Abstract
Arginine deprivation has gained increasing traction as a novel and safe antimetabolite strategy for the treatment of several hard-to-treat cancers characterised by a critical dependency on arginine. Small cell lung cancer (SCLC) displays marked arginine auxotrophy due to inactivation of the rate-limiting enzyme argininosuccinate synthetase 1 (ASS1), and as a consequence may be targeted with pegylated arginine deiminase or ADI-PEG20 (pegargiminase) and human recombinant pegylated arginases (rhArgPEG, BCT-100 and pegzilarginase). Although preclinical studies reveal that ASS1-deficient SCLC cell lines are highly sensitive to arginine-degrading enzymes, there is a clear disconnect with the clinic with minimal activity seen to date that may be due in part to patient selection. Recent studies have explored resistance mechanisms to arginine depletion focusing on tumor adaptation, such as ASS1 re-expression and autophagy, stromal cell inputs including macrophage infiltration, and tumor heterogeneity. Here, we explore how arginine deprivation may be combined strategically with novel agents to improve SCLC management by modulating resistance and increasing the efficacy of existing agents. Moreover, recent work has identified an intriguing role for targeting arginine in combination with PD-1/PD-L1 immune checkpoint inhibitors and clinical trials are in progress. Thus, future studies of arginine-depleting agents with chemoimmunotherapy, the current standard of care for SCLC, may lead to enhanced disease control and much needed improvements in long-term survival for patients.
Introduction
Small cell lung cancer (SCLC) is a notoriously lethal subtype of smoking-related thoracic cancer, accounting for 10–15% of global lung cancer incidence with a 5-year survival rate of less than 7%.Citation1 Treatments for SCLC have advanced slowly in the last few decades with much scope for improvement. Harnessing fundamental and novel biological insights will be critical to transforming the SCLC therapeutic landscape.
Derived from neuroendocrine precursor cells, SCLC is characterised by rapid tumor growth, genomic instability and early metastatic spread underlying the poor survival outcomes.Citation2,Citation3 On a molecular level, in addition to universal TP53 and RB1 inactivation, SCLC is classified into four distinct subtypes according to the expression of ASCL1 (SCLC-A), NEUROD1 (SCLC-N), POU2F3 (SCLC-P) or YAP1 (SCLC-Y).Citation4,Citation5 This molecular reappraisal of SCLC offers the possibility of novel personalised therapies. Inducing arginine deprivation is a promising therapeutic strategy for multiple cancer types including SCLC that are deficient in the arginine biosynthetic enzyme argininosuccinate synthetase 1 (ASS1). In the current review, we provide an update on arginine deprivation as a specific antimetabolite therapy focusing on SCLC both as monotherapy and in combination with selected therapies based on emerging preclinical and clinical data.
Critical Use of Arginine in Healthy and Cancerous Cells
Background
Arginine (2-amino-5-guanidinovaleric acid) is a semi-essential amino acid in mammalian cells.Citation6 As one of the 20 most common amino acids in humans, it is a highly versatile molecule serving as the precursor of key molecules such as urea, nitric oxide, proline, polyamines or proteins.Citation7 Additionally, arginine can trigger secretion of molecules such as growth hormones, insulin or glucagon.Citation8 This metabolite is a core component of the urea cycle, the only known pathway in humans capable of recycling and eliminating the nitrogen-containing molecule ammonia.Citation9,Citation10
Arginine biosynthesis takes place within the urea cycle from the conversion of ornithine to citrulline in the mitochondria catalysed by the ornithine transcarbamylase (OTC) enzyme.Citation11 Citrulline can also be sourced from glutamine through a series of sequential intestinal enzymatic reactions which release the metabolite to the blood circulation.Citation12 Citrulline and aspartate undergo condensation to argininosuccinate by the ATP-dependent enzyme ASS1, the rate-limiting enzyme of the urea cycle. The last step is the conversion of argininosuccinate into arginine and fumarate, a member of the TCA cycle, by argininosuccinate lyase (ASL).Citation8 Arginine biosynthesis accounts for only 10–15% of whole-body arginine production, implying that cells rely primarily on the transport of extracellular arginine and therefore the cell microenvironment to maintain arginine levels.Citation13
