Abstract
Purpose
To examine whether there is central sensitization in patients with phantom limb pain (PLP) after traumatic limb amputation.
Methods
Seventeen patients after unilateral lower limb amputation secondary to trauma were enrolled. Ten patients had chronic PLP, while the other seven patients had no PLP. Tactile-sensation threshold, cold- and warm-sensation thresholds, cold- and heat-pain thresholds, electrical-sensation threshold (EST), and electrical-pain threshold on the distal residual limb and the symmetrical site on the sound limb were measured in all tested patients. Their thresholds were compared within the PLP and non-PLP group, and between the groups.
Results
The novel findings included: 1) electrical-pain threshold was only decreased in the sound limb in the PLP group and there was no difference between two limbs in the non-PLP group, suggesting central sensitization in patients with PLP; and 2) EST was increased on the affected limb as compared to the sound limb within the PLP group, but there were no significant differences in EST between the PLP and non-PLP group. There were in general no significant differences in other tested thresholds within the groups and between groups.
Conclusion
Our results demonstrate central sensitization in the patients with PLP after traumatic limb amputation.
Keywords:
Introduction
Phantom limb pain (PLP) refers to a painful sensation of an amputated or deafferented limb. About 60%–80% of patients experience PLP after amputation.Citation1 PLP is often described as shooting, stabbing, squeezing, throbbing, and burning, and is primarily localized in distal parts of the missing limb. Severe phantom pain is debilitating to patients, and yet is challenging for physicians who manage it.Citation2 PLP has been viewed as a maladaptive response of the nervous system to nerve damage. Mechanisms of phantom pain are not well understood. Possible mechanisms include peripheral and spinal changes and cerebral reorganization after sensory loss from limb amputation;Citation1–Citation4 self-consciousness of body image;Citation5 and persistence of “labelled” somatosensory area after amputationCitation6 and central sensitization.Citation7
A recent studyCitation8 showed that local cortical representation was maintained after hand amputation, while chronic phantom pain was related to disrupted interregional connectivity. The altered connectivity was postulated to be driven by nociceptive or top-down inputs. This finding is further supported by another possible mechanism for PLP. It is believed that cognitive–emotional sensitivity and memory play a role in the persistence of phantom pain, which is particularly important if PLP is secondary to a traumatic event.Citation9 A recent hypermnesia–hyperarousal model is proposed specifically to account for pain persistence and pain sensitization following a traumatizing event.Citation10 In case of severe traumatization, such as traumatic limb amputation, intense hypermnesia and hyperarousal are likely imprinted on the victim’s memories, and result in central sensitization and pain chronification. This theoretical model is able to account for chronic phantom pain and pain catastrophizing in some patients with a psychological sensitivity, but not in others.Citation11 However, comparisons of phantom pain assessment between patients with and without PLP are rarely available in the literature.
Quantitative sensory testing (QST) has been used to compare sensory thresholds between affected and sound limbs of amputee subjects with and without PLP in a number of studies, including mechanical,Citation11–Citation14 thermal,Citation11,Citation13,Citation15 and electricalCitation16 thresholds. Only in one studyCitation15 were thermal thresholds compared between the affected and sound limbs in subjects with and without PLP separately. No statistically significant difference in all thermal thresholds (cold- and warm-sensation thresholds, cold- and heat-pain thresholds) was found between the affected and sound limbs in each group. Since there is no correlation between responses to noxious heat, cold, and electrical stimulation, a battery of tests is recommended to be used to assess pain sensitivity.Citation17
The aim of this study was to examine sensory thresholds (mechanical, thermal, and electrical thresholds) in a cohort of subjects after limb amputation with and without chronic phantom pain. To examine the aforementioned link between traumatization, central sensitization, and PLP, we limited subject enrollment to lower limb amputation after trauma only. Electrical-pain threshold has been used to reflect central sensitization.Citation18–Citation20 It was hypothesized that electrical thresholds would be lower in subjects with PLP than in those without PLP after traumatic injury, ie, central sensitization, and that there would be no difference in thermal and mechanical thresholds.
