Abstract
The epidermal growth factor receptor (EGFR) is a promising therapeutic target in non-small cell lung cancer, and several therapeutic agents that target this receptor, including EGFR tyrosine kinase inhibitors and monoclonal antibodies to EGFR, have been developed. Such monoclonal antibodies have shown efficacy in combination with chemotherapy and radiotherapy. Nimotuzumab (h-R3) is a humanized monoclonal antibody to EGFR, and its effects in combination with radiation have been sufficiently promising to warrant further investigation in several types of cancer. Furthermore, the typical severe dermatologic toxicities associated with other monoclonal antibodies to EGFR have not been observed with nimotuzumab. We here summarize the results of preclinical studies as well as of previous and ongoing clinical trials of nimotuzumab for the treatment of non-small cell lung cancer.
Introduction
Lung cancer is the most common cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) accounting for ~75% of all lung cancer cases.Citation1 Platinum-based chemotherapy regimens are the standard first-line treatment for individuals with advanced NSCLC, but the efficacy of such regimens has reached a plateau.Citation2 New strategies based on a better understanding of tumor biology are thus needed to improve the efficacy of treatment. The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is abnormally upregulated or activated in a variety of tumors.Citation3 Deregulation of receptor tyrosine kinases as a result of overexpression or activating mutations is frequently associated with human cancers and results in the promotion of cell proliferation or migration, inhibition of cell death, or the induction of angiogenesis.Citation4 The EGFR has thus been identified as an important target in cancer therapy.Citation5 Two different types of agent for EGFR inhibition are currently in use or under development for NSCLC: tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs).
Among EGFR-TKIs, gefitinib and erlotinib have been extensively evaluated in NSCLC, and sensitivity to these drugs has been associated with the presence of somatic mutations in the tyrosine kinase domain of EGFR or with amplification of the EGFR gene.Citation6–Citation8 However, these agents have yet to show effective synergy with chemotherapy. Various mAbs to EGFR are also undergoing preclinical studies and clinical trials of their efficacy as anticancer agents. Cetuximab is a chimeric monoclonal antibody that inhibits EGFR by binding to the extracellular domain of the receptor. The relatively modest anticancer activity of cetuximab administered as monotherapy in NSCLC patients has prompted evaluation of its use in novel combination regimens.Citation9 The safety of cetuximab in combination with the platinum-based two-drug regimens in common use for the treatment of NSCLC has been established in several Phase II studies.Citation10–Citation15 Recently, the addition of cetuximab to the regimen of cisplatin and vinorelbine was found to result in improved overall survival in patients with advanced NSCLC positive for EGFR expression.Citation16 Subsequent biomarker analysis has shown that patients with high EGFR expression fared better on cetuximab plus chemotherapy than on chemotherapy alone, regardless of histology. The median overall survival in this high-expression group treated with cetuximab plus chemotherapy was 12.0 months, compared with 9.6 months for patients treated with chemotherapy alone (hazard ratio [HR]: 0.73; P = 0.011). In contrast, the patients who had low EGFR expression showed no benefit from the addition of cetuximab to chemotherapy.Citation17 The combination of cetuximab with radiotherapy in patients with locally advanced NSCLC has also shown promising results in a Phase II studyCitation18 and is now being evaluated in a confirmatory trial (Clinicaltrials. gov: NCT00533949).
Nimotuzumab (h-R3) is a humanized mouse mAb to EGFR that binds to the extracellular domain of the receptor and thereby inhibits EGF binding.Citation19 In preclinical studies, nimotuzumab has shown antitumor effects in various types of cancer and has been found to enhance the antitumor efficacy of radiation in EGFR-expressing NSCLC cell lines.Citation20,Citation21 Nimotuzumab has been approved in more than 20 countries for various indications including pediatric and adult glioma as well as head and neck cancer, nasopharyngeal carcinoma, and esophageal cancer, and it is in clinical trials for various tumor types including NSCLC as well as colorectal, pancreatic, cervical, and breast cancer. This review summarizes the results of preclinical and clinical studies of nimotuzumab and also describes ongoing clinical trials of this drug in NSCLC patients. We also propose future directions for clinical development in this setting.
