Abstract
Despite proven clinical utility, buprenorphine has not been used widely for the treatment of chronic pain. Questions about “ceiling effect” or bell-shaped curve observed for analgesia in preclinical studies and potential withdrawal issues on combining with marketed μ-agonists continue to hinder progress in expanding full potential of buprenorphine in the treatment of cancer and noncancer pain. Mounting evidence from clinical studies and conclusions drawn by a panel of experts strongly support superior safety and efficacy profile of buprenorphine vs marketed opioids. No ceiling on analgesic effect has been reported in clinical studies. The receptor pharmacology and pharmacokinetics profile of buprenorphine is complex but unique and contributes to its distinct safety and efficacy. The buprenorphine pharmacology also allows it to be combined with other μ-receptor opioids for additivity in efficacy. Transdermal delivery products of buprenorphine have been preferred choices for the management of pain but new delivery options are under investigation for the treatment of both opioid dependence and chronic pain.
Introduction
Opioids are the market leaders for treatment of moderate to severe chronic pain among adults, amounting to over $10 billion in global sales. The opioids are emerging as the primary option for cancer pain treatment as approximately 70% of cancer patients and 85% of those suffering from cancer-related pain eventually require management with opioids.Citation1,Citation2 The use of opioids is also increasing for treatment of chronic nonmalignant pain with established benefits in inflammatory, ischemic, visceral, musculoskeletal, and neuropathic pain.Citation3,Citation4 Despite rising opioid prescriptions (11.8% in 2010 in US), many patients feel nonsatisfactory response to treatment options.Citation5 In addition, long-term use of opioid therapy leads to the development of tolerance and hyperalgesia limiting their clinical utility in controlling chronic pain. Chronic use of opioids also accounts for other side effects such as respiratory depression, constipation, dependence, and abuse potential. With a growing senior population (projected to be approximately 25% by 2020 in major markets), there is constant demand for more efficacious and safer treatment options for patients.
Structure and pharmacology
Buprenorphine () is a semi synthetic derivative of an opiate alkaloid thebaine that is isolated from the poppy Papaver somniferum. Buprenorphine is a hydrophobic molecule and carries a complex chemical structure with multiple chiral centers. Buprenorphine was introduced in the early 1980s as an opioid analgesic in Europe and subsequently for the treatment of opioid addiction in France in 1996. It is available in the US for the treatment of opioid addiction maintenance programs, and for the treatment of chronic pain.
Interactions with opioid receptors (ORs)
Buprenorphine has a distinct profile, significantly different from morphine, codeine, fentanyl, or methadone. It is a potent but partial agonist of μ-opioid receptor (μ-OR), showing a high affinity but low intrinsic activity (). High potency and slow off rate (half-life of association/dissociation is 2–5 hours)Citation6 help buprenorphine displace other μ-agonists such as morphine, methadone from receptors and overcome opioid dependence issues. Buprenorphine is approximately 25–100 times more potent than morphine. The slow dissociation from μ-receptor also accounts for its prolonged therapeutic effect to treat opioid dependence as well as pain.
Figure 2 Implications of buprenorphine interactions with opioid receptors. Buprenorphine is a partial and potent agonist of μ-opioid receptor.
Abbreviation: ORL1, opioid receptor-like 1.
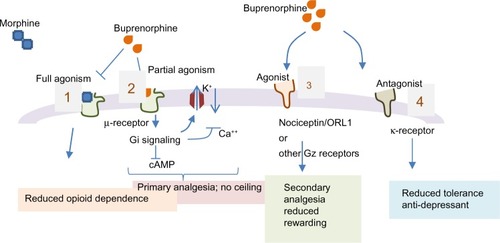
The in vitro profile of buprenorphine against ORs is captured in .Citation7 The clinical relevance of interactions of buprenorphine with different ORs is not fully resolved but the knowledge on its unique profile is improving with emerging data. Buprenorphine is a potent κ-receptor antagonist (Ki =6 nM) and this is believed to resist depression.Citation8,Citation9 Buprenorphine acts as a “chaperone” ligand and increases μ-receptor expression on membrane surfaces.Citation2,Citation10,Citation11,Citation12 Buprenorphine is also an agonist for nociceptin or OR-like 1 (ORL1) that has a unique interaction with pain processing. Activation of the ORL1 receptor in the dorsal horn is analgesic, but cerebral ORL1 activation blunts antinociception as seen in animal models.Citation12 It has been suggested that μ-receptor mediated antinociception can be reduced by the ORL1 agonist activity residing in the same molecule.Citation13,Citation14 The relevance of ORL1 activation by buprenorphine under clinical setting is, however, not clear particularly at pharmacological doses to control pain. Additional mechanisms have also been proposed for the analgesic effects of buprenorphine. In interesting studies, peripheral administration of naloxone antagonizes buprenorphine’s dose response curve while supraspinal intracerebroventricular (icv) administration of naloxone shows no effect against subcutaneous (sc) administration of buprenorphine in antinociceptive tests. Similarly, icv buprenorphine produces antinociception and intraperitoneal buprenorphine is antagonized by intraperitoneal naloxone, but not by icv naloxone in rat formalin test.Citation15 These results suggest a different supraspinal mechanism of action for buprenorphine. Pertussis toxin which prevents ligand-induced activation of G-protein-coupled receptors (GPCRs), antagonizes morphine, and fentanyl but has no effect on buprenorphine mediated analgesia. Further mechanistic studies suggest the involvement of heterotrimeric guanine nucleotide-binding regulatory protein Gz. Supraspinal administration of Gz antisense had no effect on morphine or fentanyl antinociception but blocked buprenorphine effect. The icv administration of okadaic acid (a protein phosphatase inhibitor) blocked buprenorphine but not morphine or fentanyl effect. Thus, supraspinal component of buprenorphine-induced antinociception does not appear to be mediated via the typical μ-opioid response but by other unique receptors.Citation15
