Abstract
Exploring resistance mechanisms in patients with EGFR-mutant non-small-cell lung cancer (NSCLC) upon disease progression on EGFR tyrosine kinase inhibitors (TKIs) has been an area of great interest as it may lead to effective next-line treatment strategies. Here we report a case of emergent MET amplification detected in a tumor sample from a patient with NSCLC harboring EGFR L858R mutation after disease progression on erlotinib. The patient subsequently had a sustained partial response to a combination of full-dose osimertinib and crizotinib with excellent tolerance but eventually had central nervous system (CNS) progression. Comprehensive genomic profiling performed on the resected brain sample continued to demonstrate MET amplification as an acquired resistance mechanism. A review of literature shows several groups have utilized similar combination regimens (erlotinib or osimertinib + crizotinib or cabozantinib), albeit with various dosing to target MET alterations in patients with EGFR-mutant NSCLC. As more actionable resistance mechanisms are identified, we envision combination TKI therapy will be readily adopted in clinical practice. Our case report adds to a growing body of evidence that combination osimertinib and crizotinib should be recommended to EGFR-mutant NSCLC patients with emergent MET amplification as acquired resistance. More importantly, as crizotinib has limited brain penetration, developing next-generation MET inhibitors with better CNS activity is urgently needed.
Introduction
Patients with EGFR-mutant non-small-cell lung cancer (NSCLC) derive great benefit from treatment with EGFR tyrosine kinase inhibitors (TKIs) but the response is often not long-lasting. Exploring resistance mechanisms has been an area of hot pursuit. In addition to the most common EGFR T790M mutation, other mechanisms of acquired resistance include MET amplification (5%–20%), HER2 amplification (5%–13%), EGFR amplification (8%), BRAF mutations (1%), and so on.Citation1 Crizotinib, a small molecule TKI against ALK, ROS1, and MET, has recently been granted a breakthrough therapy designation by the US Food and Drug Administration for patients with metastatic NSCLC harboring MET exon 14 skipping alterations after platinum-based chemotherapy. For patients with MET-amplified NSCLC, as well as other tumor types, crizotinib has also been reported to be an effective therapy, demonstrating that MET amplification is indeed an actionable genomic alteration. Here we report a case of emergent MET amplification detected in a tumor sample from a patient with NSCLC harboring EGFR L858R mutation after disease progression on erlotinib. The patient subsequently had a sustained partial response (PR) to a combination of full-dose osimertinib and crizotinib with excellent tolerance but eventually had central nervous system (CNS) progression highlighting the need to develop next-generation MET inhibitors with better CNS activity.
Case presentation
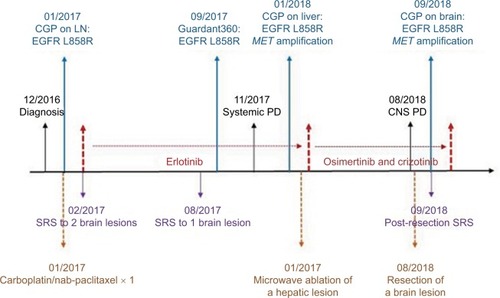
This is a 55-year-old African–American male never-smoker who was diagnosed with stage IV NSCLC in December 2016 after presenting with self-palpable lymph node in the neck. His disease burden involved a dominant right apex lung mass, bilateral lung nodules, extensive lymphadenopathy, multiple liver lesions, one single bony lesion in the T6 vertebral body, and two brain lesions. He first received one cycle of carboplatin/nab-paclitaxel in January 2017. When comprehensive genomic profiling (CGP) on his lymph node biopsy specimen revealed EGFR L858R mutation and equivocal EGFR amplification (7 copies) by Foundation Medicine (Cambridge, MA, USA), his treatment was switched to erlotinib in early February 2017 (). In the interim, he also received stereotactic radiation to two brain lesions. The patient had a PR to erlotinib, but in July 2017 was found to have a new brain lesion for which he received additional stereotactic radiation while being continued on erlotinib. His scan in September 2017 was concerning for mild disease progression so liquid biopsy was obtained via Guardant360 (Guardant Health, Redwood City, CA, USA) which showed EGFR L858R without T790M mutation. A follow-up scan in November 2017 confirmed disease progression in his lung and liver. He then underwent biopsy of a liver mass and CGP revealed EGFR L858R, EGFR amplification (17 copies), MET amplification (12 copies), among other non-actionable genomic alterations (; ).