Mechanism of action
Nimotuzumab was originally isolated as a mouse immunoglobulin (Ig) G2a antibody, designated R3, which was developed in response to human placental EGFR at the Molecular Immunology Center in Havana, Cuba. The R3 mAb was humanized to reduce its immunogenicity and slow its rate of clearance from the body in humans by grafting its complementarity determining regions onto a human IgG1 molecule, thereby generating h-R3-nimotuzumab.Citation19
Whereas it shows similar preclinical and clinical activity when compared with other mAbs to EGFR for certain indications, nimotuzumab has one major advantage: it does not show severe skin toxicity or induce severe hypomagnesemia or gastrointestinal adverse events.Citation22,Citation23 The lack of such side effects has been hypothesized to be due to differences in binding affinity for EGFR between nimotuzumab and other mAbs. The dissociation constant (Kd) for the binding of nimotuzumab to EGFR is thus >10 times that of other mAbs to EGFR including cetuximab and panitumumab ().Citation24–Citation26 The binding properties of nimotuzumab and cetuximab were studied in vitro on chip surfaces with cells expressing EGFR at various levels. Whereas cetuximab bound in a monovalent manner to cells with low EGFR expression levels, nimotuzumab required bivalent binding for stable attachment to the cell surface, with the result that it bound selectively to cells with high levels of EGFR expression ().Citation27 Both antibodies bound bivalently to and accumulated to similar extents on cells with high EGFR expression levels. In situations where EGFR density is low, such as in skin, cetuximab would thus be expected to be more active than nimotuzumab. Its reliance on bivalent binding for effective EGFR blockade allows nimotuzumab to bind robustly to tumor cells overexpressing EGFR while sparing normal cells with a low level of EGFR expression and thereby avoiding unwanted toxicities.
Figure 1 Schematic representation of the binding properties of nimotuzumab and cetuximab as a function of EGFR density. For cells with a low EGFR density, such as skin cells, both mAbs rely on one arm to bind to the EGFR epitope (monovalent binding) and to exert their effects. The level of EGFR blockade achieved is determined by the specific binding affinity of each mAb, which is lower for nimotuzumab than for cetuximab. For cells with a high EGFR density, such as certain tumor cells, the mAbs bind via both arms (bivalent binding). Bivalent binding results in the formation of a more stable interaction given that it is dictated by the avidity of binding rather than binding affinity.
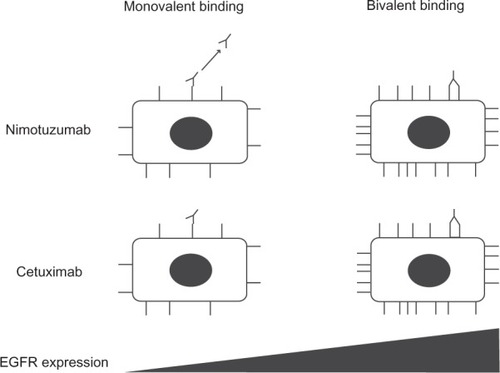
Table 1 Comparison of the anti-EGFR mAbs cetuximab and nimotuzumab
Another possible explanation for the reduced side effects of nimotuzumab has been suggested from the crystal structure of the Fab fragment of nimotuzumab bound to the extracellular region of EGFR.Citation26 Nimotuzumab recognizes domain III of the extracellular region of EGFR, and its target site overlaps with both the surface patch recognized by cetuximab and the binding site for epidermal growth factor (EGF). The epitope recognized by nimotuzumab is displaced toward the carboxyl-terminus of EGFR domain III compared with the cetuximab binding site. Nimotuzumab sterically interferes with EGF binding, while permitting EGFR domain I to approach domain III and adopt the active conformation. In contrast, cetuximab blocks the binding of EGF and clashes with domain I, preventing the receptor from adopting its active conformation. By interfering only with ligand-dependent EGFR activation, nimotuzumab reduces EGFR signaling to the basal, ligand-independent level, which is required for the survival of normal epithelial cells. Further studies are warranted to determine whether the low toxicity profile of nimotuzumab is indeed explained by the precise structural nature of the interaction between nimotuzumab and the extracellular region of EGFR.