Table 1 Buprenorphine – binding affinity (Ki, nM) for opioid receptors
Pharmacokinetics profile
Buprenorphine is a lipophilic molecule (LogP=4.98) with low aqueous solubility. The compound shows high volume of distribution and distributes well in tissues including brain. The protein binding for buprenorphine in human plasma is approximately 96%, not to the albumin but to the α- and β-globulin fractions. Buprenorphine has very low plasma concentrations and this is not believed to influence competition between globulin binding sites.Citation10 Buprenorphine is extensively metabolized in the liver, and the major metabolite norbuprenorphine () occurs through Cyp3A4 mediated N-dealkylation.Citation16,Citation17 Both buprenorphine and norbuprenorphine undergo rapid glucuronidation at the phenolic site by UGT2B7 and UGT1A1 in the liver.Citation16,Citation18 The plasma levels of conjugate metabolites buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide can exceed the parent drug levels. In human, norbuprenorphine rarely exceeds 10% of buprenorphine blood concentrations (Cmax).Citation19
The relative bioavailability of buprenorphine given intramuscular (im), sublingual solution or sublingual tablet is 70%, 49%, and 29%, respectively, assuming 100% for intravenous (iv) dosing.Citation20–Citation23 Sublingual and transdermal formulations tend to show long half-life (20–73 hours). The prolonged terminal half-life of buprenorphine can in part be due to enterohepatic recirculation as observed for nonhuman species. With a sublingual formulation, buprenorphine shows onset of effects at 30–60 minutes postdosing and the peak clinical effects are observed at 1–4 hours. The duration of effect may last for 6–12 hours at low dose (<4 mg) and 24–72 hours at higher dose (>16 mg). The longer effect at higher buprenorphine sublingual dose may be linked to sustained, effective drug levels for extended duration because of its slower elimination and enterohepatic recirculation (see for approved doses of sublingual buprenorphine).
Table 2 Marketed buprenorphine products
Buprenorphine is eliminated primarily via a stool (as free forms of buprenorphine and norbuprenorphine) while 10%–30% of the dose is excreted in urine as conjugated forms of buprenorphine and norbuprenorphine. Buprenorphine is a preferred opioid for treatment of pain in patients with compromised renal function. Buprenorphine is also safer in patients with a failing liver.Citation24
Cyp inhibition and drug–drug interaction potential
Buprenorphine and its Cyp3A4 mediated metabolite norbuprenorphine are rapidly converted to conjugate. Buprenorphine and norbuprenorphine do not inhibit Cyps at therapeutic doses, and as a result have fewer drug interactions.Citation25 Drugs that inhibit Cyp3A4 do not seem to influence the pharmacokinetics (PK) profile of buprenorphine significantly and glucuronidation is generally associated with limited drug interactions.Citation12 However, caution should be used when buprenorphine is co-administered with other drugs that inhibit Cyp3A4. The combination of buprenorphine with benzodiazepine or other central nervous system (CNS) depressants should be administered with caution as it may lead to severe or even fatal respiratory depression.Citation26
Pharmacology of metabolites
The reported Ki values for ORs for buprenorphine and metabolites vary significantly based on the experimental conditions and the laboratories conducting experiments.Citation7,Citation18,Citation27 Buprenorphine-3-glucuronide is a μ-, δ-, and ORL1 agonist, whereas norbuprenorphine-3-glucuronide is a κ- and ORL1 ligand. All metabolites except norbuprenorphine-3-glucuronide are analgesic and contribute to the observed buprenorphine profile in clinic.Citation18,Citation28 Neither buprenorphine nor the glucuronide metabolites reduce respiratory rates, although norbuprenorphine-3-glucuronide has been demonstrated to reduce tidal volume in animal models.Citation18,Citation29 Norbuprenorphine is a potent μ-agonist and contributes to respiratory depression.Citation30
Safety of buprenorphine vs other opioids
The primary side effects of buprenorphine are similar to other μ-opioid agonists (eg, nausea, vomiting, and constipation), but the intensity of these side effects is reduced significantly compared to full agonist. The superiority of buprenorphine over other opioids in safety was recently addressed.Citation12
Respiratory depression
Buprenorphine has a ceiling effect on respiratory depression and remains one of the safest opioids to curtail this adverse effect as concluded by a panel of experts reviewing opioid pharmacology.Citation10,Citation12,Citation31–Citation33 Typically, 1%–11% of patients on opioid therapy suffer from respiratory depression that seems to be more pronounced in seniors, obese, or individuals with sleep apnea or neuromuscular disease. Respiratory depression associated with buprenorphine may be partly related to its metabolite, norbuprenorphine, and not to the parent drug. Interestingly, buprenorphine prevents and reverses respiratory depression in rats that are given lethal injections of norbuprenorphine.Citation34 In another study, much higher safety window (13.5-fold) is reported for buprenorphine than for fentanyl (1.2-fold) when comparing analgesia and respiratory distress doses in rat.Citation35
The combination of buprenorphine with sedative drugs such as benzodiazepine or alcohol has been reported to affect respiratory depression adversely. Buprenorphine–benzodiazepine combination, however, seems safer than methadone–benzodiazepine for respiratory distress.Citation26 Caution should, however, be exercised in combination therapy of buprenorphine with CNS depressants.
