Abstract
Background
Various biomarkers have been shown to predict prognosis in various types of cancers. However, these biomarkers have not been studied in advanced small cell lung cancer (SCLC). The modified Glasgow prognostic score (mGPS) is based on serum albumin level and C-reactive protein (CRP). The prognostic nutritional index (PNI) is a combination of serum albumin level and absolute lymphocyte count. This study aimed to evaluate the prognostic value of mGPS and PNI in SCLC.
Methods
We retrospectively reviewed and calculated mGPS and PNI for patients with stage IIIB or IV SCLC who initiated platinum-based combination chemotherapy between November 2007 and June 2016. We compared overall survival (OS) and progression-free survival (PFS) between high and low groups of these two biomarkers. Univariate and multivariate Cox hazard analyses assessed the prognostic value of these biomarkers.
Results
We reviewed 97 SCLC patients. The OS of patients with mGPS 0–1 and higher PNI was significantly longer than that of those with mGPS 2 and lower PNI. The PFS of mGPS 0–1 was significantly longer than that of mGPS 2, while there was no significant difference in PFS according to PNI. Multivariate analyses found mGPS 0–1 (hazard ratio [HR] 2.34, 95% confidence interval [CI] 1.27–4.31, P<0.01) and higher PNI (HR 0.50, 95% CI 0.31–0.78, P<0.01) as prognostic factors for longer OS. However, neither biomarker was predictive of PFS.
Conclusion
Our study was a small retrospective study; however, the data demonstrate that pretreatment mGPS and PNI are independent predictors of OS in patients with advanced SCLC. The pretreatment assessment of mGPS and PNI may be useful for identification of patients with poor prognosis. We recommend pretreatment measurement of serum albumin, C-reactive protein, and absolute lymphocyte count.
Introduction
The incidence of small cell lung cancer (SCLC) has been declining in Japan, but still accounts for 10%–15% of lung cancer cases.Citation1 The majority of Japanese patients with lung cancer are diagnosed when the disease has already become regionally advanced or metastatic. SCLC is characterized by aggressive progression, early metastases and poor prognosis, despite being highly sensitive to chemotherapy.
Complex diagnostic and prognostic tools are cumbersome to use in clinical practice; clinicians require tools that are simple and straightforward. Recently, various laboratory biomarkers have been developed based on systemic inflammation or nutritional status. These biomarkers have been demonstrated to predict prognosis in various cancers.
The modified Glasgow prognostic score (mGPS)Citation2–Citation4 is based on a combination of serum albumin level and C-reactive protein (CRP). The prognostic nutritional index (PNI)Citation5 is based on a combination of serum albumin level and absolute lymphocyte count. These two tools have been shown to predict prognosis for many solid tumors. In contrast to a large number of studies for non-SCLC, there have been only a few studies that report validated mGPSCitation6 and PNICitation7,Citation8 for SCLC. These studies included patients at various stages of disease, undergoing various treatment protocols.
This study aimed to evaluate these two prognostic biomarkers for patients with advanced SCLC who had received platinum-based combination chemotherapy.
Patients and methods
Patient selection and study design
We retrospectively selected patients who met all the following criteria: 1) histologically or cytologically diagnosed SCLC; 2) clinical stage IIIB or IV in the seventh TNM classification of lung cancer by the Union for International Cancer Control,Citation9 because our practice did not adopt the staging system of the Veterans Administration Lung Study Group;Citation10 3) initiation of platinum-based combination regimen as the first-line chemotherapy between November 2007 and June 2016 at our institution, and 4) available pretreatment blood test and sufficient laboratory data. The exclusion criteria were as follows: 1) patients who had received non-platinum chemotherapy as the first-line therapy; 2) patients who had received concurrent or sequential curative-intent thoracic radiotherapy, though we accepted patients with concurrent palliative radiotherapy, and 3) patients who had initiated chemotherapy at other institutions and thereafter transferred to our hospital.