Preclinical data
The growth-inhibitory potential of nimotuzumab has been tested in vitro and in vivo. Nimotuzumab was found to exhibit a maximum antiproliferative activity of 40% in A431 cells (a human vulvar epidermoid cell line with a high level of EGFR expression) in vitro.Citation20 Analysis of the cell cycle profile of nimotuzumab-treated A431 cells revealed arrest in G1 phase accompanied by a decrease in the proportion of cells in S phase. This observation, together with the absence of an apparent hypodiploid peak (reflecting apoptotic cells), suggests that nimotuzumab acts principally as a cytostatic rather than cytotoxic agent in vitro. Furthermore, the growth of established subcutaneous A431 tumors in severe combined immunodeficient (SCID) mice was markedly reduced by nimotuzumab as a result of inhibition of both tumor cell proliferation and tumor angiogenesis.Citation20
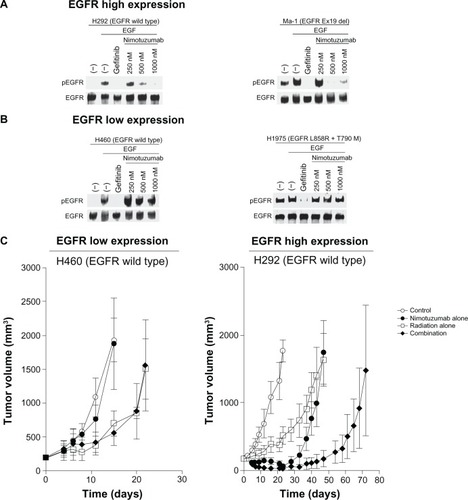
We examined the effects of nimotuzumab in combination with ionizing radiation on human NSCLC cell lines of differing EGFR status.Citation21 Nimotuzumab inhibited the EGF-induced or constitutive phosphorylation of EGFR in H292 and Ma-1 cells (), both of which manifest high levels of surface EGFR expression, consistent with the mode of action of this antibody. In contrast, nimotuzumab did not block EGF-induced or constitutive EGFR phosphorylation in H460 and H1975 cells (), both of which exhibit a low level of surface EGFR expression. Given that H292 and H460 cells both harbor wild-type EGFR alleles whereas Ma-1 and H1975 cells possess mutant EGFR alleles, these observations suggest that the inhibitory effect of nimotuzumab on EGFR signaling depends on the level of EGFR expression at the cell surface, regardless of EGFR mutation status. Consistent with our in vitro results, we found that nimotuzumab enhanced the antitumor effect of radiation on tumors formed by H292 or Ma-1 cells (both with high levels of surface EGFR expression) in nude mice (). Such enhancement was not apparent for tumors formed by H460 cells (low level of surface EGFR expression) (). These results thus suggest that EGFR mutation is not the major determining factor for enhancement of the antitumor effect of radiation by nimotuzumab, and that such enhancement depends on the tumor expression level of EGFR.
Figure 2 Effects of nimotuzumab on EGFR phosphorylation in NSCLC cells in vitro and on the response of NSCLC cells to radiation in vivo. (A and B) Effect of nimotuzumab on EGFR phosphorylation in NSCLC cells. H292 and Ma-1 cells (both of which express EGFR at a high level) (A) as well as H460 and H1975 cells (both of which express EGFR at a low level) (B) were deprived of serum overnight and then incubated first for 15 minutes in the absence or presence of gefitinib (10 µM) or the indicated concentrations of nimotuzumab and then for an additional 15 minutes in the additional absence or presence of EGF (100 ng/mL). Cell lysates were then subjected to immunoblot analysis with antibodies to the Tyr1068-phosphorylated form of EGFR (pEGFR) or to total EGFR. H292 and H460 cells possess wild-type EGFR alleles, whereas Ma-1 cells harbor a deletion in exon 19 (Ex19 del) of EGFR and H1975 cells harbor both L858R and T790M mutations in EGFR. (C) Effect of nimotuzumab on the response of NSCLC cells to radiation. H460 and H292 cells were injected subcutaneously into athymic nude mice. Treatment was initiated when tumors in each group achieved an average volume of ~170–200 mm3. Mice were treated with a single dose of nimotuzumab (1.0 mg per mouse) intraperitoneally, a single dose of γ-radiation (10 Gy), or neither (control) or both modalities, and tumor volume was determined at the indicated times thereafter.
Copyright (1997), with permission from Elsevier.
Abbreviations: EGFR, epidermal growth factor receptor; EGF, epidermal growth factor; NSCLC, non-small cell lung cancer.
The growth-inhibitory effects of nimotuzumab alone and in combination with radiation on xenografts formed by EGFR-expressing U87MG (human glioblastoma multiforme) cells in NMRI nude mice were also investigated.Citation28 Consistent with our results, coadministration of nimotuzumab increased the radiosensitivity of subcutaneous U87MG tumors, resulting in a significant delay in tumor growth. Furthermore, the addition of nimotuzumab to radiation reduced the size of tumors formed by U87MG cells in the brain to a greater extent than did radiation alone, and it inhibited by 40%–80% the increased tumor cell invasion provoked by radiotherapy as well as promoted tumor cell apoptosis. Whereas nimotuzumab also reduced the size of tumor blood vessels and the number of proliferating cells in subcutaneous tumors, cetuximab had no significant antiangiogenic or antiproliferative effects in this model. These results demonstrated the superiority of combined treatment with nimotuzumab and radiation vs radiation alone for glioblastoma multiforme.