Figure 1 Copy number plot for the liver biopsy sample tested before combination therapy of osimertinib and crizotinib.
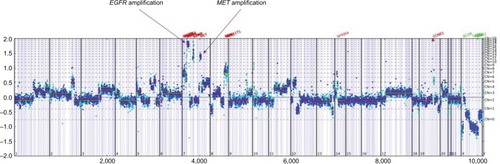
Table 1 List of genomic alterations detected from three different metastatic sites over the clinical course
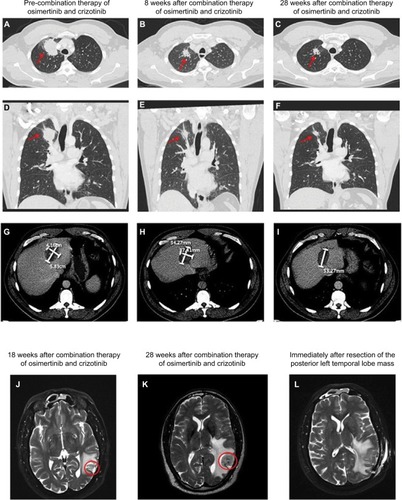
The patient subsequently transferred care to us. He had successful microwave ablation of a hepatic lesion at the end of January 2018. For his systemic treatment, erlotinib was switched to a combination of osimertinib and crizotinib in early February 2018 in an attempt to target both EGFR and MET alterations. We started the patient on 80 mg daily osimertinib and 250 mg daily crizotinib with close monitoring for toxicity. The main reason to choose osimertinib over erlotinib in the absence of T790M mutation is better tolerability of osimertinib particularly when it is combined with another TKI. Ten days later, crizotinib dose was titrated up to 250 mg twice daily given excellent tolerance. Restaging scans around 8 weeks showed interval significant decrease in size of the right apex lung mass and slight decrease in size of a hepatic mass. He had a sustained PR as follow-up scans ~20 weeks later showed further improvement of target lesions (−32% by RECIST 1.1) (). However, despite good extracranial response after 28 weeks of combination therapy, the patient experienced CNS progression with increase in size of a pre-existing lesion () and development of three new punctate lesions. He underwent resection of the enlarging brain metastasis () and CGP performed on this tumor specimen again identified EGFR L858R, EGFR amplification (42 copies), and MET amplification (35 copies) without revealing additional mechanisms of resistance (). Following resection, the patient received stereotactic radiation to the surgical bed and other untreated brain lesions. He remained on full-dose osimertinib and crizotinib without any toxicity. At the time of this manuscript submission, his combination therapy has been ongoing for 35 weeks. The schematic summary of treatment course is shown in . The patient has provided a written informed consent to have the case details published, and institutional approval is not required to publish this case report.
Figure 2 CT images showing the right apex lung mass before (A & D), 8 weeks after (B & E), and 28 weeks after (C & F) combination therapy of osimertinib and crizotinib. The red arrows indicate the right apex lung mass seen on the axial view (A–C) as well as the coronal view (D–F). CT images showing a hepatic mass before (G), 8 weeks after (H), and 28 weeks after (I) combination therapy of osimertinib and crizotinib. This patient also received microwave ablation of this hepatic mass prior to initiation of the combination therapy. MRI images showing the posterior left temporal lobe mass 18 weeks after (J) and 28 weeks after (K) combination therapy of osimertinib and crizotinib. The red circles clearly indicate disease progression at 28 weeks as demonstrated by the interval increase in size of the mass. Of note, this mass had previously received stereotactic radiation. The patient subsequently underwent total resection of the mass (L).