Methods
Subjects
Seventeen amputee subjects ( for characteristics) volunteered in this experiment. Inclusion criteria included: 1) greater than 18 years of age; 2) unilateral lower limb amputation secondary to trauma, below-the-knee amputation, or above-the-knee amputation (AKA); 3) amputation at least 6 months prior to the experiments; and 4) being stable on current pain regime for at least 2 weeks if there is phantom pain. Exclusion criteria included: 1) amputation secondary to other etiologies, such as diabetes mellitus; 2) pain disorders other than PLP (eg, infection); 3) with other severe medical problems, including neurological, pulmonary, cardiovascular, renal, or hepatic disease; 4) with preexisting psychiatric disorders; 5) alcohol or drug abuse; 6) with a pacemaker (contraindication for electrical stimulation); 7) residual limb skin issues, such as inflammation and wound; and 8) diagnosis of neuroma at the residual limb. All subjects gave informed written consent prior to participation. This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston and TIRR Memorial Hermann Hospital.
Table 1 Characteristics of subjects
QST
QST was performed in both affected and contralateral sound limbs, including tactile-sensation threshold (TST), electrical-sensation threshold (EST), electrical-pain threshold (EPT), and thermal thresholds. To standardize QST, thresholds on the residual limb were measured at 5 cm above the distal residual limb, 5 cm lateral to the midline of the limb, and on the symmetrical site of the contralateral sound limb. After the target areas were localized and marked with a marker, subjects were instructed to stay relaxed in a chair with arms and legs comfortably supported in a symmetrical position. The order of QST was randomized and balanced in two limbs. QST was performed as described in our recent studies.Citation19,Citation20
TST
TST was tested using Von Frey filaments (Touch-Test Sensory Evaluator; North Coast Medical Inc., Gilroy, CA, USA). After a target area was marked and exposed, subjects were instructed to close their eyes. The experimenter pressed different thicknesses of filaments at a 90° angle against the marked site until they bowed for approximately 1.5 seconds and then removed the filaments. Testing began with the thinnest filament (1.65 grams), then moved to the next thicker monofilament. An explicit response of touch sensation was defined as TST.
EST and EPT
The same trimmed electrodes were used to measure EST and EPT (electrical stimulator 7SA; Digitimer, Hertfordshire, UK). As previously described, a pair of electrodes (about 2.54 cm2 [1 inch square]) was placed parallel and 5 mm apart next to each other on a target area. For EST, the intensity of electrical stimulation was started from zero and gradually increased in steps of 0.1 mA until the subject explicitly felt electrical stimulation. EPT was then measured. The intensity started from the EST and increased in steps of 1 mA until the subject first felt the electrical stimulation to be painful. To improve consistency among subjects, they were advised that the pain threshold level was equivalent to 1 on the 0–10 visual analog scale. Three repetitions were made and the average was used for both EST and EPT.
Thermal thresholds
A Medoc PATHWAY system was used to measure thermal thresholds (warm sensation, cold sensation, heat pain, and cold pain). The established “Limits Full Series” protocol was selected. Briefly, the protocol contains a series of tests in the following order: four tests of cold-sensation threshold (CST), four tests of warm-sensation threshold (WST), three tests of cold-pain threshold (CPT), and three tests of heat-pain threshold (HPT). The 30×30 ATS (advanced thermal stimulation) probe was secured and centered on a target area. All subjects had an education session prior to the protocol. The averaged value was used for each threshold.
Data analysis and statistical analysis
Descriptive statistics were used. The objective of this study was to compare sensory assessment of both affected and sound legs in amputee subjects with and without phantom pain. Paired t-tests were used to compare thresholds between two limbs in all tested patients. The cohort of amputee subjects were further categorized into two subgroups: with and without phantom pain. Paired t-tests were again used to compare thresholds within each group. Furthermore, independent t-tests were used to compare thresholds between groups if different patterns of results were obtained using data from all subjects or from subgroups. The alpha level required for all statistical significance was set at 0.05. Data are reported as means ± standard errors within the text and in the tables and figure.