Constipation
Based on reported data from clinical studies, buprenorphine exhibits much lower incidence (1%–5%) of constipation than observed with full μ-agonists.Citation2,Citation36,Citation37 Unlike other opioids, buprenorphine does not cause spasm of the sphincter of Oddi and may be a preferred choice, along with nonsteroidal anti-inflammatory drugs, in the management of biliary colic and/or pancreatitis.Citation38
Cognitive and psychomotor effects
Opioid use can impair cognitive function and driving ability. Addiction to opioids can influence dependability. The addition of alcohol or sedatives may worsen the cognitive and driving ability. Comparative studies done report that buprenorphine may have better visual, psychomotor or cognitive function vs morphine, methadone or fentanyl.Citation12,Citation39 In many cases, buprenorphine effect on cognitive and psychomotor function was comparable to placebo.Citation40
Immunosuppression
Opioids seem to trigger unique biochemical communication between brain and the immune system. The reported data suggest that while exogenous opioids suppress the immune system, the endogenous opioids stimulate it. The implications of opioid evoked immunosuppression are particularly relevant during the postoperative period when the pain and susceptibility to infection are high; for sufferers of chronic pain who administer opioids for extended periods; and for patients with immunosuppressive disease such as AIDS, transplant patients, and the elderly, who are predisposed to opportunistic infections.Citation41 The potent opioids such as morphine and fentanyl reduce antibody production, reduce natural killer cell activity, and impair the cytokine expression and phagocytic activity of white cells.Citation12 The immunosuppressive effect is accentuated in presence of corticosteroids or other immunosuppressive drugs. Some immunosuppression in morphine may also emerge through non-μ-receptor mediation as the effect is not reversed by naltrexone.Citation42,Citation43 Unlike morphine, buprenorphine does not reduce natural killer-cell function, increase cortisol, reduce adrenocorticotropic hormone levels, or alter norepinephrine or serotonin levels after injection in the brain. Most of the studies showing lack of immunosuppressive effect of buprenorphine have been conducted in animals and their clinical relevance needs to be established. However, in immunosuppressed patients, opioids (morphine, fentanyl) treatment may be avoided and buprenorphine should be considered in the scheme of options.Citation10,Citation12,Citation43–Citation45
Hypogonadism
Chronic use of μ-receptor agonists has been associated with hypogonadism and fatigue. With time, hypogonadism can lead to osteopenia and loss of muscle mass. Use of morphine and fentanyl is reported to reduce testosterone levels and testosterone replacement therapy is often recommended. Even at high doses, buprenorphine seems to have minimal effect on sexual hormone levels.Citation46–Citation49
QTc prolongation vs methadone
Based on reported data, methadone-maintenance treatment has been associated with QTc prolongation (approximately 29% patients) with approximately 5% showing QTc interval of <500 ms. The risk of QTc prolongation seems particularly high at doses of <120 mg. In contrast, buprenorphine-maintenance therapy for opioid dependence does not seem to be associated with QTc prolongation. Torsades de pointes or sudden cardiac deaths occur four times more frequently with methadone than with buprenorphine.Citation50–Citation53 All reported QTc studies on buprenorphine seem to be on opioid maintenance therapy. Since the dose needed for analgesic effect is generally lower, it should also improve therapeutic window for cardiac safety.
Seniors’ treatment
Multiple studies undertaken on elderly patients (age 65 years and above) indicate that PK profile, efficacy results, or adverse events of buprenorphine did not alter with age. Many elderly patients tend to suffer from chronic diseases such as arthritis, diabetes, cardiovascular issues, or cancer. For all opioids except buprenorphine, half-life of the parent drug and its metabolites increased in elderly and those with renal impairment.Citation2,Citation10,Citation54–Citation56 For elderly on multiple medications, drug–drug interactions mediated through Cyp enzymes are not uncommon. However, buprenorphine and its Cyp3A4 mediated metabolite are rapidly converted to conjugate. Drugs that inhibit Cyp3A4 do not seem to influence the PK profile of buprenorphine significantly and glucuronidation is generally associated with limited drug interactions. Buprenorphine also seems to be the select opioid that is not associated with fracture in elderly.Citation57
Patients with renal failure
Buprenorphine is largely eliminated through bile in nonrenal pathway. The levels of buprenorphine and its metabolite as well as pain rating do not seem to change for patients on dialysis.Citation58,Citation59 The drug is also relatively safe in patients with liver failure.Citation60 The data favor selection of buprenorphine as preferred opioid during emergency or intensive care hospitalization.
Tolerance and hyperalgesia
The clinical usefulness of opioids is often hampered by the development of tolerance after chronic treatment. Although tolerance to the antinociceptive effect of buprenorphine has been demonstrated, the onset is slower than tolerance to morphine. In a retrospective study involving nearly 900 cancer and noncancer patients buprenorphine produced less analgesic tolerance than fentanyl, as measured by an opioid escalation index.Citation61–Citation64 Multiple mechanisms have been proposed to explain opioid tolerance observed with μ-agonists such as morphine.Citation2,Citation10 Among others, a proposed mechanism of tolerance indicates increased activity of the anti-opiate peptides in the brain (eg, ORL1 ligand Orphanin-FQ/nociceptin (OFQ/N) and dynorphin). Buprenorphine may also control secondary hyperalgesia through activation of ORL1 receptor, antagonism of κ-receptorCitation62 or other pathways.Citation65
Abuse potential and withdrawal
Buprenorphine is a partial agonist and has fewer rewarding effects compared to another μ-agonists and blocks psychological dependence. Despite these properties, there have been reports of abuse particularly by new users.Citation66,Citation67 To counter potential misuse, a combination of buprenorphine with the opioid antagonist naloxone in a ratio of 4 to 1, has been used in sublingual administration. Naloxone has poor bioavailability (approximately 3%) on sublingual dosing. Thus, when the buprenorphine/naloxone tablet is taken in sublingual form, the buprenorphine opioid agonist effect predominates, and the naloxone does not precipitate opioid withdrawal in the opioid-addicted user. Naloxone via the parenteral route, however, has good bioavailability. If the sublingual buprenorphine/naloxone tablets are crushed and injected by an opioid-addicted individual, the naloxone effect predominates and can precipitate the opioid withdrawal syndrome.
