Abstract
The objective of this study was to understand outcomes of patients treated with ALK inhibitors, especially when ALK inhibitors are followed by other ALK inhibitors. A systematic literature review was conducted in PubMed, Embase, and Cochrane through July 17, 2017. Conference abstracts (three meetings in past 2 years) also were searched. Of 504 unique publications, 80 met inclusion criteria (47 clinical trials, 33 observational studies). Observational studies have the potential to provide information for ALK inhibitors used sequentially. Ten observational studies reported median overall survival of crizotinib-led sequences ranging from 30.3 to 63.75 months from initiation of crizotinib; 49.4–89.6 months from metastatic non-small-cell lung cancer diagnosis; and 15.5–22.0 months from initiation of the second-generation ALK inhibitor after initial crizotinib. Sequencing of ALK inhibitors may benefit patients progressing on initial ALK inhibitors.
Introduction
ALK is a member of the insulin receptor superfamily,Citation1 and oncogenic EML4-ALK fusion variants represent molecular targets in non-small-cell lung cancer (NSCLC). ALK fusions have been identified in both squamous and adenocarcinoma histologic subtypes, with a higher frequency observed in adenocarcinoma.Citation2,Citation3 Overall, ALK fusions occur in 3%–5% of patients with metastatic NSCLC.Citation4
Prior to 2011, when the first ALK tyrosine kinase inhibitor was approved, the standard of care for patients with ALK-positive NSCLC was chemotherapy, and outcomes were poor, with median overall survival (OS) of ~12 months.Citation5,Citation6 Crizotinib was approved by the United States Food and Drug Administration (FDA) under accelerated approval in 2011 and was the first ALK inhibitor approved for patients with ALK-positive advanced NSCLC.Citation7
Although patients with ALK-positive advanced NSCLC initially respond to ALK inhibitors, resistance eventually often develops in these patients.Citation8 One of the mechanisms of acquired resistance is a mutation in the kinase domain of ALK, although other resistance mechanisms have also been reported, such as activation of alternative pathways (EGFR, KIT, and IGF-IR), ALK amplification, and epithelial–mesenchymal transition.Citation9 In some patients, the mechanism of acquired resistance remains unknown.Citation9
To address resistance, additional ALK inhibitors have been introduced. Ceritinib was approved by the FDA in April 2014Citation10 for the treatment of patients with ALK-positive metastatic NSCLC who have progressed on or are intolerant to crizotinib, and in May 2017 it received approval for expanded use to include first-line treatment.Citation11 Subsequently, alectinib received FDA approval in December 2015 for the treatment of patients with ALK-positive metastatic NSCLC who have progressed on or are intolerant to crizotinibCitation12,Citation13 and in November 2017 for first-line treatment.Citation14 Brigatinib received FDA approval in April 2017 for the treatment of patients with ALK-positive metastatic NSCLC who have progressed on or are intolerant to crizotinib.Citation15
The current standard of care for treating ALK-positive NSCLC is the use of ALK inhibitors. Multiple available ALK inhibitors allow the possibility of sequencing these agents to extend patient benefit and improve outcomes. The available ALK inhibitors have different potencies, differential penetration into the central nervous system, unique safety profiles, and different “spectrums” of activity against particular acquired resistance mutations.
Outcomes of ALK inhibitors are well documented in controlled clinical trials; however, less is known about the outcomes associated with sequencing. We hypothesized that sequencing of ALK inhibitors will benefit survival outcomes of patients. Herein, we report the first systematic literature review with an aim to understand the outcomes of patients treated with ALK inhibitors, especially when an ALK inhibitor is followed by another ALK inhibitor.
Material and methods
Electronic literature searches were conducted in PubMed, Embase, and the Cochrane Library databases through July 17, 2017 for real-world and clinical trial evidence for drug sequencing/treatment patterns and the related outcomes associated with the use of ALK inhibitors. Additional studies not published in the peer-reviewed literature were identified by searching online conference abstracts of three professional societies for the previous 2 calendar years: the American Society of Clinical Oncology (2016 and 2017), the European Society of Medical Oncology (2015 and 2016), and the International Association for the Study of Lung Cancer World Conference on Lung Cancer (2015 and 2016). The electronic database searches were also supplemented by a review of the bibliographic reference lists of relevant literature review articles.
The search terms for the medical library databases included Medical Subject Heading, Emtree, and free-text terms, including disease terms (carcinoma, non-small-cell lung; non-small-cell lung cancer; non-small-cell lung carcinoma; non-small-cell lung cancer), terms to identify drug sequencing/treatment patterns (practice pattern, prescribing pattern, treatment pattern), terms to identify the agents of interest (crizotinib, Xalkori, PF-02341066, ceritinib, Zykadia, LDK378, alectinib, Alecensa, CH5424802, brigatinib, AP26113, ALK inhibitor), various terms to identify study types and outcomes of interest, and terms to identify observational studies and clinical trials (Table S1). The search was limited to English-language studies of humans and had no date limit.