ASS1 Modulation in Cancer
ASS1 inactivation confers arginine auxotrophy in cancer cells and thereby susceptibility to arginine-depleting agents.Citation14 Loss of ASS1 and sensitivity to arginine deprivation has been observed in numerous cancers including melanoma, hepatocellular carcinoma (HCC), malignant pleural mesothelioma, glioblastoma, sarcomas and haematological cancers.Citation15–19 Other malignancies, such as ovarian and colorectal cancer express high levels of ASS1 compared to corresponding normal tissues.Citation20 In SCLC, Kelly et al identified that 45% of tumor samples and 50% of cell lines were ASS1 negative highlighting the importance of arginine biosynthesis downregulation for this malignancy.Citation21 The mechanisms leading to altered ASS1 expression differ. Transcription factors such as c-Myc and HIF-1a interacting with an E-box located at the ASS1 gene promoter are known to modulate the expression of ASS.Citation22 ASS1 expression is also under epigenetic regulation with promoter methylation first being identified in mesothelioma cell lines and primary tumors as a driver of ASS1 deficiency.Citation16,Citation23 Moreover, ADI-PEG20 treatment induced ASS1 re-expression in an MPM cell line linked to decreased methylation of the ASS1 promoter.Citation24 Although the exact mechanism of ASS1 expression in SCLC is yet to be fully understood, Chalishazar et al showed that MYC directly affects the arginine dependency of SCLC cell lines.Citation25 As such, MYC-driven cells were more sensitive to arginine deprivation, the reverse of that described for melanoma and highlighting cell-of-origin as a key determinant of the arginine metabolome.Citation8,Citation26
Several studies have highlighted the functional and metabolic changes as a consequence of ASS1 dysregulation in cancer (). To summarise, ASS1 downregulation correlates frequently with more aggressive and chemoresistant cancer phenotypes including increased proliferation, migration, invasion, and metabolic changes affecting glycolysis and the TCA cycle.Citation27 Low levels of ASS1 were associated with worse overall survival for several cancer types including ovarian cancer, mesothelioma, non-small cell lung cancer, bladder cancer and osteosarcoma.Citation28–32 On the other hand, in gastric cancer, ASS1 expression has been linked with tumorigenesis, chemoresistance and poor outcomes.Citation33
Table 1 Functional and Metabolic Effects of ASS1 Modulation in Cancer
Targeting Arginine for Cancer Treatment
A deeper understanding of the role of arginine and ASS1 in tumorigenesis has accelerated the exploitation of arginine auxotrophy for personalised anticancer therapy. Several enzyme therapeutics have been developed to have a synthetically lethal effect in arginine-dependent and urea cycle-dysregulated tumor cells.Citation34
Arginase (ARG)
ARG is an enzyme that catalyses the conversion of arginine into ornithine and urea. Recombinant human ARGI has been conjugated with polyethylene glycol of 5kDa to increase its half-life creating the clinically usable molecule: rhArg-PEG (BCT-100). Xu et al evaluated the potency of rhArg-PEG (BCT-100) on several SCLC cell lines, revealing apoptosis induced by oxidative stress, G1 cell cycle arrest, and reduced arginine concentrations correlating with low levels of ASS1 and/or OTC.Citation35 rhArg-PEG (BCT-100) has been studied in several phase I and II clinical trials in patients with HCC, melanoma, prostate adenocarcinoma or leukaemia; however, no data are available specifically in SCLC.Citation36–39 Another rhArgPEG that is cobalt-substituted (Co-Arg1-PEG or pegzilarginase) also displayed robust activity in 8/12 patient-derived xenograft (PDx) models of SCLC and the related Merkel cell cancer, an aggressive skin neuroendocrine cancer.Citation40 There was a good correlation between SCLC PDx sensitivity to pegzilarginase (4mg/kg) monotherapy and negative or low ASS1 expression by immunohistochemistry, consistent with multiple cancer cell lines studies. A clinical trial of pegzilarginase in a dose-expansion cohort of patients with SCLC has completed accrual and results are awaited (ClinicalTrials.gov Identifier: NCT03371979).