Phase I studies of nimotuzumab as a single agent for treatment of solid tumors
The first Phase I trial for nimotuzumab enrolled twelve Cuban patients with advanced epithelial tumors (four ovarian adenocarcinomas as well as four breast, two lung, one stomach, and one renal carcinoma) and administered a one-time intravenous dose of 50, 100, 200, or 400 mg of nimotuzumab.Citation29 No serious adverse events were observed. Seven of the twelve participants developed mild or moderate adverse reactions, consisting mostly of tremors, fever, vomiting, nausea, dryness of mouth, asthenia, hypertension, and flushing. No acneiform rash or other dermatologic toxicity was detected. The area under the curve (AUC) and elimination half-life for the drug increased linearly with dose. Whereas plasma clearance of nimotuzumab decreased as the dose increased from 50 to 200 mg, it was similar at doses of 200 and 400 mg.
Another Phase I trial was performed to assess the pharmacodynamic effects of escalating weekly intravenous doses of nimotuzumab ranging from 100 to 800 mg administered alone in Caucasian patients with advanced solid cancer.Citation30 Tumor and skin biopsy specimens were collected before and 3 weeks after the onset of treatment for immunohistochemical assessment of the expression of EGFR and its downstream signaling molecules. Seventeen patients were enrolled, including one who was not treated with nimotuzumab; most of the patients had colon (n = 11) or rectal (n = 2) cancer. Although one dose-limiting-toxicity (DLT) was observed at the dose of 100 mg (fatigue of grade 3), dose escalation to 800 mg was not associated with any other DLT. No toxicity of grade 4 was observed, and only three patients developed an acneiform rash of grade 1 (18.7%). Among the 16 patients treated with nimotuzumab, one achieved a partial response (PR, 6.2%) and eight had stable disease (SD, 50.0%). The median time to progression (TTP) was 2.4 months. No substantial changes in EGFR, AKT, ERK, or Ki67 immunostaining were observed between the tumor or skin biopsy specimens obtained before treatment onset and those obtained after. Nimotuzumab has thus shown a favorable safety profile when administered as a single agent at doses up to 800 mg in patients with solid tumors.
A Phase I trial was also performed to evaluate the safety profile, maximum tolerated dose (MTD), and DLT for nimotuzumab in patients with advanced solid tumors in Japan.Citation31 Nimotuzumab was given intravenously at weekly doses of 100, 200, or 400 mg, with four patients enrolled at each dose level. The major adverse event was skin rash of grade 1 or 2 (58%, 7/12), and no toxicities of grade 3 or 4 or DLTs were observed. The MTD was thus not determined. Of the 12 patients evaluable for response, SD and progressive disease (PD) were observed in eight (67%) and four (33%) patients, respectively, with no objective responses being achieved. Median TTP was 4 months. Nimotuzumab was thus well tolerated at doses up to 400 mg weekly in Japanese patients with advanced solid tumors.
Collectively, these studies demonstrated that nimotuzumab can be administered at doses up to 800 mg per week with moderate toxicity. Importantly, characteristic adverse events seen with other EGFR-targeting antibodies, such as skin toxicities and hypomagnesemia, were not observed with nimotuzumab.
Nimotuzumab in combination with radiotherapy for NSCLC
Palliative radiotherapy for advanced NSCLC
Preclinical data have suggested that nimotuzumab enhances the antitumor activity of ionizing radiation.Citation21 It has also shown activity and been well tolerated when administered together with radiation in several clinical trials. A Phase I clinical trial of weekly nimotuzumab (100, 200, or 400 mg) in combination with external radiotherapy (30 or 36 Gy in 3-Gy fractions) was performed in Canadian patients diagnosed with stage IIB, III, or IV NSCLC unsuitable for curative therapy.Citation32 The most common adverse events were fatigue, anorexia, chills, pain, and hypophosphatemia (all of grades 1 or 2 in most patients). No severe skin or allergic toxicity was noted. Of 17 patients evaluable for response within the radiation field, twelve showed a PR and five had SD; thus, none had PD. Median progression-free survival (PFS) was 16 weeks, median overall survival (OS) was 60 weeks, and the 1-year survival rate was 52%. The study did not identify the MTD for this therapeutic combination of nimotuzumab and radiation.