Results
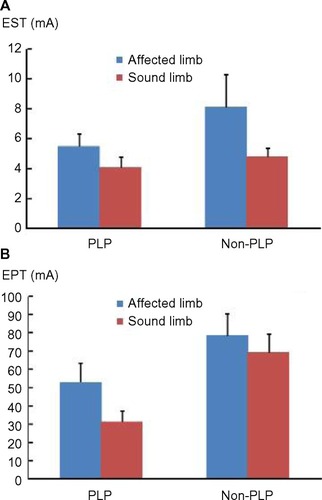
Seventeen amputee subjects with AKA or below-the-knee amputation participated in the study. Ten subjects had PLP, while the remaining seven subjects had no phantom pain. Sensory thresholds averaged for all tested subjects, subjects with phantom pain, and subjects without phantom pain are summarized in . In all tested subjects, EST was significantly greater on the affected limb than on the sound limb (6.59 ± 1.03 mA versus 4.36 ± 0.47 mA, t=0.012). Similarly, EPT was significantly greater on the affected limb than on the sound limb (63.59 ± 8.07 mA versus 46.87 ± 7.01 mA, t=0.001). CPT was significantly decreased on the affected limb as compared to the sound limb (3.16°C ± 1.78°C versus 7.91°C ± 2.05°C, t=0.033). Paired t-tests revealed no statistical significance in TST, CST, WST, and HPT in all tested subjects. To examine whether the same pattern of results was obtained in subjects with and without phantom pain, paired t-tests were performed for each group (with and without pain) individually. Statistically significant difference in EST and EPT was only found in subjects with phantom pain, but not in subjects without phantom pain. CPT was not significantly different between two limbs in both groups. The same pattern of results was obtained for other thresholds (TST, CST, WST, HPT).
Table 2 Tactile, electrical, and thermal thresholds were averaged within the PLP group, the non-PLP group, and across all tested patients
Independent t-tests were then performed for EST, EPT, and CPT for each limb between groups. EST was significantly greater on the affected limb than on the sound limb in the PLP group (5.51 ± 0.84 mA versus 4.07 ± 0.71 mA, P=0.010), but both were not significantly different from EST values in the non-PLP group ( and ). The pattern of results was different for EPT. EPT was significantly smaller on the sound limb in the phantom pain group (31.29 ± 5.80 mA) than on the other three limbs: 1) the affected limb (53.04 ± 10.25 mA) of the PLP group; 2) the affected limb (78.65 ± 11.51 mA) of the non-PLP group; and 3) the sound limb (69.10 ± 10.28 mA) of the non-PLP group (P<0.01). The EPT values of these three limbs were not significantly different among each other (). These results suggest that the sound limb in the PLP group was sensitized. According to independent t-tests, CPT was significantly greater on the sound limb in the PLP group than CPT on the affected and sound limbs in the non-PLP group (P<0.04) (). As mentioned above, there was no difference in CPT between the affected limb and the sound limb within the PLP group or the non-PLP group.
Discussion
Tactile, electrical, and thermal thresholds were measured in all 17 subjects after traumatic lower limb amputation with and without phantom pain. The novel findings included: 1) EPT was only decreased in the sound limb in the PLP group, suggesting central sensitization; and 2) EST was increased on the affected limb as compared to the sound limb within the PLP group, but there were no difference in EST between the PLP group and non-PLP group. Other confirmatory findingsCitation15 included greater CPT on the sound limb in the PLP group, and no difference in other sensory or pain thresholds (TST, CST, WST, HPT).
