216298059
- HoffmanDLSadoskyADukesEMAlvirJHow do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?Pain2010149219420120303665
- JensenMPSmithDGEhdeDMRobinsinLRPain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe painPain200191331732211275389
- KapstadHHanestadBRLangelandNRustøenTStavemKCutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgeryBMC Musculoskelet Disord200895518426591
- PaulSMZelmanDCSmithMMiaskowskiCCategorizing the severity of cancer pain: further exploration of the establishment of cutpointsPain20051131–2374415621362
- ZelmanDCDukesEBrandenburgNBostromAGoreMIdentification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathyPain20051151–2293615836967
- ZelmanDCHoffmanDLSeifeldinRDukesEMDevelopment of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritisPain20031061–2354214581108
- ContiPCPinto-FiamenguiLMCunhaCOContiACOrofacial pain and temporomandibular disorders: the impact on oral health and quality of lifeBraz Oral Res201226Suppl 112012323318754
- TölleTDukesESadoskyAPatient burden of trigeminal neuralgia: results from a cross-sectional survey of health state impairment and treatment patterns in six European countriesPain Pract20066315316017147591
- PiggMSvenssonPDrangsholtMListTSeven-year follow-up of patients diagnosed with atypical odontalgia: a prospective studyJ Orofac Pain201327215116423630687
- ZigmondASSnaithRPThe Hospital Anxiety and Depression ScaleActa Psychiatr Scand19836763613706880820
- Headache Classification Subcommittee of the International Headache SocietyThe International Classification of Headache Disorders: 2nd editionCephalalgia200424Suppl 1916014979299
- Van GrootelRJvan der BiltAvan der GlasHWLong-term reliable change of pain scores in individual myogenous TMD patientsEur J Pain200711663564317118682
- HirschfeldGZernikowBVariability of “optimal” cut points for mild, moderate, and severe pain: neglected problems when comparing groupsPain2013154115415923182623
- CairnsBEThe influence of gender and sex steroids on craniofacial nociceptionHeadache200747231932417300382
- PallerCJCampbellCMEdwardsRRDobsASSex-based differences in pain perception and treatmentPain Med200910228929919207233
- CarlssonGEEkbäckGJohanssonAOrdellSUnellLIs there a trend of decreasing prevalence of TMD-related symptoms with ageing among the elderly?Acta Odontol Scand201472871472024666243
- JohanssonAUnellLCarlssonGESöderfeldtBHallingARisk factors associated with symptoms of temporomandibular disorders in a population of 50- and 60-year-old subjectsJ Oral Rehabil200633747348116774504
- KomiyamaOObaraRIidaTAge-related associations between psychological characteristics and pain intensity among Japanese patients with temporomandibular disorderJ Oral Sci201456322122525231149
- GerritsMMvan OppenPvan MarwijkHWPenninxBWvan der HorstHEPain and the onset of depressive and anxiety disordersPain20141551535924012953
- De HeerEWGerritsMMBeekmanATThe association of depression and anxiety with pain: a study from NESDAPLoS One2014910e10690725330004
- GerritsMMVogelzangsNvan OppenPvan MarwijkHWvan der HorstHPenninxBWImpact of pain on the course of depressive and anxiety disordersPain2012153242943622154919
- McCrackenLMLearning to live with the pain: acceptance of pain predicts adjustment in persons with chronic painPain199874121279514556
- McCrackenLMEcclestonCCoping or acceptance: what to do about chronic pain?Pain20031051–219720414499436
- McCrackenLMVowlesKEEcclestonCAcceptance of chronic pain: component analysis and a revised assessment methodPain20041071–215916614715402
- ShachamSA shortened version of the Profile of Mood StatesJ Pers Assess19834733053066886962
- ZakrzewskaJMLinskeyMETrigeminal neuralgiaBMJ2014348g47424534115
- KomiyamaOWangKSvenssonPArendt-NielsenLKawaraMDe LaatAThe influence of psychological state on the masseteric exteroceptive suppression reflex and somatosensory functionClin Neurophysiol2008119102321232818768348
- KomiyamaOObaraRUchidaTPain intensity and psychosocial characteristics of patients with burning mouth syndrome and trigeminal neuralgiaJ Oral Sci201254432132723221157
- WongWSChenPPYapJMakKHTamBKFieldingRAssessing depression in patients with chronic pain: a comparison of three rating scalesJ Affect Disord20111331–217918721565408
- YapAUTanKBChuaEKTanHHDepression and somatization in patients with temporomandibular disordersJ Prosthet Dent200288547948412473996
- YapAUChuaEKHoeJKClinical TMD, pain-related disability and psychological status of TMD patientsJ Oral Rehabil200229437438011966972
- YapAUDworkinSFChuaEKListTTanKBTanHHPrevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patientsJ Orofac Pain2003171212812756927
- ThorneFMMorleySProspective judgments of acceptable outcomes for pain, interference and activity: patient-determined outcome criteriaPain2009144326226919446958
- FarrarJTYoungJPLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain200194214915811690728
- EmshoffREmshoffIBertramSEstimation of clinically important change for visual analog scales measuring chronic temporomandibular disorder painJ Orofac Pain201024326226920664827
- DworkinRHTurkDCFarrarJTCore outcome measures for chronic pain clinical trials: IMMPACT recommendationsPain20051131–291915621359
- ZakrzewskaJMJassimSBulmanJSA prospective, longitudinal study on patients with trigeminal neuralgia who underwent radiofrequency thermocoagulation of the gasserian ganglionPain199979151589928776
- LeeJYChenHIUrbanCDevelopment of and psychometric testing for the Brief Pain Inventory-Facial in patients with facial pain syndromesJ Neurosurg2010113351652320151778