Two independent reviewers screened the titles and abstracts according to predefined inclusion and exclusion criteria (Table S2). Full-text articles of selected records were obtained, and the two independent reviewers further screened each article according to the same predefined inclusion and exclusion criteria. Data extraction by a single researcher included study design, patient characteristics, line/sequence of therapy, and outcomes, including treatment duration, response rates, median OS, and median progression-free survival (PFS). A separate researcher conducted quality control of data extraction.
Results
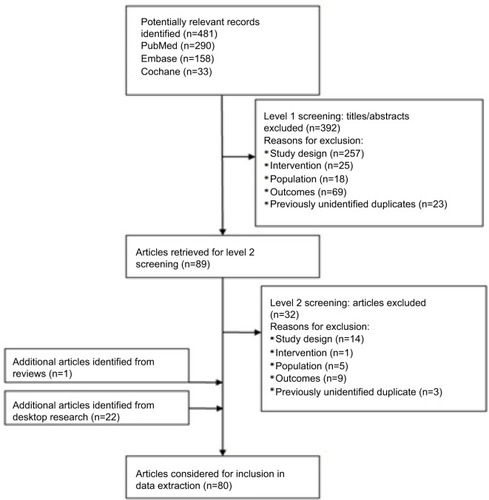
The electronic literature database search identified 481 unique records. One additional article was identified following a review of the bibliographic reference lists of relevant literature review articles. Twenty-two additional abstracts were identified from the search of professional societies and associated conferences. Of the 504 unique articles/abstracts identified, 80 publications met the inclusion criteria (). Of the 80 publications, 47 were from clinical trials and 33 were from observational studies. Studies were heterogeneous regarding study design, data source, sample size, timeframe of observation, and outcomes collected, including PFS and OS. A detailed overview of the PFS and OS outcomes in the observational studies of ALK inhibitors used after an initial ALK inhibitor is shown in and , respectively. The online supplement provides a list of the 80 publications included (Table S3).
Table 1 Results of observational studies of an ALK inhibitor after initial ALK inhibitor – median PFS
Table 2 Results of observational studies of an ALK inhibitor after initial ALK inhibitor – median OS
Evidence base of first use of an ALK inhibitor with or without prior chemotherapy (ALK inhibitor naïve)
A total of 45 publications assessed outcomes of first use of an ALK inhibitor with or without prior chemotherapy in patients who were ALK inhibitor naïve. Of the 45 publications, 27 were from clinical trialsCitation16–Citation42 and 18 were from observational studies.Citation43–Citation60 In clinical trials, median PFS ranged from 7.7 monthsCitation30 to 25.9 months,Citation19 median OS ranged from 20.3 monthsCitation30 to 39.1 months,Citation20 and objective response rate (ORR) ranged from 46%Citation27 to 100%.Citation36 In the observational studies, median PFS ranged from ~7 months (reported as 28 weeks)Citation51 to 17.7 months (from diagnosis of advanced NSCLC)Citation58 and median OS ranged from 11.2 monthsCitation54 to ~104 months (reported as 416 weeks);Citation47 note that data from Nosaki et alCitation47 were presented in a conference abstract and thus not all data may have been included.
Evidence base of use of second or subsequent ALK inhibitor (ALK inhibitor followed by another ALK inhibitor)
A total of 38 publications assessed outcomes of use of an ALK inhibitor after an initial ALK inhibitor. Of the 38 publications, 25 were from clinical trialsCitation35–Citation37,Citation40–Citation42,Citation61–Citation79 and 13 were from observational studies.Citation44–Citation48,Citation50,Citation54,Citation57,Citation80–Citation84 All 38 publications reported on use of a second-generation ALK inhibitor after initial crizotinib therapy; one publication of an observational study also included a population that used a second-generation ALK inhibitor after an initial second-generation ALK inhibitor (ceritinib after initial alectinib),Citation47 and one publication of an observational study mentioned two patients who received crizotinib after alectinib.Citation84 The efficacy data reported in clinical trials are from initiation of the second-generation ALK inhibitor only; as noted, all sequences were of a second-generation ALK inhibitor after initial crizotinib therapy. In the 25 publications from clinical trials, median PFS from initiation of the second-generation ALK inhibitor ranged from 5.4 monthsCitation77 to 15.6 months,Citation70 median OS ranged from 14.9 monthsCitation75 to 26.0 months,Citation61,Citation65 and ORR ranged from 33.0%Citation42 to 80.0%;Citation36 estimated 12-month survival rates ranged from 63.8%Citation75 to 83.0%.Citation36 Only seven clinical trial publications reported treatment duration of the second-generation ALK inhibitor, which ranged from 8.1 weeksCitation76 to 38.6 weeks.Citation37