Typically, the withdrawal syndrome following the abrupt cessation of long-term buprenorphine treatment emerges within 3–5 days of the last dose, and mild withdrawal features continue for up to several weeks. Treatment with opioid antagonist (naloxone) can be commenced within days of the cessation of low dose buprenorphine treatment without precipitating severe opioid withdrawal. This enables patients to transfer promptly to naloxone treatment, and avoid relapse and treatment drop-out.Citation10
A summary of comparative safety profile of buprenor-phine with other opioids is captured in .
Table 3 Comparison of safety profile of buprenorphine with other opioids
Efficacy of buprenorphine
Buprenorphine in treatment for opioid dependence
Buprenorphine has been used extensively for treatment of opioid addiction and this also accounts for a significant portion of its market revenues. The main signs experienced during the initial stages of opioid withdrawal include nausea, vomiting, diaphoresis, yawning, fatigue, aches and pain, diarrhea, mydriasis, and piloerection. Cravings initiate 4–6 hours after the last dose of short-acting and at 12–24 hours after last dose of long-term opioids. This is followed by anxiety, diaphoresis, agitation, and the other symptoms. Peak withdrawal discomfort is usually experienced after 36–72 hours and decreases thereafter.Citation68,Citation69 Consciousness is usually unimpaired, and opioid withdrawal is not life threatening, even if untreated in outpatient or inpatient settings. A Cochrane review of 13 studies concluded “buprenorphine is an effective intervention for the treatment of opioid dependence”.Citation70
Buprenorphine’s high binding affinity and low intrinsic activity can induce withdrawal in opioid dependent patients that are using full μ-agonists (methadone, heroin, and morphine) by displacing opioids from the receptor.Citation69,Citation71 To control opioid dependence, buprenorphine treatment is initiated at the appearance of withdrawal symptoms. The patients on opioids are encouraged to abstain from use for until at least 12–24 hours or until the emergence of withdrawal symptoms. The patients are started on a low dose of transdermal or sublingual formulation of buprenorphine. If clinical signs remain controlled, buprenorphine is titrated upwards to individualized dose. A small randomized controlled trial (N=32) showed no significant difference in withdrawal symptoms between buprenorphine and buprenorphine/naloxone.Citation72 The consensus panel recommends that buprenorphine/naloxone be used for the induction, stabilization, and maintenance phases of opioid dependence treatment for most patients. For pregnant women, monotherapy of buprenorphine is recommended. In summary, buprenorphine is an effective detoxifying agent for opioid dependence and is equivalent to or better than methadone.Citation70 The buprenorphine has also exhibited utility for longer-term opioid maintenance. Its lower abuse potential and good safety profile make it particularly appealing to family physicians.
Buprenorphine in pain treatment
Buprenorphine is a partial agonist at μ-receptor. The data in suggests that the affinity of buprenorphine for ORL1 receptor is approximately 50 times lower than for μ-receptor and many preclinical reports indicate that ORL1 agonism may contribute to reducing its antinociception effect at high concentration. The significance of ORL1 activation and implication on pronociceptive effect are not validated in clinic.Citation10,Citation12,Citation28 In addition, a number of studies suggest that buprenorphine dose needs for treatment of pain are much lower compared to doses used for the treatment of opioid addiction. The typical analgesic dose of buprenorphine is 0.3–0.6 mg (im or iv), and its analgesic effects last approximately 6 hours.Citation67 A review of clinical trials showed that buprenorphine was effective in 25/26 trials.Citation73 Contrary to previous concerns, no analgesic ceiling effect and no antagonism on a combination of buprenorphine with pure μ-OR agonists is seen within the therapeutic dose range in humans.Citation12,Citation28 Some authors believe that false myths about buprenorphine based on unconfirmed animal data and early clinical research came into textbooks on pharmacology and pain approximately 30 years ago and have been difficult to eradicate.Citation28 The fact is that in clinical practice there is no bell-shaped dose–response curve, there is no plateau on the dose–response curve, and there is no antagonist effect from buprenorphine on other μ-opioid agonists.Citation28 This was also the conclusion by a panel of world experts convened to review pharmacology of buprenorphine and available evidence.Citation2,Citation10,Citation74
Effective long-term pain relief requires sustenance of opioid plasma levels for extended period to prolong duration of analgesic action and reduce potential adverse effects. The introduction of slow release, transdermal drug delivery systems offered a number of advantages. Depending upon the formulation and the pain model, buprenorphine has been reported to be approximately 25–100 times more potent than morphine.Citation75–Citation78 The conversion calculations for buprenorphine to morphine suggest an equipotency ratio of 1:110–1:115 to accomplish the same degree of analgesia.Citation77–Citation80
Transdermal buprenorphine product butrans® (5, 10, and 20 μg/h) is useful in controlling pain. The published observations suggest that 20 μg/h butrans® may be equivalent to 12–16 μg/day sublingual dose. At the same time 5 μg/h dose of buprenorphine may be a high start dose for frail, elderly patients.