Pegylated Arginine Deiminase
Arginine Deiminase (ADI) is an enzyme that can be found in several organisms (Streptococcus faecalis, Pseudomonas putida, Mycoplasma hominis, Mycoplasma arginini). This enzyme catalyzes the conversion of arginine into citrulline and ammonia. ADI has been formulated with polyethylene glycol (20kDa) to optimise the circulating half-life of the drug known as ADI-PEG20 (pegargiminase). In 2002, Ensor et al published the first study linking ASS1 expression to the efficacy of ADI-PEG20 in melanoma and liver cancer cell lines, leading to early phase clinical trials and FDA designation of ADI-PEG20 as an orphan drug for the treatment for malignant melanoma and HCC.Citation15,Citation41,Citation42 ADI-PEG20 is also cytotoxic to SCLC cell lines in vitro inducing caspase-independent cell death and autophagy. SCLC xenografts were also sensitive to ADI-PEG20 (5IU) with both small and established tumors responding in an ASS1-dependent fashion on par with that seen in SCLC PDx models with pegzilarginase.Citation21
Based on the promising preclinical data, a Phase II two-arm, non-randomised study of pegargiminase enrolled 22 patients with histologically confirmed SCLC in which <5% of tumor cells expressed ASS1 by immunohistochemistry.Citation43 Cohort 1 enrolled 9 patients with “sensitive” disease who had disease control for at least 90 days after 1 previous line of chemotherapy. Cohort 2 enrolled 13 patients with “refractory” disease who had either progressed while on chemotherapy or within 90 days of completing treatment. Both cohorts received treatment with weekly intramuscular ADI-PEG20 (320IU/m2 or 36.8mg/m2) and the primary endpoint for clinical efficacy was tumor response, defined as complete response or partial response by RECIST 1.1 criteria. Although, the study was terminated early as there was poor recruitment to the sensitive disease cohort and no radiologic responses to pegargiminase monotherapy, activity was identified in the refractory cohort with a patient experiencing temporary disease stabilisation and normalisation of serum LDH, a known prognostic and predictive factor in SCLC.Citation44
Sensitivity and Resistance to Arginine-Depleting Agents
The effects of arginine deprivation on tumor cell molecular mechanisms and the metabolome are under active investigation and vary according to cell type. Much of the mechanistic data derives from studies of ADI-PEG20 and is discussed in further detail below and in . Broadly, a number of outcomes have been reported as follows: cell cycle arrest, caspase activation, PARP cleavage, autophagy, nucleotide and polyamine metabolism rewiring, and modulation of RAS-ERK and PI3K-mTOR activity.Citation45 In SCLC, much remains to be elucidated; however, several areas are of specific interest relating to the products of arginine catabolism.
Table 2 Functional and Metabolic Effects of ADI-PEG20
First, nitric oxide synthase (NOS) converts arginine into NO, a small reactive molecule with pleiotropic effects in host and cancer cells.Citation46 Indeed, NOS has a dominant role along with NOTCH in activating downstream soluble guanylate cyclase, a key pathway of platinum resistance in SCLC. Pharmacological depletion of NO via NOS blockade re-sensitized SCLC to chemotherapy.Citation47 NO also possesses key innate and adaptive immune signalling properties, with myeloid cell arginase – a known immunosuppressive pathway – directly competing with NOS for arginine.Citation48,Citation49 Furthermore, inhibiting myeloid arginase and NOS has been shown to enhance tumor immunity in a variety of cancer models.Citation50 Interestingly, bronchoalveolar-derived leucocytes from patients with SCLC produce 6-fold greater levels of NO than those from patients with NSCLC.Citation51 Also, arginase-1 (ARG1) and ARG2 are expressed in SCLC in TAMs and tumor cells, respectively, with higher levels of ARG2 when compared to NSCLC, reinforcing the functional and clinical analysis of SCLC which is dependent on downstream polyamine biosynthesis.Citation25,Citation52–54