A similarly designed Korean trial documented almost identical results.Citation33 Treatment consisted of nimotuzumab (100, 200, or 400 mg) administered weekly with palliative radiotherapy (30 to 36 Gy at 3 Gy/day). Of 15 patients evaluable for response within the radiation field, seven showed a PR and two had SD. Median TTP was 5.4 months, and median OS was 9.8 months. Although one patient developed a DLT (pneumonia of grade 4) at the nimotuzumab dose of 200 mg, the MTD was not reached at doses up to 400 mg. These two studies thus showed that combination therapy with nimotuzumab and radiation is well tolerated and feasible. A randomized Phase II study comparing nimotuzumab plus palliative radiation with palliative radiation alone is currently ongoing (Clinicaltrials.gov: NCT00369447).
Brain metastases occur in 30%–50% of patients with NSCLC and are associated with a worse prognosis and poorer quality of life. Historically, whole-brain radiation therapy (WBRT) alone was offered for management of brain metastases. A randomized Phase II trial comparing weekly nimotuzumab (200 mg) plus WBRT (40 Gy in 2-Gy fractions) with WBRT alone was performed in patients with advanced NSCLC and brain metastasis.Citation34 Disease control rate as a primary endpoint was 91.6% (eleven patients had SD, one showed PD) for the nimotuzumab-radiation arm, compared with 44.4% (four patients had SD, five showed PD) for the radiation group (P = 0.0039). A randomized, double-blind, Phase II study comparing WBRT (30 Gy, in 10 fractions) together with either weekly nimotuzumab (200 mg) or placebo in NSCLC patients with brain metastasis has a target enrollment of 80 patients and is being conducted internationally (Clinicaltrials.gov: NCT00872482). The primary efficacy endpoint is the difference in intracranial disease progression over 6 months.
Curative chemoradiation for locally advanced NSCLC
Inoperable NSCLC of stage III constitutes ~30% of all newly diagnosed cases of NSCLC, with the standard of care being combined-modality treatment with both platinum-based chemotherapy and thoracic radiation therapy (TRT).Citation35,Citation36 Recent clinical trials have shown that concurrent chemotherapy and radiotherapy (chemoradiation) yields outcomes superior to those of sequential therapy in such patients, with a 3-year survival rate of ~15%–20% and median OS of 15–20 months,Citation37–Citation39 but the efficacy of such regimens has reached a plateau.
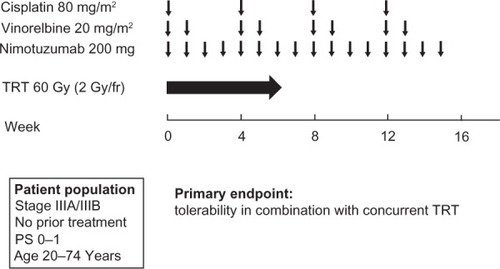
Given the preclinical data showing a synergistic effect of nimotuzumab and radiationCitation21 and the promising clinical results of nimotuzumab administration in combination with palliative radiotherapy,Citation32,Citation33 a Phase II study of nimotuzumab in combination with cisplatin, vinorelbine, and concurrent TRT was performed for stage III NSCLC in Japan (JapicCTI-090825).Citation40 Treatment consisted of a chemoradiotherapy phase with two cycles of cisplatin (80 mg/m2), vinorelbine (20 mg/m2), and nimotuzumab (200 mg) followed by a consolidation phase with two cycles of these drug regimens (). The primary endpoint was tolerability of the drug therapy in combination with concurrent TRT, which was determined from the percentage of patients who completed 60 Gy of radiotherapy within 8 weeks, completed two cycles of chemotherapy, and received more than 75% of the planned total dose of nimotuzumab. Forty patients, the target sample size, were enrolled. Thirty-four patients (87%) met the criteria for treatment tolerability, and 38 patients (97%) completed 60 Gy of radiotherapy within 8 weeks. Infusion reactions, a skin rash of grade ≥3, radiation pneumonitis of grade ≥3, and nonhematologic toxicities of grade 4 were not observed. For the preliminary efficacy analysis, 37 patients were evaluable with appropriate radiotherapy planning. The response rate was 70%, and the median PFS was 11.1 months. The combination of nimotuzumab with platinum-based chemotherapy and concurrent TRT is thus feasible and shows promising activity for locally advanced NSCLC.
Figure 3 Treatment scheme for the chemoradiotherapy phase and consolidation phase of a Phase II study of nimotuzumab in combination with cisplatin, vinorelbine, and concurrent thoracic radiotherapy (TRT) in patients with stage III NSCLC.Citation40
Nimotuzumab in combination with chemotherapy for advanced NSCLC
Platinum-based doublets with third-generation agents are considered the standard first-line treatment for advanced NSCLC. However, recent advances in cancer cell biology have provided a basis for the development of specific molecularly targeted therapeutic agents, including antibodies to the extracellular domain of EGFR, for the treatment of patients with advanced NSCLC.