Efficacy in moderate to severe pain
Buprenorphine has been studied for treatment of cancer and noncancer pain in large patients. The reports indicate marked improvement in pain intensity from 62 mm to 16 mm in visual analog scale of 0–100 (0 – no pain, 100 – severe pain) over 2-week treatment. A starting buprenorphine dose range of 35–70 μg/h; with the majority (74%) starting at 35 μg/h was preferred using transdermal, sublingual, or parenteral dosing. Studies report good to very good pain relief in 85% patients and improvement in sleep quality (48%) with low (3%) discontinuations. Pain relief with buprenorphine seems equivalent to that of morphine, hydromorphone, oxycodone, fentanyl, and methadone. In opioid naïve patients, low dose (17 μg/h) buprenorphine treatment has been reported to reduce pain intensity and improve quality of life. In a comparative study, transdermal buprenorphine reduced pain intensity to a larger extent (mean difference −16.2 in visual analog scale) compared to morphine with significantly lower adverse events (nausea, constipation, and vomiting) and discontinuations relative to morphine. Another review also indicates that buprenorphine treatment gives equivalent analgesic effect but markedly reduced adverse events and discontinuations relative to fentanyl and morphine.Citation80–Citation82
Efficacy in neuropathic pain
Unlike other μ-agonists, buprenorphine seems to block secondary hyperalgesia from central sensitization in human pain conditions.Citation83 Buprenorphine has shown efficacy in neuropathic pain in human studies.Citation84,Citation85 In two case studies, buprenorphine gave positive response where transdermal fentanyl failed.Citation63,Citation86 In this small patient study,Citation63 transdermal buprenorphine vs oral morphine gave equianalgesia of 110:1 to 115:1. Similarly, approximately 40% patients with various central neuropathic syndromes (usually considered refractory to opioid analgesia) responded to buprenorphine starting with a low dose (8.75 μg/h) and titrating escalation. In another double-blind study, buprenorphine (iv) showed efficacy in postthoracotomy pain with high response (69%) at doses ranging from 35 to 70 μg/h. Although there are no randomized clinical trials comparing buprenorphine with other opioids, a consensus panel stated that there is significant evidence that buprenorphine effectively relieves neuropathic pain and offers distinct benefit.Citation10,Citation36,Citation63 More studies are needed to compare and define the utility of buprenorphine in different neuropathic syndromes.
Efficacy in broad pain phenotypes
Preclinical studies establish effectiveness of buprenorphine under varying pain conditions including formalin injection, cold temperature tail flick, and diffuse noxious inhibitory control tests.Citation85 Human studies indicate buprenorphine to be effective in broader pain conditions (bone pain, heat pain, pain related to nerve growth factor injections, and cold pressor pain) than fentanyl which was effective only in cold pressor pain. Less dramatic differences were reported in another comparative pain study between fentanyl and buprenorphine.Citation87 The data, however, support the potential utility of buprenorphine in a range of painful conditions.
Buprenorphine (butrans® or butoxone) has also been used successfully to treat musculoskeletal, visceral, as well as for chronic headaches. Furthermore, possibly because of kappa antagonism, buprenorphine seems to have antidepressive and antianxiety properties, which also helps in pain management.Citation9
Combination and rotation with opioids
Recent studies indicate that buprenorphine could be effectively and safely combined with full μ-agonists, and switching between buprenorphine and another opioid provides comparable pain relief using equianalgesic doses.Citation2,Citation10,Citation12 The results indicate that adding opioids to patients currently receiving buprenorphine therapy is safe and effective, while the addition of buprenorphine to patients receiving other opioid therapy should be used more cautiously. Buprenorphine has been combined with other opioids (morphine, tramadol) and has demonstrated additivity. In one study, supra-additive analgesia has been reported in combination study of buprenorphine with oxycodone or hydromorphone. Although buprenorphine has demonstrated very high affinity for μ-receptors, it occupies fewer receptors for analgesia, which leads to a significant receptor reserve for other μ-agonists. Buprenorphine increases μ-receptor expression, which allows other μ-agonists to interact with receptors.
Supplemental dosing with an opioid is the main treatment suggested to manage breakthrough pain in cancer patients. Combination of immediate release or iv morphine and a basal analgesic regime of transdermal buprenorphine has been used as an effective and safe treatment. Clinically, the treatment shows an additive analgesic effect, without any safety relevant issues.Citation10,Citation88 No cross tolerance was observed during rotation between buprenorphine and fentanyl.Citation10,Citation89 Future studies will need to confirm combination therapy and the role of buprenorphine in opioid rotation.
Marketed buprenorphine
Transdermal buprenorphine formulations (butrans®) have been available in the US since 2011 as 5, 10 and 20 μg/h dose for 7-day delivery patches. Buprenorphine has also been available in most European countries since 2001 as transtec® in 35, 52.5 and 70 μg/h transdermal patches that deliver dose over 96 hours. Transdermal buprenorphine is approved and indicated for the management of moderate to severe chronic pain in patients requiring a continuous, around-the-clock opioid analgesic for an extended period. Transdermal patches show high variability in PK and take approximately 60 hours to reach steady state. Slow onset with transdermal formulations and inability to adjust initial dose levels may limit the utility in some cases. The supplemental addition of immediate release full μ-agonist is used as a co-therapy in many cases. Transdermal patches may also cause erythema and pruritus.
Sublingual formulations of buprenorphine are available in the US market as monotherapy (generic subutex®; tablet) and a combination therapy of buprenorphine with naloxone (generic suboxone®, zubsolv®, temgesic®, as a tablet or film) for opioid addiction. Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA discontinued distribution of buprenorphine/naloxone sublingual tablets in March 2013, as a result of concerns over accidental or unsupervised pediatric exposure compared to the film formulation. Subutex® was discontinued previously in 2011. Sublingual tablets are very common but can take up to 10 minutes to dissolve. Many suffer from after taste, dissolve time, and dispensing restrictions. Branded formulations such as suboxone sublingual film (Reckitt Benckiser Pharmaceuticals Inc.), bunavail® (BioDelivery Sciences, Raleigh, NC, USA) mucoadhesive film and zubsolv® (Orexo, Uppsala, Sweden) sublingual tablet were launched during 2011–2015. Mucoadhesive film bunavail seems to have much better taste but dissolution time may be higher per individual observations.
Buprenex® as an injectable (iv or im) approved in US for moderate pain.
No oral buprenorphine product is in the market currently. Low bioavailability (poor absorption, high first pass effect) and uncertainty on GI safety have been limiting factors. Some oral formulations are under investigation ().
Table 4 Buprenorphine products under development
These buprenorphine products are classified as Schedule III controlled. The and gives a summary of marketed and under development buprenorphine formulations.