Of the 13 publications from observational studies, there was a heterogeneous makeup in study design, population, and the method in which results were reported. Combined PFS of a crizotinib-led sequence was reported in four publicationsCitation45,Citation46,Citation82,Citation84 and ranged from 17.0 monthsCitation45,Citation82 to 35.2 months.Citation46 In general, median combined PFS was defined as the sum of PFS of the two ALK inhibitors and did not include the interval from discontinuation of crizotinib to initiation of the second-generation ALK inhibitor or postprogression use of crizotinib. In some instances, patients may have been allowed to receive chemotherapy in the interval between use of the two ALK inhibitors. Five observational study publicationsCitation44–Citation46,Citation82,Citation84 of crizotinib-led sequences reported PFS of crizotinib ranging from 6.1 monthsCitation84 to 10.7 months.Citation46 Seven publicationsCitation44–Citation46,Citation48,Citation80,Citation82,Citation84 reported PFS from initiation of the second-generation ALK inhibitor ranging from 4.6 monthsCitation48 to 24.7 months.Citation44
Ten publications from observational studiesCitation44,Citation45,Citation47,Citation48, Citation50,Citation54,Citation80,Citation82–Citation84 of crizotinib-led sequences reported median OS. Of these ten publications, four reported median OS for the sequence as not reached.Citation44,Citation50,Citation54,Citation83 Median follow-up for the sequence was reported as follows: not reported,Citation83 21.3 months,Citation44 21.4 months,Citation50 and 44.4 months.Citation54 Four publicationsCitation45,Citation47,Citation82,Citation84 reported median OS from initiation of crizotinib (ie, for the full ALK sequence) ranging from 30.3 monthsCitation82 to ~64 months (reported as 255 weeks).Citation47 The 64 months reported by Nosaki et alCitation47 was in patients receiving alectinib or ceritinib after initial crizotinib; however, this publication was a conference abstract and thus limited data are reported. Four publicationsCitation48,Citation54,Citation82,Citation84 reported median OS from diagnosis of metastatic disease ranging from 49.4 monthsCitation82 to 89.6 months.Citation54 The 89.6 months reported by Duruisseaux et alCitation54 were in 84 patients who received a second-generation ALK inhibitor at some point after progressing on initial crizotinib. Three publicationsCitation45,Citation48,Citation80 reported median OS of the second-generation ALK inhibitor after crizotinib ranging from 15.5 monthsCitation80 to 22.0 months.Citation45 One publication of a second-generation ALK inhibitor after an initial second-generation ALK inhibitor (ceritinib after initial alectinib) reported median OS for the sequence as not reached; median duration of follow-up was not reported.Citation47
Survival rates were reported in five publications from observational studies.Citation46,Citation48,Citation50,Citation54,Citation80 Twelve-month survival rates in crizotinib-led sequences were reported as 59.9%Citation50 and 92.9%Citation54 from first dose of crizotinib.
Specific sequences
Ceritinib after initial crizotinib
When reviewing specific sequences, 12 publications described results of ceritinib after initial crizotinib; 8 were from clinical trials,Citation37,Citation40–Citation42,Citation75–Citation77,Citation79 and 4 were from observational studies.Citation48,Citation80–Citation82 The clinical trials reported results for only the ceritinib portion of the sequence. Median PFS ranged from 5.4 monthsCitation77 to 6.9 months,Citation37,Citation41 median OS ranged from 14.9 monthsCitation75 to 20.0 months,Citation79 and ORR ranged from 33% to 63%.Citation42
In the four observational study publications, combined median PFS was reported in only one publication and was reported as 17.4 months.Citation82 Combined PFS for sequential treatment with crizotinib and ceritinib did not include postprogression use of crizotinib or the interval between crizotinib discontinuation and start of ceritinib, in which patients could have received cytotoxic chemotherapy.Citation82 In patients in which crizotinib was discontinued and ceritinib immediately initiated (ie, no intervening treatment), median combined PFS was 17.0 months.Citation82 Median PFS while patients were on ceritinib ranged from 7.8 monthsCitation82 to 12.9 months.Citation80 Only one study reported median PFS while patients were on crizotinib, which was reported as 8.2 months.Citation82
Finally, median OS was reported as 30.3 months from initiation of crizotinib,Citation82 15.5 monthsCitation80 and 20.4 monthsCitation48 from initiation of ceritinib, and 49.4 monthsCitation82 and 51.0 monthsCitation48 from diagnosis of metastatic disease.