Baseline characteristics included sex, age, clinical stage, Eastern Cooperative Oncology Group Performance Status (ECOG-PS),Citation11 smoking status, height, body weight and therapeutic data. Current smokers were arbitrarily defined as patients who had smoked within a year prior to the diagnosis. Pretreatment laboratory data obtained within 2 weeks prior to chemotherapy included: complete blood count, hemoglobin, red cell distribution width, creatinine clearance estimated by Cockcroft–Gault formula with addition of 0.2 mg/dL to serum creatinine concentration,Citation12 serum albumin, sodium concentration, lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and CRP. PNI was calculated according to the following formula as previously described:Citation5 10×serum albumin value (g/dL)+0.005×peripheral lymphocyte count (/μL). Elevated CRP and hypoalbuminemia were combined to generate the mGPS. If CRP was >1.0 mg/dL and albumin <3.5 g/dL, we assigned a score of 2; CRP >1.0 mg/dL only was assigned a score of 1 and absence of these two abnormalities was assigned a score of 0.Citation13 Progression-free survival (PFS) and overall survival (OS) were calculated from the first day of chemotherapy to the date of documented progressive disease or death. Response to chemotherapy was based on Response Evaluation Criteria in Solid Tumors version 1.1.Citation14 The data cut-off date was March 31, 2017.
The Osaka Police Hospital ethics committee approved our study (number 685). Informed consent was waived because the study was retrospective with de-identified data.
Data analysis
We expressed continuous variables as mean ± standard deviation (SD) and discrete and categorical variables as frequency. We used Fisher’s exact test for comparison of relative frequencies and Welch’s t-test for comparison of continuous variables. Using the receiver operating characteristic curve and the outcome variable of response to chemotherapy, we selected the optimal cut-off values of PNI that provided the closest point to the left upper hand corner of the graph, and then divided our patients into two groups. We compared the survival times by the Kaplan–Meier method and the log-rank test. We used the Cox proportional hazard model in order to investigate the association between biomarkers and PFS or OS. We included all variables with a P value <0.1 in univariate analysis in the subsequent multivariate analyses. The results were described as hazard ratios (HR) and 95% confidence interval (CI). A P value <0.05 was defined as statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).Citation15
Results
During the study period, 104 patients with stage IIIB or IV SCLC received chemotherapy. Among them, seven patients were excluded from the analysis due to lack of pretreatment albumin value. Thus, 97 patients met the inclusion and exclusion criteria. As of the data cut-off date, 6 patients were still alive, 13 were lost to follow-up, 78 were confirmed dead and 88 had experienced progressive disease during or after first-line chemotherapy. Concurrent with first-line chemotherapy, five patients received palliative radiotherapy. Three patients were palliated by thoracic radiotherapy for superior vena cava syndromes or tracheal stenosis, and two were treated for sciatica by lumbar irradiation.
In order to divide our patients into two groups, receiver operating characteristic curve analysis defined 44.3 (sensitivity 0.59, specificity 0.59, area under the curve 0.60, 95% CI 0.47–0.72) as the optimal cut-off value for PNI. Patients with mGPS 0–1 had better ECOG-PS (P=0.02) and higher body mass index (23.5±3.7 vs 21.3±4.2, P=0.02) than those with mGPS 2. Patients with high PNI (≥44.3) had better ECOG-PS (P<0.01) and higher body mass index (24.1±3.6 vs 21.2±3.8, P<0.01). They were likely to receive cisplatin-based first-line regimen (P=0.02) and second-line chemotherapy (P=0.01). Compared with patients with mGPS 2, those with mGPS 0–1 had higher serum albumin levels and lower CRP values. Patients with high PNI (≥44.3) had higher absolute lymphocyte counts, higher serum albumin levels and lower CRP levels than those with low PNI (<44.3) ().
Table 1 Clinical manifestations according to mGPS and PNI at first-line chemotherapy
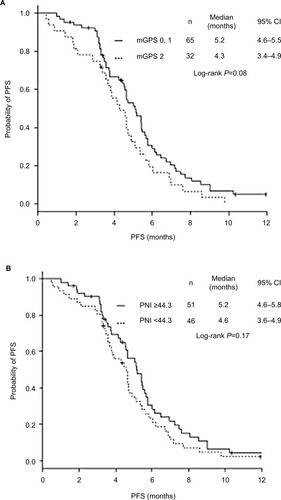
The OS of patients with mGPS 0–1 and high PNI (≥44.3) was significantly longer than that of those with mGPS 2 and low PNI (<44.3) (). There was no significant difference in PFS according to mGPS and PNI ().
Figure 1 Kaplan–Meier curves of overall survival according to mGPS and PNI.
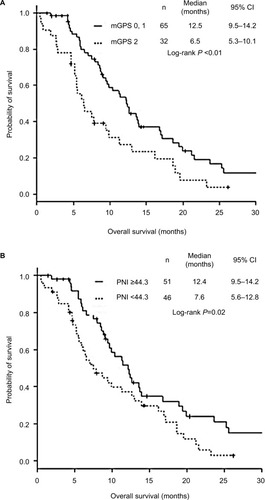
Figure 2 Kaplan–Meier curves of PFS according to mGPS and PNI.