New buprenorphine products in development pipeline
Among the investigational drugs, attempts are being made to improve oral delivery with enteric coated (REL 1028) or nanoparticle (NTC 510) formulations. There are also attempts to deliver once a week/month sc formulation (CAM 2038) and six month subdermal (probuphine) implants.
Probuphine – is an investigational subdermal implant containing buprenorphine HCl for the long-term maintenance treatment of opioid addiction. The drug being developed by Braeburn Pharmaceuticals, New York, NY, USA/Titan Pharmaceuticals, Inc., San Francisco, CA, USA reported encouraging P-III results for long-term maintenance therapy for opioid addiction. Data from 6-month double-blind, efficacy and safety study suggest the implant to be noninferior to sublingual formulation in efficacy while showing benefits in compliance, misuse and abuse. Titan plans to submit an New Drug Application (NDA) toward the end of 2015.
REL-1028 (BuTab) represents novel oral formulations of modified release buprenorphine, being developed for both chronic pain and opioid dependence indications, is designed to overcome the significant first pass metabolism in the upper gastrointestinal tract to allow for enteric coated oral administration in traditional capsule or tablet form.
NTC-510 (NanoBUP) is being developed by Nanotherapeutics as a combination of buprenorphine and naloxone for oral delivery. It uses a proprietary nanoparticle delivery system as an immediate delivery capsule. In Phase-I, the drug combination is claimed to give 60%–70% relative bioavailability vs sublingual formulation. The investigational drug completed Phase-I studies in 2009/2010 and seem to have been studied in Phase IIa for the treatment of pain following dental surgery of third molars. No results are reported.
Belbuca (BioDelivery Sciences) is a mucoadhesive buccal film undergoing NDA review for treatment of moderate to severe chronic pain.
Summary
Buprenorphine has unique pharmacology and markedly distinct profile vs other opioids. Data from preclinical and clinical studies establish that buprenorphine carries highly discriminatory profile vs known μ-agonists (morphine, fentanyl, and methadone). Safety of buprenorphine is much superior over the marketed opioids – i) ceiling effect on respiratory depression; much lower risk vs other opioids; ii) lower constipation risk vs other opioids; iii) safest opioid for CNS, immune-suppression issues; iv) anti-hyperalgesic profile and low tolerance issues; v) low clearance through renal path; no clinically relevant changes in patients with renal impairment; vi) safer option for seniors; minimal drug–drug interactions and minimal influence on PK. Buprenorphine continues to be used as an effective treatment for opioid addiction during induction, stabilization, and maintenance phases. The recognition that there is no “ceiling effect” on analgesia and that buprenorphine can be combined with marketed opioids without concerns about withdrawal or precipitation is beginning to help patients suffering from moderate to severe pain. In addition, ability to switch buprenorphine or rotate opioids has added flexibility and options for optimal pain relief. Buprenorphine has demonstrated efficacy and improved therapeutic window in cancer, nonmalignant and neuropathic pain conditions. Given its superior safety and efficacy profile, buprenorphine can now be considered as a first line therapy for the treatment of a wide range of chronic pain conditions. Ideal buprenorphine formulations may deliver quick onset and persistent plasma levels for extended duration as mono or combination therapy. New buprenorphine formulations (sc or subdermal implants) for sustained, once a week/month delivery of drug are under investigation. Oral delivery of buprenorphine is challenging but can potentially offer convenience, sustained release, quick onset, and ease of dose adjustments – key needs for patients suffering from cancer, postsurgical or other chronic pain conditions. Since buprenorphine dose needs for pain treatment are relatively low, a combination of innovation and modern delivery technologies is likely to present novel solutions to improve absorption and related issues. The oral delivery can also improve on the limitations of marketed (sublingual and transdermal) options discussed earlier. Learnings from ongoing clinical investigations of oral buprenorphine formulations and continuing research in laboratories is likely to open new avenues. Additional clinical studies will also be needed to expand efficacy, improve compliance, and enhance safety of buprenorphine or its combination partner.
Disclosure
The authors report no conflict of interest in this work.
References
- FinePGMiaskowskiCPaiceJAMeeting the challenges in cancer pain managementJ Support Oncol20042Suppl 452215605922
- KressHGClinical update on the pharmacology, efficacy and safety of transdermal buprenorphineEur J Pain20091321923018567516
- BreivikHCollettBVentafriddaVCohenRGallacherDSurvey of chronic pain in Europe: prevalence, impact on daily life, and treatmentEur J Pain20061028733316095934
- PortenoyRKFarrarJTBackonjaMMCleelandCSYangKFriedmanMLong term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry studyClin J Pain20072328729917449988
- SitesBDBeachMLDavisMAIncreases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among usersReg Anesth Pain Med201439161224310049
- VirkMSArttamangkulSBirdsongWTWilliamsJTBuprenorphine is a weak partial agonist that inhibits opioid receptor desensitizationJ Neurosci200929227341734819494155
- KhroyanTVWuJPolgarWEBU08073 a buprenorphine analog with partial agonist activity at μ receptors in vitro but long-lasting opioid antagonist activity in vivo in miceBr J Pharmacol201517266868024903063
- KarpJFButtersMABegleyAESafety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adultsJ Clin Psychiatry2014758e785e79325191915