Discussion
MET amplification has been well documented to confer acquired resistance in patients with EGFR-mutant NSCLC after progression on various EGFR TKIs. Crizotinib has known activity against MET amplification. In fact, we have previously reported a case of high MET amplification (30 copies) in a patient who progressed on osimertinib and then responded to crizotinib symptomatically.Citation2 Other agents, such as cabozantinib, capmatinib, emibetuzumab, glesatinib, merestinib, telisotuzumab vedotin, tepotinib, and savolitinib, can also target MET amplification. It is worth mentioning that the cutoff for MET amplification varies by detection method. Using fluorescent in situ hybridization, a MET/CEP7 (centromeric enumeration probe for chromosome 7) ratio ≥2 defines high-level amplification, whereas CGP reports gene copy number per cell ≥6 as amplification.Citation3 We have previously investigated focal (<20 megabase pair) vs non-focal MET amplification in 545 NSCLC cases and found that focal MET amplification was associated with higher MET copy number (median 11 vs 7 copies) and lower co-occurrence of other driver mutations.Citation4 Whether or not this can serve as a prognostic or predictive biomarker for patients receiving anti-MET targeted therapies has yet to be elucidated.
The use of combination TKI therapy to dual-target primary and resistance genomic alterations when patients progress on targeted therapies is emerging in clinical practice. For instance, Scheffler et al successfully treated an NSCLC patient with erlotinib at 100 mg daily in combination with crizotinib at 250 mg twice daily when rebiopsy showed high-level MET amplification (MET/CEP7 ratio: 8.3) emerging in the setting of EGFR L858R mutation. The patient had a dramatic response to this combination therapy.Citation5 Gainor et al reported a NSCLC case with EGFR L858R mutation and de novo high-level MET amplification (MET/CEP7 ratio: >15) that was primarily resistant to erlotinib but had an excellent response to the combination of erlotinib at 100 mg daily and crizotinib at 250 mg daily. The lower than standard doses were chosen given concern for increased exposure of erlotinib by crizotinib, and this patient tolerated the treatment well with only grade 1 rash and diarrhea.Citation6 Li et al also used the same combination therapy to target pre-existing EGFR L858R mutation and secondary MET amplification in a patient who had progressed on erlotinib. While this patient also had an excellent response, the combination erlotinib at 150 mg daily and crizotinib at 250 mg daily resulted in severe vomiting and rash.Citation7 Osimertinib is now moving to the front-line setting for metastatic NSCLC patients with activating EGFR mutations based on the FLAURA trial showing its superior efficacy and tolerability (lower rates of serious adverse events) to gefitinib or erlotinib. The combination of osimertinib and MET inhibitors has proven to be safe and efficacious. This was first demonstrated by York et al in the management of an African American NSCLC patient with emergent MET amplification (MET/CEP7 ratio: 3.67) after EGFR T790M mutation. The patient received 80 mg daily osimertinib and 200 mg twice daily crizotinib at first with repeat scans after four months of therapy showing significant improvement. Notably, when serum carcinoembryonic antigen (CEA) started to rise, the dose of crizotinib was increased to 250 mg twice daily resulting in reduction of the CEA level. The most notable toxicity was grade 2 fatigue.Citation8 Kang et al reported a case of acquired MET amplification with pre-existing EGFR L858R and T790M mutations that achieved a PR to full-dose osimertinib and crizotinib after becoming resistant to gefitinib and then osimertinib. This patient subsequently developed multiple MET secondary-site mutations and responded symptomatically when crizotinib was switched to cabozantinib at 80 mg daily.Citation9 Most recently, Deng et al treated a Hispanic male patient with a combination of osimertinib at 80 mg daily and crizotinib at 250 mg daily in the setting of severe hepatic dysfunction when liquid biopsy revealed MET amplification (5.3 copies) in addition to three pre-existing EGFR mutations including EGFR L858R, R776C, and T790M. The dose of crizotinib had to be reduced to 200 mg daily due to grade 3 neutropenia, nausea, and lower extremity edema. This combination therapy had provided clinical benefit for 6 months until the patient experienced disease progression with rising MET copy number to 22.4 copies.Citation10
While our patient derived good extrancranial response to full-dose osimertinib and crizotinib without experiencing any toxicity, he eventually progressed in the brain 28 weeks into combination therapy. Interestingly, CGP performed on the resected brain specimen did not reveal additional resistance mechanisms suggesting MET amplification likely continued to contribute to acquired resistance. This is not entirely surprising as crizotinib, a type Ia MET inhibitor, is well known to have poor brain penetration. Switching to a different MET inhibitor with potentially better CNS activity, such as cabozantinib, a type II MET inhibitor, can be considered should the patient develop further CNS progression.Citation11,Citation12
Conclusion
As more actionable resistance mechanisms are identified in EGFR-mutant NSCLC patients, we envision combination TKI therapy will be readily adopted in clinical practice. Understanding the safety profile of each agent and drug interactions will be essential to provide effective yet tolerable combination therapy. Notably, we have successfully treated a patient with full-dose osimertinib and alectinib when liquid biopsy detected a novel in-frame PLEKHA7-ALK fusion after the patient had developed resistance to sequential erlotinib, afatinib, and osimertinib.Citation13 Taken together, our case report adds to a growing body of evidence that combination of osimertinib and crizotinib should be recommended to EGFR-mutant NSCLC patients with emergent MET amplification as acquired resistance. More importantly, developing next-generation MET inhibitors with better CNS activity is urgently needed.