Univariate Cox hazard analysis revealed the following factors to be favorable prognostic markers (): for longer OS – no brain metastasis (HR 1.98, 95% CI 1.03–3.81, P=0.04), no liver metastasis (HR 1.73, 95% CI 1.04–2.89, P=0.03), no adrenal gland metastasis (HR 1.85, 95% CI 1.01–3.40, P=0.046), better ECOG-PS (HR 3.33, 95% CI 2.05–5.41, P<0.01), higher albumin (HR 0.41, 95% CI 0.26–0.64, P<0.01), lower LDH (×10−2) (HR 1.21, 95% CI 1.08–1.37, P<0.01), lower ALP (×10−2) (HR 1.08, 95% CI 1.01–1.16, P=0.04), lower CRP (HR 1.05, 95% CI 1.01–1.09, P=0.02), mGPS 0, 1 (HR 1.92, 95% CI 1.19–3.07, P=0.01) and higher PNI (×10−1) (HR 0.51, 95% CI 0.35–0.73, P<0.01); for longer PFS – no brain metastasis (HR 2.03, 95% CI 1.08–3.83, P=0.03), no adrenal gland metastasis (HR 2.05, 95% CI 1.04–4.06, P=0.04), better ECOG-PS (HR 2.00, 95% CI 1.27–3.16, P<0.01), higher sodium concentration (×10−1) (HR 0.56, 95% CI 0.36–0.86, P<0.01), higher albumin (HR 0.59, 95% CI 0.40–0.88, P=0.01), lower LDH (×10−2) (HR 1.12, 95% CI 1.01–1.24, P=0.04), lower ALP (×10−2) (HR 1.10, 95% CI 1.02–1.19, P=0.02), lower CRP (HR 1.05, 95% CI 1.01–1.09, P=0.02) and higher PNI (×10−1) (HR 0.72, 95% CI 0.52–0.98, P=0.04).
Table 2 Univariate Cox hazard analysis of factors associated with OS and PFS
Multivariate analyses found mGPS 0–1 (HR 2.34, 95% CI1.27–4.31, P<0.01) and higher PNI (×10−1) (HR 0.50, 95% CI 0.31–0.75, P<0.01) to be favorable prognostic factors for longer OS, in addition to absence of brain metastasis and ECOG-PS (). Neither mGPS nor PNI was a prognostic factor for PFS ().
Table 3 Multivariate Cox hazard analysis of the association between OS and mGPS or PNI
Table 4 Multivariate Cox hazard analysis of the association between PFS and mGPS or PNI
Discussion
This study demonstrated that mGPS and PNI are useful as prognostic factors for OS in advanced SCLC treated with the standard first-line regimen of platinum-based chemotherapy. We demonstrated that these simple and user-friendly prognostic tools are useful for patients with advanced SCLC treated with chemotherapy alone.
Both mGPS and PNI were expected to be independent prognostic factors for OS in patients with SCLC treated with chemotherapy alone. We evaluated this by two means: first, multivariate Cox proportional hazard analysis detected both biomarkers as independent prognostic factors; second, Kaplan–Meier curves and log-rank tests showed that OS in patients with mGPS 2 and low PNI (<44.3) was significantly lower than that of patients with mGPS 0–1 and high PNI (≥44.3). Our results were similar to some previous studies, but different from other studies (). Two studies showed mGPS as an independent prognostic factor for OS in SCLC patients.Citation6,Citation16 Although their patient backgrounds were different from ours, their result was consistent with ours. Compared with our study, including 33% (n=32) of mGPS 2, the proportions of mGPS 2 were much lower in those two previous studies: a Chinese and a Japanese study included only 3.1% (n=11)Citation6 and 22.9% (n=73),Citation16 respectively. Unlike our study that enrolled patients treated with chemotherapy alone, the Chinese study included 46.2% of patients treated with thoracic radiotherapyCitation6 and the Japanese study included 13.5% patients treated with supportive care alone.Citation16
Table 5 Review of multivariate analyses for prediction of survival outcomes in patients with SCLC
Use of PNI as a prognostic factor for OS in SCLC is controversial. There have been two studies evaluating PNI as a prognostic factor for SCLC. One Chinese study demonstrated that PNI was a significant prognostic factor, independent of stage, performance status and LDH.Citation8 This study and ours detected PNI and PS as common independent prognostic factors. Another Chinese study included 919 patients, but failed to find PNI and PS as independent prognostic factors by multivariate analysis. In that study, patients with PNI ≥45 survived significantly longer than those with PNI <45.Citation7
As a predictive marker for the efficacy of platinum-based first-line chemotherapy for SCLC, neither mGPS nor PNI was promising. In addition to a statistically insignificant difference in PFS between mGPS 0–1 vs 2 and high vs low PNI groups, neither mGPS nor PNI was found to be a significant predictive factor by multivariate analysis. To our knowledge, there are no studies investigating the association of mGPS and PNI with the efficacy of chemotherapy.