- MelloNKMendelsonJHBehavioral pharmacology of buprenorphineDrug Alcohol Depend1985143–42833033888577
- PergolizziJBögerRHBuddKOpioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone)Pain Pract20088428731318503626
- ZakiPAKeithDEJrBrineGACarrollFIEvansCJLigand-induced changes in surface mu-opioid receptor number: relationship to G protein activation?J Pharmacol Exp Ther200029231127113410688632
- DavisMPTwelve reasons for considering buprenorphine as a frontline analgesic in the management of painJ Support Oncol201210620921922809652
- KhroyanTVPolgarWEJiangFZaveriNTTollLNociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonistsJ Pharmacol Exp Ther2009331394695319713488
- SpagnoloBCaloGPolgarWEActivities of mixed NOP and mu-opioid receptor ligandsBr J Pharmacol2008153360961918059322
- DingZRaffaRBZhe Ding and Robert B Raffa. Identification of an additional supraspinal component to the analgesic mechanism of action of buprenorphineBr J Pharmacol200915783184319422392
- KobayashiKYamamotoTChibaKHuman buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4Drug Metab Dispos1998268188219698298
- IribarneCPicartDDréanoYBailJPBerthouFInvolvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomesLife Sci19976022195319649180349
- BrownSMHoltzmanMKimTKharaschEBuprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically activeAnesthesiology201111561251126022037640
- HuestisMAConesEJPirnaySOUmbrichtAPrestonKLIntravenous buprenorphine and norbuprenorphine pharmacokinetics in humansDrug Alcohol Depend2013131325826223246635
- BrewsterDHumphreyMJMcleavyMABiliary excretion, metabolism and enterohepatic circulation of buprenorphineXenobiotica1981111891967293215
- NathRPUptonRAEverhartETBuprenorphine pharmacokinetics: relative bioavailability of sublingual tablet and liquid formulationsJ Clin Pharmacol199939661962310354966
- KuhlmanJJLalaniSMagluiloJHuman pharmacokinetics of intravenous, sublingual, and buccal buprenorphineJ Anal Toxicol1996203693788889672
- SchuhKJJohansonCEPharmacokinetic comparison of the buprenorphine sublingual liquid and tabletDrug Alcohol Depend1999561556010462093
- CiccozziAAngelettiCBaldascinoGHigh dose of buprenorphine in terminally ill patient with liver failure: efficacy and tolerabilityJ Opioid Manag20128425325922941853
- ZhangWRamamoorthyYTyndaleRFSellersEMInteraction of buprenorphine and its metabolite norbuprenorphine with cytochromes p450 in vitroDrug Metab Dispos200331676877212756210
- McCance-KatzEFSullivanLENallaniSDrug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a reviewAm J Addict201019141620132117
- HuangPKehnerGBCowanALiu-ChenLYComparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonistJ Pharmacol Exp Ther200129768869511303059
- ButlerSBuprenorphine – clinically useful but often misunderstoodScand J Pain20134148152
- GueyePNBorronSWRisedePBuprenorphine and midazolam act in combination to depress respiration in ratsToxicol Sci200265110711411752690
- OhtaniMKotakiHNishitatenoKSawadaYIgaTKinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphineJ Pharmacol Exp Ther199728114284339103526
- DahanAAartsLSmithTWIncidence, reversal, and prevention of opioid-induced respiratory depressionAnesthesiology2010112122623820010421
- DahanAYassenARombergRBuprenorphine induces ceiling in respiratory depression but not in analgesiaBr J Anaesth200696562763216547090
- NielsenSDietzePLeeNDunlopATaylorDConcurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatmentAddiction2007102461662217286641
- MégarbaneBMarieNPirnaySBuprenorphine is protective against the depressive effects of norbuprenorphine on ventilationToxicol Appl Pharmacol2006212325626716169027
- YassenAOlofsenEKanJDahanADanhofMPharmacokinetic-pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in ratsPharm Res200825118319317914664
- GriessingerNSittlRLikarRTransdermal buprenorphine in clinical practice – a post-marketing surveillance study of 13,179 patientsCurr Med Res Opin2005211147115616083522
- ShiptonEASafety and tolerability of buprenorphineBuddKRaffaRBuprenorphine – The Unique Opioid AnalgesicStuttgartThieme Verlag KG200592101
- CuerJCDapoignyMAjmiSEffects of buprenorphine on motor activity of the sphincter of Oddi in manEur J Clin Pharmacol19893622032042721544
- SoykaMHockBKagererSLehnertRLimmerCKuefnerHLess impairment on one portion of a driving-relevant psychomotor battery in buprenorphine-maintained than in methadone-maintained patients: results of a randomized clinical trialJ Clin Psychopharmacol200525549049316160628
- ShmygalevSDammMWeckbeckerKBerghausGPetzkeFSabatowskiRThe impact of long-term maintenance treatment with buprenorphine on complex psychomotor and cognitive functionDrug Alcohol Depend20111172–319019721353749
- KapitzkeDVetterICabotPJEndogenous opioid analgesia in peripheral tissues and the clinical implications for pain controlTher Clin Risk Manag20051427929718360571
- VallejoRde Leon-CasasolaOBenyaminROpioid therapy and immunosuppression: a reviewAm J Ther200411535436515356431
- WangJBarkeRARoySTranscriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphineJ Biol Chem2007282107164717117227776
- SacerdotePOpioids and the immune systemPalliat Med200620Suppl 1S9S1516764216
- SacerdotePOpioid-induced immunosuppressionCurr Opin Support Palliat Care200821141818685388
- AloisiAMCeccarelliICarlucciMHormone replacement therapy in morphine-induced hypogonadic male chronic pain patientsReprod Biol Endocrinol201192621332999
- BliesenerNAlbrechtSSchwagerAWeckbeckerKLichtermannDKlingmullerDPlasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependenceJ Clin Endocrinol Metab200590120320615483091
- HallinanRByrneAAghoKMcMahonCGTynanPAttiaJHypogonadism in men receiving methadone and buprenorphine maintenance treatmentInt J Androl200932213113917971165
- HallinanRByrneAAghoKMcMahonCTynanPAttiaJErectile dysfunction in men receiving methadone and buprenorphine maintenance treatmentJ Sex Med20085368469218093096