Disclosure
VWZ has received honoraria from AstraZeneca, Roche-Foundation Medicine, Roche/Genentech, and Takeda, and consulting fees from TP Therapeutics. ABS and SMA are employees of Foundation Medicine, Inc., a wholly owned subsidiary of Roche. SIO has received honoraria from Astra-Zeneca, Pfizer, Roche-Foundation Medicine, Roche/Genentech and Takeda, and has stock ownership in TP Therapeutics. The authors report no other conflicts of interest in this work.
References
- FeniziaFDe LucaAPasqualeREGFR mutations in lung cancer: from tissue testing to liquid biopsyFuture Oncol201511111611162326043215
- OuSIAgarwalNAliSMHigh MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progressionLung Cancer201698596127393507
- SchildhausHUSchultheisAMRüschoffJMET amplification status in therapy-naïve adeno-and squamous cell carcinomas of the lungClin Cancer Res201521490791525492085
- OuSHPavlickDStephensPJGemonic analysis of non-small cell lung cancer (NSCLC) cases with focal and non-focal MET amplificationAbstract OA 12.08, IASLC 18th World Conference on Lung Cancer2017
- SchefflerMMerkelbach-BruseSBosMSpatial tumor heterogeneity in lung cancer with acquired epidermal growth factor receptor-tyrosine kinase inhibitor resistance: targeting high-level MET-amplification and EGFR T790M mutation occurring at different sites in the same patientJ Thorac Oncol2015106e40e4326001148
- GainorJFNiederstMJLennerzJKDramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-mutant lung cancer harboring de novo MET amplificationJ Thorac Oncol2016117e83e8526988570
- LiYQSongSSJiangSHZhangXYCombination therapy of erlotinib/crizotinib in a lung adenocarcinoma patient with primary EGFR mutation plus secondary MET amplification and a novel acquired crizotinib-resistant mutation MET G1108CAnn Oncol201728102622262428961830
- YorkERVarella-GarciaMBangTJAisnerDLCamidgeDRTolerable and effective combination of full-dose crizotinib and osimertinib targeting MET amplification sequentially emerging after T790M positivity in EGFR-mutant non-small cell lung cancerJ Thorac Oncol2017127e85e8828274743
- KangJChenH-JWangZOsimertinib and cabozantinib combinatorial therapy in an EGFR-mutant lung adenocarcinoma patient with multiple MET secondary-site mutations after resistance to crizotinibJ Thorac Oncol2018134e49e5329128427
- DengLKiedrowskiLARaveraEChengHHalmosBResponse to dual crizotinib and osimertinib treatment in a lung cancer patient with MET amplification detected by liquid biopsy who acquired secondary resistance to EGFR tyrosine kinase inhibitionJ Thorac Oncol2018139e169e17230166014
- ReungwetwattanaTLiangYZhuVOuS-HIThe race to target Met exon 14 skipping alterations in non-small cell lung cancer: the why, the how, the WHO, the unknown, and the inevitableLung Cancer2017103273728024693
- KlempnerSJBorgheiAHakimianBAliSMOuS-HIIntracranial activity of cabozantinib in MET exon 14-positive NSCLC with brain metastasesJ Thorac Oncol201712115215627693535
- SchrockABZhuVWHsiehWSReceptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitorsJ Thorac Oncol20181391312132329883838