Alectinib after initial crizotinib
A total of 15 publications described results of alectinib after initial crizotinib; 11 were from clinical trials,Citation35,Citation61–Citation69,Citation78 and 4 were from observational studies.Citation44,Citation46,Citation57,Citation84 The clinical trials reported results for just the alectinib portion of the sequence. Median PFS ranged from 8.0 monthsCitation64 to 13.9 months,Citation78 median OS was reported as 22.7 monthsCitation64 and 26.0 months,Citation61,Citation65 and ORR ranged from 44.0%Citation62 to 72.2%.Citation35 The estimated 12-month survival rate was 71.0%.Citation63
Of the four observational studies, two publications reported combined median PFS as 18.2 monthsCitation84 and 35.2 months.Citation46 Asao et alCitation46 defined combined PFS as the sum of the PFS of the two ALK inhibitors without the interval between the ALK inhibitors, ie, treatment duration with cytotoxic chemotherapy between the two ALK inhibitors was excluded. In Watanabe et al,Citation84 combined PFS did not include postprogression use of crizotinib or the interval from discontinuation of crizotinib to initiation of alectinib. Median PFS from initiation of alectinib ranged from 15.2 monthsCitation84 to 24.7 months.Citation44 Median PFS while patients received crizotinib ranged from 6.1 monthsCitation84 to 10 months.Citation45
Median OS was reported to be not reachedCitation44 (median follow-up of 21.3 months) and 48.6 monthsCitation84 from initiation of crizotinib and 51.1 monthsCitation84 from diagnosis of metastatic disease. Estimated 12-month survival was reported to be 38.6% in patients on crizotinib and 60.0% in those on ceritinib;Citation46 the estimated 5-year survival for the sequence was 77.8%.Citation46
Brigatinib after initial crizotinib
Six publications described results of brigatinib after initial crizotinib, all of them clinical trials.Citation36,Citation70–Citation74 There were no observational studies found in the literature that assessed brigatinib after initial crizotinib. Median PFS from initiation of brigatinib ranged from 8.8 monthsCitation70 to 15.6 months,Citation70 and ORR ranged from 45%Citation70,Citation73 to 80%.Citation36 Median OS was not reported in any of the six publications. Estimated 12-month survival rates ranged from 71%Citation73 to 83%.Citation36
Any second-generation ALK inhibitor after initial crizotinib
Five publications from observational studies described outcomes of a second-generation ALK inhibitor after initial crizotinib.Citation45,Citation47,Citation50,Citation54,Citation83 In these publications, either the second-generation ALK inhibitor was not specified or the results were combined for more than one ALK inhibitor. All five publications were from observational studies. Median PFS was reported in only one publication and was 17 months combined for the sequence, 10 months for crizotinib, and 7 months from initiation of the second ALK inhibitor (alectinib or ceritinib).Citation45 Median OS was 40.0 monthsCitation45 and ~64 monthsCitation47 for the sequence, 22 months from initiation of the second ALK inhibitor,Citation45 and 89.6 months from diagnosis of metastatic disease.Citation54 Two publications reported median OS as not reached for the specific sequence being studiedCitation54,Citation83 (median follow-up not reported in Roeper et alCitation83 and 44.4 months in Duruisseaux et alCitation54). Estimated 12-month survival was reported to be 59.9% from the start of a sequence of ceritinib, alectinib, or brigatinib after initial crizotinibCitation50 and 92.9% from the start of a sequence of ceritinib or alectinib after initial crizotinib.Citation54
Ceritinib after initial alectinib
Nosaki et alCitation47 reported median OS as not being reached in patients who received ceritinib after initial alectinib; median follow-up time was not reported. Note these data were reported from a conference abstract with limited information.
Discussion
In this systematic literature review, we aimed to understand the outcomes of patients treated with ALK inhibitors, especially when an ALK inhibitor is followed by another ALK inhibitor.
The identified clinical trials of patients who were ALK inhibitor naïve reported median PFS ranging from 7.7 to 25.9 months and median OS ranging from 20.3 to 39.1 months. Observational studies reported median PFS ranging from 7 to 17.7 months and median OS ranging from 11.2 to 104 months.
In clinical trials of a second-generation ALK inhibitor used after initial crizotinib, median PFS from initiation of the second-generation ALK inhibitor ranged from 5.4 to 15.6 months and median OS ranged from 14.9 to 26.0 months. In observational studies of an ALK inhibitor followed by another ALK inhibitor, median PFS ranged from 4.6 to 35.2 months and median OS ranged from 15.5 to 89.6 months.