- KrantzMJMartinJStimmelBMehtaDHaigneyMCQTc interval screening in methadone treatmentAnn Intern Med2009150638739519153406
- AnchersenKClausenTGossopMHansteenVWaalHPrevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: a mortality assessment studyAddiction2009104699399919392907
- AthanasosPFarquharsonALComptonPPsaltisPHayJElectrocardiogram characteristics of methadone and buprenorphine maintained subjectsJ Addict Dis2008273313518956527
- WedamEFBigelowGEJohnsonRENuzzoPAHaigneyMCQT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trialArch Intern Med2007167222469247518071169
- HandCWSearJWUppingtonJBallMJMcQuayHJMooreRABuprenorphine disposition in patients with renal impairment: single and continuous dosing, with special reference to metabolitesBr J Anaesth19906432762822328175
- IribarneCBerthouFCarlhantDInhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitorsDrug Metab Dispos19982632572609492389
- SeripaDPilottoAPanzaFMateraMGPilottoAPharmacogenetics of cytochrome P450 (CYP) in the elderlyAgeing Res Rev20109445747420601196
- VestergaardPRejnmarkLMosekildeLFracture risk associated with the use of morphine and opiatesJ Intern Med20062601768716789982
- BögerRHRenal impairment: a challenge for opioid treatment? the role of buprenorphinePalliat Med200620Suppl 1S17S2316764217
- FilitzJGriessingerNSittlRLikarRSchuttlerJKoppertWEffects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphineEur J Pain200610874374816426877
- LikarRTransdermal buprenorphine in the management of persistent pain – safety aspectsTher Clin Risk Manag20062111512518360586
- KoppertWIhmsenHKorberNWehrfritzASittlRSchmelzMDifferent profiles of buprenorphine induced analgesia and antihyperalgesia in a human pain modelPain2005118152216154698
- VanderahTWGardellLRBurgessSEDynorphin promotes abnormal pain and spinal opioid antinociceptive toleranceJ Neurosci2000207074707910995854
- LikarRSittlRTransdermal buprenorphine for treating nociceptive and neuropathic pain: four case studiesAnaesth Analg2005100781785
- LouisFTransdermal buprenorphine in pain management – experiences from clinical practice: five case studiesInt J Clin Pract200660101330133416981980
- LutfyKCowanABuprenorphine: a unique drug with complex pharmacologyCurr Neuropharmacol20042439540218997874
- WalshSLEissenbergTThe clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinicDrug Alcohol Depend200370Suppl 2S13S2712738347
- McNicholasLClinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. A Treatment Improvement Protocol TIP 40Rockville, MDUS Department of Health and Human ServicesDHHS Publication No. (SMA) 04–393920041147
- FarrellMOpiate withdrawalAddiction19948911147114757841858
- DucharmeSFraserRGillKUpdate on the clinical use of buprenorphine: in opioid-related disordersCan Fam Physician201258374122267618
- GowingLAliRWhiteJBuprenorphine for the management of opioid withdrawalCochrane Database Syst Rev20062CD00202516625553
- StrainECPrestonKLLiebsonIABigelowGEBuprenorphine effects in methadone-maintained volunteers: effects at two hours after methadoneJ Pharmacol Exp Ther199527226286387853176
- StrainECHarrisonJABigelowGEInduction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble-filmsClin Pharmacol Ther201189344344921270789
- RaffaRBHaideryMHuangHMThe clinical analgesic efficacy of buprenorphineJ Clin Pharm Ther201439657758325070601
- PergolizziJAloisiAMDahanACurrent knowledge of buprenorphine and its unique pharmacological profilePain Pract201010542845020492579
- AtkinsonRESchofieldPMellorPThe efficacy in sequential use of buprenorphine and morphine in advanced cancer painDoyleDOpioids in the Treatment of Cancer PainInternational Congress and Symposium SeriesLondonRoyal Society of Medicine Services19901468187
- JasinskiDRPevnickJSGriffithJDHuman pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addictionArch Gen Psychiatry1978354501516215096
- SittlRLikarRPoulsen-NautrupBEquipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and non-cancer pain: results of a retrospective cohort studyClin Ther20052722523715811486
- SittlRTransdermal buprenorphine in clinical practiceBuddKRaffaRBuprenorphine – The Unique Opioid AnalgesicStuttgartThieme Verlag KG200592101
- SittlRTransdermal buprenorphine in cancer pain and palliative carePalliat Med200620Supp 1s25s3016764218
- BöhmeKLikarREfficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain. A randomised, double-blind, placebo-controlled studyPain Clinic2003152193202
- SittlRGriessingerNLikarRAnalgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: A multicenter randomized, double-blind, placebo-controlled studyClin Ther200325115016812637117
- SorgeJSittlRTransdermal buprenorphine in the treatment of chronic pain: Results of a phase III, multicenter, randomized, double-blind, placebo-controlled studyClin Ther200426111808182015639693
- InduruRRDavisMPBuprenorphine for neuropathic pain – targeting hyperalgesiaAm J Hosp Palliat Care200926647047319666890
- BenedettiFDose-response relationship of opioids in nociceptive and neuropathic postoperative painPain1998742052119520235
- HansGBuprenorphine – a review of its role in neuropathic painJ Opioid Manag20073419520617957979
- AndresenTStaahlCOkscheAMansikkaHArendt-NielsenLDrewesAMEffect of transdermal opioids in experimentally induced superficial, deep and hyperalgesic painBr J Pharmacol201116493494521182491
- WolffRFAuneDTruyersCHernandezAVMissoKRiemsmaRKleijnenJSystematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe painCurr Med Res Opin201228583384522443154
- MercadanteSVillariPFerreraPSafety and effectiveness of intravenous morphine for episodic breakthrough pain in patients receiving transdermal buprenorphineJ Pain Symptom Manage20063217517916877185
- CowanAFriderichsEStraßburgerWRaffaRBBasic pharmacology of buprenorphineBuddKRaffaRBBuprenorphine – The Unique Opioid AnalgesicStuttgartThieme Verlag KG200592101