Our study has several limitations. First, it was a single-centered, small-scale and retrospective study. Moreover, 13% of patients were lost to follow-up. The small number of patients and the high proportion lost to follow-up may account for the failure to identify the two biomarkers as significant predictive markers for the efficacy of chemotherapy. By contrast, both mGPS and PNI may be true prognostic markers for OS, because, despite the small size of the study, these indices were statistically significant and clinically relevant. Multicenter, prospective, large-scale studies should be conducted to validate this result. Second, patient selection was based on the availability of pretreatment blood tests. Our study excluded 6.7% of patients (7/104) due to lack of pretreatment laboratory data, a number that was much lower than reported in previous studies. Two Chinese studies excluded 22% (101/460)Citation6 and 40% (492/1216)Citation8 of SCLC patients during selection. Hong et al excluded 12% (146/1216) of patients owing to incomplete laboratory data.Citation8 Serum albumin measurement is not routine in clinical practice.
Conclusion
This was a small retrospective study, but we found that pretreatment mGPS and PNI were independent prognostic factors for OS in patients with advanced SCLC treated with chemotherapy. The pretreatment assessment of mGPS and PNI may be useful for identification of patients with poor prognosis. We recommend pretreatment testing for CRP, serum albumin and absolute lymphocyte count.
Acknowledgments
We are grateful to Son-Ho Kim, Yuki Nakatani, Narumi Noda, Kanako Nishimatsu and Shouko Ikuta, Saori Ikebe and Hideyasu Okada at the Department of Respiratory Medicine, Osaka Police Hospital, for their detailed medical records, diagnosis, treatment and care of their patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- KinoshitaFLItoYNakayamaTTrends in lung cancer incidence rates by histological type in 1975–2008:A Population-Based Study in Osaka, JapanJ Epidemiol2016261157958627150013
- ForrestLMMcMillanDCMcArdleCSAngersonWJDunlopDJEvaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancerBr J Cancer20038961028103012966420
- McMillanDCAn inflammation-based prognostic score and its role in the nutrition-based management of patients with cancerProc Nutr Soc200867325726218452641
- McMillanDCCrozierJECannaKAngersonWJMcArdleCSEvaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancerInt J Colorectal Dis200722888188617245566
- OnoderaTGosekiNKosakiGPrognostic nutritional index in gastrointestinal surgery of malnourished cancer patientsNihon Geka Gakkai Zasshi198485910011005 Japanese6438478
- ZhouTHongSHuZA systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancerTumour Biol201536133734325256672
- HongXCuiBWangMYangZWangLXuQSystemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancerTohoku J Exp Med2015236429730426250537
- HongSZhouTFangWThe prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patientsTumour Biol20153653389339725527156
- Rami-PortaRCrowleyJJGoldstrawPThe revised TNM staging system for lung cancerAnn Thorac Cardiovasc Surg20091514919262443
- GreenRAHumphreyECloseHPatnoMEAlkylating agents in bronchogenic carcinomaAm J Med19694645165255791000
- Common Toxicity Criteria (CTC), CTC Version 2.01999 Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdfAccessed October 28, 2017
- AndoYMinamiHSakaHAndoMSakaiSShimokataKAdjustment of creatinine clearance improves accuracy of Calvert’s formula for carboplatin dosingBr J Cancer1997768106710719376268
- ProctorMJTalwarDBalmarSMThe relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome StudyBr J Cancer2010103687087620717110
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- KandaYInvestigation of the freely available easy-to-use software ‘EZR’ for medical statisticsBone Marrow Transplant201348345245823208313
- KurishimaKWatanabeHIshikawaHSatohHHizawaNModified glasgow prognostic score in patients with small-cell lung cancerMol Clin Oncol20177112112428685088