In sequencing observational studies of an ALK inhibitor used after an initial ALK inhibitor, median OS from initiation of the first ALK inhibitor, ie, for the ALK sequence, has varied and has been reported as 30.3 months,Citation82 40 months,Citation45 48.6 months,Citation84 and ~64 months.Citation47 Median OS has been consistently reported to be ~50 months from time of diagnosis of metastatic disease in several observational studies of ALK inhibitors used in sequence,Citation48,Citation82,Citation84 indicating that sequential use of ALK inhibitors may be clinically beneficial to patients. There are currently several examples of median OS being reported as “not reached” in studies of the full sequence of an ALK inhibitor after an initial ALK inhibitor. This is not surprising given that only relatively recently multiple ALK inhibitors became available. As sequential ALK inhibitors are utilized and survival data mature, we expect that additional outcomes data will become available to help inform treatment decisions for improved outcomes of patients with ALK-positive NSCLC. Important to note is that lorlatinib, a third generation ALK inhibitor, recently became available in the USA. Lorlatinib is indicated for the treatment of patients with ALK-positive metastatic NSCLC whose disease has progressed on crizotinib and at least one other ALK inhibitor; or alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease,Citation85 which further supports the sequencing of ALK inhibitors. Approval was based on a phase 2 study in which lorlatinib demonstrated substantial overall and intracranial response both in treatment-naive patients with ALK-positive NSCLC, and in those who had progressed on crizotinib, second-generation ALK inhibitors, or after up to three previous ALK inhibitors.Citation86
The evidence base is broader and more mature for crizotinib-led sequences than for second-generation-led sequences. The amount of research of crizotinib-led sequences is not unexpected given that crizotinib was the first ALK inhibitor on the market. Additional research is needed to understand the survival outcomes of second-generation ALK inhibitors as initial therapy.
This study adds to the current literature in that it is the first systematic review of sequencing of ALK inhibitors. A robust methodology was used that included a study protocol, multiple broad electronic databases searching for both clinical trials and observational studies, and that did not limit by date. In addition, two reviewers independently screened all titles, abstracts, and full-text articles using predefined inclusion and exclusion criteria. This robust methodology enables the reproducibility of the review.
It is important to note in the interpretation of retrospective studies reporting median OS or “combined” PFS for a sequence that immortal time bias must be considered. In studies of sequential therapy conducted retrospectively, patients who do not survive to receive the second treatment are not included in the analysis. Patients who received both ALK inhibitors are selected for having lived long enough and for having stable enough disease (in some instances related in part to chemotherapy after the first ALK inhibitor) that they were able to receive both ALK inhibitors in sequence. Therefore, the observed value for combined PFS and OS reported in these studies is likely to be biased upward from what may be expected at the outset for patients treated according to such a sequential treatment plan; however, as no prospectively designed studies have evaluated this question to date, retrospective studies are currently the best available evidence.
Another important consideration in interpreting the findings from this systematic review is that the cutoff date of July 17, 2017 did not allow for inclusion of the final OS data from PROFILE 1014, which is the first long-term study with mature OS data for an ALK-positive NSCLC population. Results showed that median OS was not reached for crizotinib and was 47.5 months for chemotherapy (median follow-up was ~46 months in each treatment arm).Citation87 Most patients (84.2%) receiving chemotherapy crossed over to crizotinib; therefore, a crossover-adjusted analysis was conducted demonstrating OS in the crizotinib arm to be significantly longer than the chemotherapy arm (HR, 0.346; 95% CI, 0.081–0.718).Citation87 At 4 years, 56.6% of crizotinib patients and 49.1% of chemotherapy patients were still alive.Citation87 Interestingly, these results were consistent with the OS data of around 50 months from observational studies of ALK inhibitors that we identified in this systematic literature review.
Owing to the data immaturity and currently available trial designs, it is not currently possible to determine which sequence confers the best long-term outcomes.
Conclusion
Subsequent use of ALK inhibitors may clinically benefit patients progressing on an initial ALK inhibitor. Crizotinib-led sequences have a broader evidence base and more mature clinical outcomes than second-generation-led sequences. No evidence was found directly comparing different ALK inhibitor sequences. Further research is warranted to directly compare ALK inhibitor sequences and to understand the outcomes of second-generation ALK inhibitors as initial ALK inhibitor therapy.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was funded by Pfizer, Inc. The abstract of this paper was presented at the International Society for Phar-macoeconomics and Outcomes Research (ISPOR) 23rd Annual International Meeting; May 19–23, 2018, as a poster presentation with interim findings. The poster’s abstract is published online at https://tools.ispor.org/ScientificPresen-tationsDatabase/Presentation/81967?pdfid=54840. In addition, the ISPOR abstract was published in Value in Health 2018;21(S1):S49–S50.
Disclosure
Elizabeth T Masters, Marc Chioda, Robin Wiltshire, and Knut Martin Torgersen are employees of Pfizer, Inc. RTI Health Solutions received funding from Pfizer to conduct this research and for manuscript development; Stephanie M Barrows, Kelly Wright, Catherine Copley-Merriman, and James A Kaye are employees of RTI Health Solutions. The authors report no other conflicts of interest in this work.