The anti-EGFR mAb cetuximab prolonged survival in patients with advanced NSCLC when combined with platinum-based chemotherapy in the FLEX Phase III trial.Citation16 A supportive study, BMS099, did not achieve its primary PFS endpoint, although an improvement in overall response rate and a favorable OS trend were observed when cetuximab was added to chemotherapy.Citation41 A meta-analysis evaluated the effect of adding cetuximab to chemotherapy for first-line treatment of advanced NSCLC. The pooled HR for OS (0.87; P = 0.004) was in favor of cetuximab plus chemotherapy, which also gave rise to a higher overall response rate (relative risk [RR]: 1.19; P = 0.013). However, the analysis failed to show the benefit of cetuximab plus chemotherapy for PFS (HR: 0.91; P = 0.06) or 1-year survival rate (RR: 1.10; P = 0.172).Citation42
A Phase I study of nimotuzumab in combination with cisplatin and docetaxel in chemotherapy-naïve patients with advanced NSCLC has begun recruitment of patients (Clinicaltrials.gov: NCT00985998). Patients will receive nimotuzumab at doses of 200, 400, or 600 mg on days 1, 8, and 15 plus cisplatin (75 mg/m2) and docetaxel (75 mg/m2) on day 1 of a 3-week cycle for up to six cycles. The primary endpoint is safety. In the second-line therapy setting, a randomized Phase II trial evaluating the efficacy of docetaxel with or without nimotuzumab in advanced NSCLC is ongoing (NCT 00983047). Docetaxel is administered at a dose of 75 mg/m2 on day 1 of a 3-week cycle for up to six cycles, whereas nimotuzumab at a dose of 200 mg per week is administered during chemotherapy and is continued after its completion until the detection of disease progression or unacceptable toxicity. The total sample size will be 52 eligible patients in each treatment arm, for a total of 104 eligible patients. The primary endpoint is OS.
Predictive biomarkers
Molecular predictors of response
Many surrogate markers, including molecular markers and physical signs, for the selection of patients who are likely to benefit from molecularly targeted therapy, including treatment with mAbs to EGFR, have been investigated. Whereas the presence of an EGFR mutation is the strongest predictor of sensitivity to EGFR-TKIs,Citation6–Citation8 the relation between EGFR mutations and the response to anti-EGFR mAbs remains unclear. A preclinical study examined the effects of gefitinib and cetuximab on NSCLC cell lines with mutant EGFR alleles in vitro but failed to reveal a significant association between EGFR mutation status and specific outcome of cetuximab treatment.Citation43 In the FLEX trial, EGFR mutation status was determined in 293 of 1125 patients, with 45 patients (15.4%) being found to harbor an EGFR-activating mutation.Citation44 The presence of such an EGFR mutation was associated with better survival in patients treated with chemotherapy with or without cetuximab but was not predictive of benefit derived from the addition of cetuximab. EGFR mutations were thus of prognostic value in this patient population. Similar findings were obtained in the BMS099 trial, in which EGFR-activating mutations were found in 17 (10%) of 167 patients but were not predictive of benefit for the addition of cetuximab to chemotherapy.Citation45 In a preclinical study, we found that nimotuzumab enhanced the antitumor efficacy of radiation in certain human NSCLC cell lines harboring either wild-type or mutant EGFR,Citation21 consistent with the notion that EGFR mutation is not the major determining factor for enhancement of the antitumor effect of radiation by nimotuzumab. Together, these various data indicate that the presence of an EGFR mutation is not a prerequisite for a response to anti-EGFR mAbs.
No clear association has emerged between the level of EGFR expression measured by immunohistochemical analysis and the response to the anti-EGFR mAbs cetuximab or panitumumab in metastatic colorectal cancer. Conflicting results regarding the relation between EGFR expression and outcome in NSCLC patients treated with mAbs to EGFR have been reported. Analysis of EGFR expression levels in tumors of patients participating in the FLEX study showed that, among those with a high level of EGFR expression, the response rate in the cetuximab-plus-chemotherapy arm was 44.4% compared with 28.1% in the chemotherapy-alone arm (P = 0.002). Among patients with a low level of EGFR expression, there was no difference in response rate between the two treatment arms (32.6% for cetuximab plus chemotherapy, vs 29.6% for chemotherapy alone).Citation17 With regard to nimotuzumab, we have shown that enhancement of the growth-inhibitory effect of radiation by nimotuzumab in NSCLC cell lines depends on the expression level of EGFR both in vitro and in a xenograft model.Citation19 Analysis of tumor samples from patients with locally advanced head and neck cancer treated with radiotherapy plus nimotuzumab did not detect an association between EGFR expression and treatment outcome, although this finding may have been due to the small number of patients.Citation20 EGFR expression was also evaluated immunohistochemically in ten NSCLC specimens in a Phase I trial of nimotuzumab plus palliative radiation therapy, but again there was no statistically significant association between EGFR expression and treatment response or outcome because of the limited sample size.Citation30 No definitive conclusions can thus be drawn at this time concerning the predictive utility of immunohistochemically determined EGFR expression level for the treatment of NSCLC patients with anti-EGFR mAbs, and further studies are therefore warranted.