To our knowledge, this is the first study that compared mechanical, thermal, and electrical thresholds in both affected and sound limbs in patients with and without PLP. Such comprehensive assessment and comparisons are advantageous. Significant difference in EPT between the affected limb and the sound limb in the PLP group can be accurately interpreted as a significant decrease in EPT on the sound limb in the PLP group after comparing with the EPT values of each limb in the non-PLP group. EPT is reported to have high test–retest reliability.Citation21 Furthermore, decrease in EPT has been viewed to reflect central sensitization.Citation18–Citation20 The primary significant finding of decreased EPT in the sound limb in the PLP group with no significant difference in other sensory thresholds between two groups suggests central sensitization as a possible mechanism mediating PLP. The finding of selective modulation of EPT was also consistent with our recent studies.Citation19,Citation20 We found that EPT was significantly increased after breathing-controlled electrical stimulation (BreEStim), but it was significantly decreased after standard electrical stimulation without changes in TSTs, thermal thresholds, and EST. These studies suggest habituation to experimentally induced pain after BreEStim, but sensitization after standard electrical stimulation.
Our finding of central sensitization with chronic PLP supports the hypothesis of a link between traumatization, central sensitization, and memory-mediated phantom pain,Citation10 indicating an important role of central memory processes in chronic phantom pain.Citation9 Traumatic injury resulting in limb amputation is usually a single event. The memory of the event could last for the rest of life. When associated with a negative emotional context, PLP could be perceived as aversive, and retriggered by a stressful life event.Citation22 On the other hand, our finding is helpful in understanding inconsistent results of phantom pain management by peripheral analgesia.Citation9
The finding of a link between central sensitization and chronic PLP suggests that mechanism-based therapeutic interventions could be developed for management of chronic phantom pain. A recently developed noninvasive, nonpharmacological voluntary BreEStim to peripheral nerves has a desensitization effect, as manifested by increased EPT after the interventions.Citation19,Citation20 In a recent case report, an AKA patient with chronic shooting phantom pain reported disappearance of PLP after BreEStim treatment.Citation23 This could be attributed to the desensitization effect of BreEStim. Future research comparing EPT values in PLP patients before and after BreEStim could further test this mechanism of PLP. In a review article, Moura et al reported that various mind–body interventions for PLP management, including hypnosis, imagery, and visual mirror feedback, are able to offer either temporary or long-term relief, either alone or in combination with conventional therapies.Citation24 Our finding supports these mind–body interventions for treatment of PLP.Citation24 Moreover, our result suggests that psychotherapy for PLP management focusing on desensitization may bring about better outcomes.
There are a few limitations in this study. Findings of the present study clearly support the role of central sensitization in patients with PLP, but not in patients without PLP. Future studies on its correlation with psychological sensitivity tests are needed to provide further support regarding the connection between PLP and cognitive–emotional sensitivity. Only subjects after traumatic amputations were enrolled to this study. Other etiologies, such as diabetes mellitus and tumor, are major causes of limb amputation. It remains unknown whether the current findings could be generalized to these populations. The findings of no difference in mechanical and thermal thresholds except for CPT are confirmatory and generally consistent with previous studies.Citation11–Citation15 The result of greater CPT on the sound limb in the PLP group than in the non-PLP group is likely related to the floor effect of CPT. The Medoc PATHWAY system sets the lower limit for CPT at 0°C for safety reasons. Four out of seven subjects in the non-PLP group and three out of ten subjects in the PLP group reached the lower limit in both affected and sound limbs. Thus, there is a possibility of large variations in the remaining subjects in each group.
Conclusion
In summary, comprehensive comparisons of sensory thresholds in patients after traumatic lower limb amputation with and without phantom pain demonstrate a primary novel finding of significantly decreased EPT in the sound limb in the PLP group. No difference in other sensory or pain thresholds was found between the PLP group and the non-PLP group. The findings support the idea of central sensitization of PLP. Mechanism-based therapeutic intervention, such as BreEStim, are promising for PLP management, either alone or in combination with conventional therapies. EPT may be used as a measurable variable to quantify phantom pain and to monitor the progress of pain management.
Author contributions
Shengai Li, Danielle H Melton, and Sheng Li conceived and designed the experiments; Shengai Li performed the experiments; Shengai Li and Sheng Li analyzed the data; Shengai Li, Danielle H Melton, and Sheng Li discussed and interpreted the data; and Shengai Li, Danielle H Melton, and Sheng Li wrote and revised the paper.