References
- MorrisSWNaeveCMathewPALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK)Oncogene19971418217521889174053
- InamuraKTakeuchiKTogashiYEML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onsetMod Pathol200922450851519234440
- WongDWLeungELSoKKThe EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRASCancer200911581723173319170230
- GerberDEMinnaJDALK inhibition for non-small cell lung cancer: from discovery to therapy in record timeCancer Cell201018654855121156280
- LeeJKParkHSKimDWComparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancerCancer2012118143579358622086654
- ShawATYeapBYSolomonBJImpact of crizotinib on survival in patients with advanced, ALK-positive NSCLC compared with Historical controlsJ Clin Oncol20112915_suppl7507
- FDANDA 202570 Accelerated ApprovalUS Food and Drug Administration2011 Available from: https://www.accessdata.fda.gov/drug-satfda_docs/appletter/2011/202570s000ltr.pdfAccessed November 16, 2017
- KatayamaRShawATKhanTMMechanisms of acquired crizotinib resistance in ALK-rearranged lung cancersSci Transl Med20124120120ra17
- LiaoBCLinCCShihJYYangJCTreating patients with ALK-positive non-small cell lung cancer: latest evidence and management strategyTher Adv Med Oncol20157527429026327925
- FDANDA 205755 Approval LetterUS Food and Drug Administration2014 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/205755Orig1s000ltr.pdfAccessed December 22, 2017
- FDANDA 205755/S-09 Supplement Approval Fulfillment of Postmarketing RequirementUS Food and Drug Administration2017 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/205755Orig1s009ltr.pdfAccessed March 7, 2018
- CDERApproval Package for Application Number 208434Orig1s000Center for Drug Evaluation and Research2015 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000Approv.pdfAccessed March 7, 2018
- EMAAlecensa (Alectinib) Authorization DetailsEuropean Medicines Agency2017 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004164/human_med_002068.jsp&mid=WC0b01ac058001d124Accessed March 7, 2018
- FDANDA 208434/S-03 Supplement Approval Fulfillment of Postmarketing RequirementUS Food and Drug Administration2017 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/208434Orig1s003ltr.pdfAccessed March 7, 2018
- Brigatinib Prescribing Information (United States)Cambridge, MAARIAD Pharmaceuticals, Inc.42017 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208772lbl.pdfAccessed December 19, 2017
- TamuraTKiuraKSetoTThree-year follow-up of an alectinib phase I/II study in ALK-positive non-small-cell lung cancer: AF-001JPJ Clin Oncol201735141515152128296581
- HidaTNokiharaHKondoMAlectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trialLancet201739010089293928501140
- PetersSCamidgeDRShawATAlectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancerN Engl J Med2017377982983828586279
- TakiguchiYHidaTNokiharaHUpdated efficacy and safety of the J-ALEX study comparing alectinib (ALC) with crizotinib (CRZ) in ALK-inhibitor naïve ALK fusion positive non-small cell lung cancer (ALK+ NSCLC)Paper presented at: Annual Meeting of the American Society of Clinical Oncology2017Chicago, IL
- TanDFelipEChowLQCeritinib as first-line therapy in patients with ALK-rearranged non-small cell lung cancer: ASCEND-1 subgroup analysis17th World Conference on Lung CancerVienna, Austria2016
- FelipEOrlovSParkKPhase 2 study of ceritinib in ALKi-naïve patients (PTS) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC): whole body responses in the overall Pt group and in PTS with baseline brain metastases (BM)Ann Oncol201627suppl_612080
- de CastroGTanDS-WCrinòLPL03.07: first-line ceritinib versus chemotherapy in patients with ALK-rearranged (ALK+) NSCLC: a randomized, phase 3 study (ASCEND-4)J Thorac Oncol2017121S7
- SoriaJCTanDSWChiariRFirst-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 studyLancet20173891007291792928126333
- CamidgeDRBangYJKwakELActivity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 studyLancet Oncol201213101011101922954507
- KimDWAhnMJShiYResults of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC)J Clin Oncol201230Suppl Abstract 7533
- ShawATJannePABesseBCrizotinib vs chemotherapy in ALK + advanced non-small cell lung cancer (NSCLC): final survival results from PROFILE 1007J Clin Oncol20163415_suppl9066
- CostaDBShawATOuSHClinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastasesJ Clin Oncol201533171881188825624436
- NishioMKimDWWuYLCrizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancerCancer Res Treat201850369170028701030
- LeiYYYangJJZhongWZClinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastasesJ Thorac Dis2015771181118826380734
- ShawATKimDWNakagawaKCrizotinib versus chemotherapy in advanced ALK-positive lung cancerN Engl J Med2013368252385239423724913
- SolomonBJCappuzzoFFelipEIntracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014J Clin Oncol201634242858286527022118