Clinical predictors of response
Patients treated with EGFR inhibitors frequently develop a rash characterized by inflammatory papules and pustules on the scalp, face, neck, and upper trunk. To evaluate the association between rash and cetuximab efficacy in patients with NSCLC, a subgroup analysis of patients from the FLEX study was performed. Patients in the chemotherapy plus cetuximab group with first-cycle rash had significantly prolonged OS compared with patients in the same treatment group without first-cycle rash (median 15.0 months vs 8.8 months; P < 0.0001).Citation46 OS for patients without first-cycle rash was similar to that of patients who received chemotherapy alone (median 8.8 months vs 10.3 months; P = 0.36). Rash may therefore be a surrogate marker of target inhibition and activity of cetuximab-based chemotherapies.
In contrast, these side effects are rare in cases of nimotuzumab use and are mainly limited to grade 1 or 2 adverse events.Citation25 Most conspicuously, the typical grade 3 or 4 skin toxicity found in other anti-EGFR drugs has thus far remained virtually absent in nimotuzumab trials.Citation22 Therefore, in contrast to the other EGFR mAbs, attempts at finding clinical predictive markers specifically for nimotuzumab have to date not been forthcoming.
Conclusions
There is a need to improve the outcome in NSCLC. Nimotuzumab is an EGFR-targeting mAb that has the potential for use as a sensitizer to radio- or chemotherapy in patients with NSCLC positive for EGFR expression. The key feature of clinical experience with nimotuzumab to date is that it does not induce the skin toxicities of grade 3 or 4 that are common to other anti-EGFR mAbs. If nimotuzumab is shown to be clinically beneficial in ongoing and upcoming Phase II and III trials, its benign side effect profile will make it an attractive therapeutic option compared with other anti-EGFR mAbs. Given that previous studies have not identified predictive molecular markers for nimotuzumab treatment, mostly as a result of a small sample size, further studies to establish such markers are also needed.
Disclosure
The authors declare no conflicts of interest in this work.
References
- HoffmanPCMauerAMVokesEELung cancerLancet2000355920247948510841143
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- BaselgaJWhy the epidermal growth factor receptor? The rationale for cancer therapyOncologist20027Suppl 42812202782
- GschwindAFischerOMUllrichAThe discovery of receptor tyrosine kinases: targets for cancer therapyNat Rev Cancer20044536137015122207
- BaselgaJArteagaCLCritical update and emerging trends in epidermal growth factor receptor targeting in cancerJ Clin Oncol200523112445245915753456
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaezJGJannePALeeJCEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience200430456761497150015118125
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- HannaNLilenbaumRAnsariRPhase II trial of cetuximab in patients with previously treated non-small-cell lung cancerJ Clin Oncol200624335253525817114658
- RobertFBlumenscheinGHerbstRSPhase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non-small-cell lung cancerJ Clin Oncol200523369089909616301597
- ThieneltCDBunnPAJrHannaNMulticenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancerJ Clin Oncol200523348786879316246975
- BorghaeiHLangerCJMillensonMPhase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non-small cell lung cancer (NSCLC): results of OPN-017J Thorac Oncol20083111286129218978564
- BelaniCPSchreederMTSteisRGCetuximab in combination with carboplatin and docetaxel for patients with metastatic or advanced-stage nonsmall cell lung cancer: a multicenter phase 2 studyCancer200811392512251718816622
- SocinskiMASalehMNTrentDFA randomized, phase II trial of two dose schedules of carboplatin/paclitaxel/cetuximab in stage IIIB/IV non-small-cell lung cancer (NSCLC)Ann Oncol20092061068107319188136
- RosellRRobinetGSzczesnaARandomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancerAnn Oncol200819236236917947225
- PirkerRPereiraJRSzczesnaACetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trialLancet200937396741525153119410716
- O’ByrneKEberhardtWEEStörkelSUse of a continuous EGFR IHC scoring method reveals association between tumor response and EGFR expression levels for patients receiving CT + cetuximab versus CT alone in the phase III trial FLEX in advanced NSCLCJ Thorac Oncol20105Suppl 7S558S559
- BlumenscheinGRJrPaulusRCurranWJPhase II study of cetuximab in combination with chemoradiation in patients with stage iiia/b non-small-cell lung cancer: RTOG 0324J Clin Oncol201129172312231821555682