Apropos drug resistance, while most patients develop anti-ADI-PEG20 antibodies compared to a minority of patients developing anti-pegzilarginase antibodies, complete responses were nonetheless reported for pegargiminase but none for pegzilarginase in patients with acute myeloid leukaemia.Citation43,Citation55,Citation56 Clearly, tumor-intrinsic rather than drug-innate mechanisms of resistance are operational with emerging rebiopsy data from several phase I/II studies providing pertinent clues. Thus, one-third of patients with the thoracic cancers mesothelioma and NSCLC progressing on pegargiminase display tumoral ASS1 re-expression.Citation30,Citation57 This is consistent with data reported using primary melanoma cell lines and also evident in patients with melanoma progressing on ADI-PEG20.Citation58,Citation59 When ASS1 is re-expressed, cancer cells no longer depend on exogenous arginine and instead upregulate the urea cycle using citrulline generated by ADI-PEG20. Recent work by Xu et al identified contactin 1 as a mediator of rhArg-PEG (BCT-100) resistance in SCLC cell lines by promoting epithelial-mesenchymal transition linked to upregulation of AKT.Citation60
In addition to specific alterations within tumor cells – and, notwithstanding the known limitations of obtaining rebiopsy tissue in SCLC – a critical component of resistance may be mediated by the stromal compartment. Thus, we showed ASS1-expressing macrophages highly upregulated in relapsing thoracic cancers linked to a pro-tumor chemokine network.Citation57 The role of macrophages in SCLC is being leveraged with the development of CD47 antibodies and this may provide opportunities for combination studies that are discussed further below.Citation61 Lastly, autophagy may provide a temporary and finite source of arginine for cancer cells under amino acid restriction. Both ADI-PEG20 and arginase induce autophagy in thoracic cancer cell lines, which may be modulated using antimalarial agents such as chloroquine and quinine.Citation21,Citation62 Notably, in a recent phase 1 study of ADI-PEG20-based combination therapy, a patient with relapsed glioblastoma multiforme exhibited prolonged re-sensitisation to arginine deprivation following the serendipitous prescription of quinine for leg cramps.Citation63,Citation64 The clinical implications of autophagy modulators in the context of arginine deprivation for cancer therapy will require further study.
Strategic Combinations Incorporating Arginine Deprivation for SCLC Therapy
The disconnect between the marked preclinical efficacy of arginine deprivation in SCLC and an apparent lack of response of ADI-PEG20 monotherapy in patients may be explained by several factors, including tumor adaptation by ASS1 re-expression and autophagy, stromal refuelling and tumor heterogeneity. To overcome these hurdles, combination treatments of ADI-PEG20 have now progressed into phase II and III studies in multiple cancers, and here we explore how these might be configured for SCLC as summarised in .
Figure 1 Future strategic combinations of arginine deprivation with personalised targeted therapeutics for SCLC management.
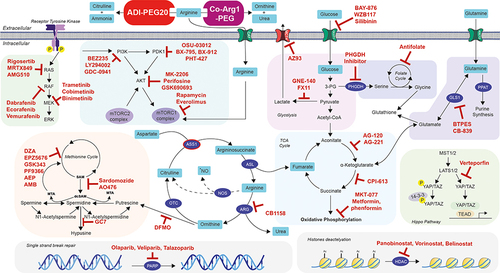
Targeting DNA Repair
PARP are enzymes that play an important role in DNA repair by transferring ADP-ribose residues to targeted proteins. Some tumors depend highly on PARP for survival and therefore PARP inhibition is an interesting therapeutic strategy for these cancers.Citation55 PARP inhibitors (olaparib, veliparib, talazoparib) have been investigated for SCLC treatment. In 2019, Van Den Borg et al showed promising preliminary evidence of the preclinical and clinical activity of these molecules for SCLC treatment especially in sensitising cells to chemotherapy.Citation65 Moreover, in lymphoma, ADI-PEG20 treatment induced the activation of PARP cleavage, a marker of apoptosis, suggesting that arginine deprivation can have an impact on PARP derived pathways.Citation19 This highlights the potential for enhanced cytotoxicity by targeting the DNA damage/repair pathway with arginine deprivation in SCLC.