- SolomonBJMokTKimDWFirst-line crizotinib versus chemotherapy in ALK-positive lung cancerN Engl J Med2014371232167217725470694
- ShawATYeapBYSolomonBJEffect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysisLancet Oncol201112111004101221933749
- KwakELBangYJCamidgeDRAnaplastic lymphoma kinase inhibition in non-small-cell lung cancerN Engl J Med2010363181693170320979469
- IwamaEGotoYMurakamiHAlectinib for patients with ALK rearrangement-positive non-small cell lung cancer and a poor performance status (Lung Oncology Group in Kyushu 1401)J Thorac Oncol20171271161116628238961
- GettingerSNBazhenovaLALangerCJActivity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trialLancet Oncol201617121683169627836716
- KimDWMehraRTanDSWActivity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trialLancet Oncol201617445246326973324
- FelipETanDS-WKimD-WWhole body and intracranial efficacy of ceritinib in ALK-inhibitor (ALKi)-naive patients (pts) with ALK-rearranged (ALK+) NSCLC and baseline (BL) brain metastases (BM): Results from ASCEND-1 and -3J Clin Oncol20163415_supple20520
- TanDSAraújoAZhangJComparative efficacy of ceritinib and crizotinib as initial ALK-Targeted therapies in previously treated advanced NSCLC: an adjusted comparison with external controlsJ Thorac Oncol20161191550155727288979
- NishioMMurakamiHHoriikeAPhase I study of ceritinib (LDK378) in Japanese patients with advanced, anaplastic lymphoma kinase-rearranged non-small-cell lung cancer or other tumorsJ Thorac Oncol20151071058106626020125
- ShawATKimDWMehraRCeritinib in ALK-rearranged non-small-cell lung cancerN Engl J Med2014370131189119724670165
- FelipEde BraudFGMaurMCeritinib plus nivolumab (NIVO) in patients (pts) with anaplastic lymphoma kinase positive (ALK+) advanced non-small cell lung cancer (NSCLC)Paper Presented at: Annual Meeting of the American Society of Clinical Oncology2017Chicago, IL
- MurakamiHOnoANakashimaKLong-term clinical outcomes of ALK inhibitors in patients with ALK-positive advanced non-small cell lung cancerJ Clin Oncol20173515_supple20542
- ItoKHatajiOKobayashiHSequential therapy with crizotinib and alectinib in ALK-rearranged non-small cell lung cancer – a multi-center retrospective studyJ Thorac Oncol201712239039627498387
- ChiariRMetroGIaconoDClinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: results of a multicenter analysisLung Cancer201590225526026395848
- AsaoTFujiwaraYItahashiKSequential use of anaplastic lymphoma kinase inhibitors in Japanese patients with ALK-rearranged non-small-cell lung cancer: a retrospective analysisClin Lung Cancer2017184e251e25828065466
- NosakiKToyozawaRTaguchiKReal-world data on treatment patterns and survival among ALK+ NSCLC patients in JapanJ Clin Oncol20173515_supple20505
- KayaniyilSHurryMWilsonJTreatment patterns and survival in patients with ALK-positive non-small-cell lung cancer: a Canadian retrospective studyCurr Oncol2016236589e597
- BergeEMLuXMaxsonDClinical benefit from pemetrexed before and after crizotinib exposure and from crizotinib before and after pemetrexed exposure in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancerClin Lung Cancer201314663664323931899
- CadranelJParkKArrietaOCharacteristics, treatment patterns, and survival among ALK+ non-small cell lung cancer (NSCLC) patients treated with crizotinib: a chart review studyLung Cancer20169891427393500
- CaoYXiaoGQiuXYeSLinTEfficacy and safety of crizotinib among Chinese EML4-ALK-positive, advanced-stage non-small cell lung cancer patientsPLoS One2014912e11400825501361
- CuiSZhaoYDongLIs there a progression-free survival benefit of first-line crizotinib versus standard chemotherapy and second-line crizotinib in ALK-positive advanced lung adenocarcinoma? A retrospective study of Chinese patientsCancer Med2016561013102126880708
- CuiSZhaoYGuAEfficacy and tolerability of crizotinib in the treatment of ALK-positive, advanced non-small cell lung cancer in Chinese patientsMed Oncol201532662625966792
- DuruisseauxMBesseBCadranelJOverall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective studyOncotarget2017813219032191728423535
- LimSHYohKALeeJSCharacteristics and outcomes of ALK+ non-small cell lung cancer patients in KoreaAsia Pac J Clin Oncol2017
- NoronhaVRamaswamyAPatilVMALK positive lung cancer: clinical profile, practice and outcomes in a developing countryPLoS One2016119e016075227637025
- YoshidaTOyaYTanakaKClinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancerLung Cancer201697434727237026
- XingPWangSHaoXZhangTLiJClinical data from the real world: efficacy of crizotinib in Chinese patients with advanced ALK-rearranged non-small cell lung cancer and brain metastasesOncotarget2016751846668467427835868