Acknowledgments
We thank Liu “Eunice” Yang, MS for her statistical consultation.
Disclosure
The authors report no conflicts of interest in this work.
References
- NikolajsenLJensenTSPhantom limb painBr J Anaesth200187110711611460799
- GiummarraMJMoseleyGLPhantom limb pain and bodily awareness: Current concepts and future directionsCurr Opin Anaesthesiol201124552453121772144
- FlorHPhantom-limb pain: characteristics, causes, and treatmentLancet Neurol20021318218912849487
- FlorHElbertTKnechtSPhantom-limb pain as a perceptual correlate of cortical reorganization following arm amputationNature199537565314824847777055
- RamachandranVSConsciousness and body image: lessons from phantom limbs, Capgras syndrome and pain asymboliaPhilos Trans R Soc Lond B Biol Sci19983531377185118599854257
- PereiraJCJrAlvesRCThe labelled-lines principle of the somatosensory physiology might explain the phantom limb phenomenonMed Hypotheses201177585385621862233
- WoolfCJCentral sensitization: implications for the diagnosis and treatment of painPain20111523 SupplS2S1520961685
- MakinTRScholzJFilippiniNHenderson SlaterDTraceyIJohansen-BergHPhantom pain is associated with preserved structure and function in the former hand areaNat Commun20134157023463013
- FlorHPainful memories. Can we train chronic pain patients to ‘forget’ their pain?EMBO Rep20023428829111943751
- EgloffNHirschiAvon KänelRTraumatization and chronic pain: a further model of interactionJ Pain Res2013676577024231792
- VaseLNikolajsenLChristensenBCognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patientsPain2011152115716221067864
- HunterJPKatzJDavisKDStability of phantom limb phenomena after upper limb amputation: a longitudinal studyNeuroscience2008156493994918755249
- HunterJPKatzJDavisKDDissociation of phantom limb phenomena from stump tactile spatial acuity and sensory thresholdsBrain2005128Pt 230832015634736
- BrauneSSchadyWChanges in sensation after nerve injury or amputation: the role of central factorsJ Neurol Neurosurg Psychiatry19935643933998482960
- HardenRNGagnonCMKhanAWallachGZereshkiAHypoesthesia in the distal residual limb of amputeesPM R20102760761120659715
- VaseLEgsgaardLLNikolajsenLSvenssonPJensenTSArendt-NielsenLPain catastrophizing and cortical responses in amputees with varying levels of phantom limb pain: a high-density EEG brain-mapping studyExp Brain Res2012218340741722349560
- JanalMNGlusmanMKuhlJPClarkWCOn the absence of correlation between responses to noxious heat, cold, electrical and ischemic stimulationPain19945834034117838590
- BendtsenLCentral sensitization in tension-type headache – possible pathophysiological mechanismsCephalalgia200020548650811037746
- LiSBerlinerJCMeltonDHLiSModification of electrical pain threshold by voluntary breathing-controlled electrical stimulation (BreEStim) in healthy subjectsPLoS One201387e7028223894632
- LiSHuTBeranMALiSHabituation to Experimentally Induced Electrical Pain during Voluntary-Breathing Controlled Electrical Stimulation (BreEStim)PLoS One201498e10472925153077
- Biurrun ManresaJANeziriAYCuratoloMArendt-NielsenLAndersenOKTest-retest reliability of the nociceptive withdrawal reflex and electrical pain thresholds after single and repeated stimulation in patients with chronic low back painEur J Appl Physiol20111111839220814801
- JensenTSKrebsBNielsenJRasmussenPImmediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb painPain19852132672783991231
- LiSMeltonDHBerlinerJCBreathing-controlled electrical stimulation could modify the affective component of neuropathic pain after amputation: a case reportJ Pain Res20125717522536094
- MouraVLFaurotKRGaylordSAMind-body interventions for treatment of phantom limb pain in persons with amputationAm J Phys Med Rehabil201291870171422286895