- MateoCMorenoEAmourKLombarderoJHarrisWPerezRHumanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activityImmunotechnology19973171819154469
- Crombet-RamosTRakJPerezRViloria-PetitAAntiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibodyInt J Cancer2002101656757512237899
- AkashiYOkamotoIIwasaTEnhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor statusBr J Cancer200898474975518253126
- CrombetTOsorioMCruzTUse of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patientsJ Clin Oncol20042291646165415117987
- RodriguezMORiveroTCdel Castillo BahiRNimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neckCancer Biol Ther20109534334920448462
- ArteagaMELedonNCasacoASystemic and skin toxicity in Cercopithecus aethiops sabaeus monkeys treated during 26 weeks with a high intravenous dose of the anti-epidermal growth factor receptor monoclonal antibody NimotuzumabCancer Biol Ther2007691390139517827980
- BolandWKBebbGNimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicityExpert Opin Biol Ther2009991199120619624281
- TalaveraAFriemannRGomez-PuertaSNimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformationCancer Res200969145851585919584289
- GarridoGTikhomirovIARabasaABivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profileCancer Biol Ther201111437338221150278
- Diaz MiqueliARolffJLemmMFichtnerIPerezRMonteroERadiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodiesBr J Cancer2009100695095819293809
- CrombetTTorresLNeningerEPharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancerJ Immunother200326213914812616105
- YouBBradeAMagalhaesJMA dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignanciesInvest New Drugs2011295996100320454832
- BokuNYamazakiKYamamotoNPhase I study of nimotuzumab, a humanized anti-epidermal growth factor receptor (EGFR) IgG1 monoclonal antibody in patients with solid tumors in Japan [abstract]J Clin Oncol200927Suppl 15se14574
- BebbGSmithCRorkeSPhase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapyCancer Chemother Pharmacol201167483784520563810
- ChoiHJSohnJHLeeCGA phase I study of nimotuzumab in combination with radiotherapy in stages IIB–IV non-small cell lung cancer unsuitable for radical therapy: Korean resultsLung Cancer2011711555920451284
- MaciasANeningerESantiestebanEPreliminary results of a phase II clinical trial of the anti EGFR monoclonal antibody Nimotuzumab in combination with whole brain radiation therapy in patients diagnosed with advanced non-small cell lung cancer tumors unresectable brain metastases [abstract]Proceedings of the 20th EORTC-NCI-AACR Annual MeetingOctober 21–24, 2008Geneva, Switzerland2008505
- DillmanROSeagrenSLPropertKJA randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancerN Engl J Med1990323149409452169587
- SauseWKolesarPTaylorSIFinal results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology GroupChest2000117235836410669675
- FuruseKFukuokaMKawaharaMPhase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancerJ Clin Oncol19991792692269910561343
- CurranWScottCLangerCLongterm benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410 [abstract]Proc Am Soc Clin Oncol2003222499
- YamamotoNNakagawaKNishimuraYPhase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105J Clin Oncol201028233739374520625120
- TanakaKOkamotoIYamamotoNPhase 2 study of nimotuzumab in combination with concurrent chemoradiotherapy (CRT) in patients with locally advanced non-small cell lung cancer (NSCLC) [abstract]Proceedings of the European Multidisciplinary Cancer CongressSeptember 23–27, 2011Stockholm, Sweden abstract 9.044
- LynchTJPatelTDreisbachLCetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099J Clin Oncol201028691191720100966
- LinHJiangJLiangXZhouXHuangRChemotherapy with cetuximab or chemotherapy alone for untreated advanced non-small-cell lung cancer: a systematic review and meta-analysisLung Cancer2010701576220149474
- MukoharaTEngelmanJAHannaNHDifferential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutationsJ Natl Cancer Inst200597161185119416106023
- GatzemeierUPaz-AresLRodrigues PereiraJvon PawelJRamlauRRohJMolecular and clinical biomarkers of cetuximab efficacy: data from the phase III FLEX study in non-small cell lung cancer (NSCLC) [abstract]J Thorac Oncol20094Suppl 1S324
- Khambata-FordSHarbisonCTHartLLAnalysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancerJ Clin Oncol201028691892720100958
- GatzemeierUvon PawelJVynnychenkoIFirst-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 studyLancet Oncol2011121303721169060