- ZhangQQinNWangJCrizotinib versus platinum-based double-agent chemotherapy as the first line treatment in advanced anaplastic lymphoma kinase-positive lung adenocarcinomaThorac Cancer2016713826816533
- GuérinASasaneMWakeleeHTreatment, overall survival, and costs in patients with ALK-positive non-small-cell lung cancer after crizotinib monotherapyCurr Med Res Opin20153181587159726029864
- BarlesiFDingemansA-MCYangJC-HUpdated efficacy and safety from the global phase II NP28673 study of alectinib in patients (PTS) with previously treated ALK+ non-small-cell lung cancer (NSCLC)Ann Oncol201627suppl_6
- OuSHAhnJSde PetrisLAlectinib in Crizotinib-Refractory ALK-rearranged non-small-cell lung cancer: a phase II global studyJ Clin Oncol201634766166826598747
- ShawATGandhiLGadgeelSAlectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multi-centre, phase 2 trialLancet Oncol201617223424226708155
- CamidgeDRSmGOuSUpdated efficacy and safety data from the phase 2 NP28761 study of alectinib in ALK-positive non-small-cell lung cancer (now available)17th World Conference on Lung CancerVienna, Austria2016
- YangJCSiOde PetrisLPooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancerJ Thorac Oncol2017
- GandhiLOuSIShawATEfficacy of alectinib in central nervous system metastases in crizotinib-resistant ALK-positive non-small-cell lung cancer: comparison of RECIST 1.1 and RANO-HGG criteriaEur J Cancer201782273328646771
- GandhiLGadgeelSShawATime to response in patients with ALK+ NSCLC receiving alectinib in the phase II NP28673 and NP28761 studiesAnn Oncol201627suppl_61209PD
- GadgeelSMShawATGovindanRPooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancerJ Clin Oncol201634344079408527863201
- GadgeelSMGandhiLRielyGJSafety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 studyLancet Oncol201415101119112825153538
- AhnM-JCamidgeDRTiseoMBrigatinib (BRG) in crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): updates from ALTA, a pivotal randomized phase 2 trialPaper presented at: Annual Meeting of the American Society of Clinical Oncology;2017Chicago, IL
- CamidgeDRBazhenovaLASalgiaRAssessment of brigatinib (AP26113) CNS activity in patients (Pts) with ALK+ NSCLC and intracranial metastases in a phase 1/2 study18th European Cancer CongressVienna, Austria2015
- GettingerSNKimDTiseoMBrigatinib activity in patients with ALK+ NSCLC and intracranial CNS metastases in two clinical trials17th World Conference on Lung CancerVienna, Austria2016
- KimD-WTiseoMAhnM-JBrigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trialJ Clin Oncol201735222490249828475456
- OuSHTiseoMCamidgeDRBrigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC) and brain metastases in the pivotal randomized phase 2 ALTA trialPaper presented at: Annual Meeting of the American Society of Clinical Oncology2017Chicago, IL
- CrinòLAhnMJde MarinisFMulticenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2J Clin Oncol201634242866287327432917
- HidaTSatouchiMNakagawaKCeritinib in patients with advanced, crizotinib-treated, anaplastic lymphoma kinase-rearranged NSCLC: Japanese subsetJpn J Clin Oncol20171727989996
- ShawATKimTMCrinòLCeritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trialLancet Oncol201718787488628602779
- HidaTNakagawaKSetoTPharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non-small-cell lung cancer with or without prior crizotinib therapyCancer Sci2016107111642164627566263
- LiuGZhangJZhouZYLiJCaiXSignorovitchJAssociation between time to progression and subsequent survival in ceritinib-treated patients with advanced ALK-positive non-small-cell lung cancerCurr Med Res Opin201632111911191827488695
- BendalyEDalalAACulverKTreatment patterns and early outcomes of ALK-positive non-small cell lung cancer patients receiving ceritinib: a chart review studyAdv Ther20173451145115628405961
- BendalyEDalalAACulverKMonitoring for and characterizing crizotinib progression: a chart review of ALK-positive non-small cell lung cancer patientsAdv Ther20173471673168528578501
- GainorJFTanDSde PasTProgression-free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinibClin Cancer Res201521122745275225724526
- RoeperJNetchaevaMLueersACImpact on OS of 2nd and 3rd generation TKI in EGFR mt+ and ALK+ patients: Results of the NOWEL networkJ Clin Oncol20173515_supple20560
- WatanabeSHayashiHOkamotoKProgression-free and overall survival of patients with ALK rearrangement-positive non-small cell lung cancer treated sequentially with crizotinib and alectinibClin Lung Cancer201617652853427318655
- LORBRENA (lorlatinib)U.S. Prescribing InformationNew York, NYPfizer Inc2018
- SolomonBJBesseBBauerTMLorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 studyLancet Oncol201819121654166730413378
- MokTSKKimD-WWuY-LOverall survival (OS) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from PROFILE 1014Paper presented at: Annual Meeting of the European Society for Medical Oncology2017Madrid, Spain