Targeting DNA Synthesis and Replication
The high proliferative capacity of SCLC and the consequent demand for increased nucleotide precursors represents an area of potential combination studies with arginine deprivation. We showed that ADI-PEG20 downregulated thymidine salvage and de novo synthesis as a mechanism underlying potentiation with the anti-folate pemetrexed in ASS1-deficient mesothelioma and bladder cell lines and consistent with the known diversion of aspartate for enhanced nucleotide synthesis in arginine auxotrophs.Citation31,Citation66 Previous work in HCC cell lines revealed potentiation by arginase with the thymidylate synthase inhibitor 5-fluorouracil.Citation67 In addition to pemetrexed, ADI-PEG20 also synergised with the histone deacetylase inhibitor panobinostat in ASS1 deficient pancreatic ductal adenocarcinoma (PDAC).Citation68 The synthetic lethality was explained by the combination of accumulating DNA damage from panobinostat and cell cycle S-phase arrest upon ADI-PEG20 treatment in ASS1 deficient PDAC. In ASS1-ve cancer models, Prudner et al highlighted the efficacy of the combination of ADI-PEG20 with docetaxel and gemcitabine both in vitro and in vivo. The study suggests that ADI-PEG20 with docetaxel translocate c-MYC to the nucleus thereby promoting hENT1 expression which induces gemcitabine uptake and increased cell death.Citation69 An ongoing phase II clinical trial is investigating the potential of this therapeutic combination for the treatment of sarcoma and SCLC (NCT03449901). Moreover, pemetrexed, gemcitabine and panobinostat have been studied in preclinical and clinical settings in SCLC raising the possibility of further combinations with arginine deprivation.Citation70–73 Similarly, arginine deprivation also modulates platinum and temozolomide cytotoxicity with evidence for additive and synergistic effects in arginine-auxotrophic cell lines.Citation17,Citation74 While platinum forms the backbone of SCLC chemotherapy, temozolomide has documented activity in relapsed disease and further studies are warranted in combination with ADI-PEG20 and rhArgPEG.Citation75
Polyamines
Polyamines are highly regulated positively charged organic molecules that are found to be dysregulated in various cancers. Polyamines are essential for several cellular functions such as cell proliferation, apoptosis regulation, gene expression and protein translation. However, the mechanisms underlying these effects are yet to be fully described and understood. Locke et al showed the rewiring of the polyamine metabolism pathway in mesothelioma upon the appearance of ADI-PEG20 resistance.Citation24 Furthermore, combination treatment with a polyamine inhibitor reversed resistance to ADI-PEG20. Chalishazar et al highlighted the importance of the polyamine pathway in MYC-driven SCLC which was highly dependent on arginine regulation and sensitive to the polyamine biosynthesis inhibitor DFMO.Citation25 Efforts are ongoing to understand the role of the polyamine pathway in arginine auxotrophic cancers with the aim of developing novel combinations for testing in the clinic, including for patients with SCLC.
Glutamine Metabolism
Phosphoribosyl pyrophosphate amidotransferase (PPAT) transfers the gamma-nitrogen atom of glutamine to 5-phosphoribosyl pyrophosphate as the rate-limiting step of purine synthesis. RNA-sequencing of patients with SCLC revealed that PPAT expression was upregulated in cancerous cells compared to normal tissue whereas glutaminase was downregulated (GLS1).Citation76 These results indicate a rewiring of glutamine metabolism in SCLC and highlight the importance of this pathway. In melanoma and sarcoma, arginine deprivation induced by ADI-PEG20 caused a compensatory upregulation of the anaplerotic glutamine-glutamate axis refuelling the TCA cycle. This dependence was confirmed by the combination of ADI-PEG20 with GSL1 inhibitor BTPES that showed a synthetic lethal effect in these cell models.Citation22,Citation27 Thus, the potency of a combination of ADI-PEG20 with a glutamine metabolism inhibitor is of interest in ASS1 deficient SCLC, thereby assessing the extent of glutamine metabolism rewiring upon arginine deprivation.
Hippo Pathway
Recent studies suggest four different molecular subtypes of SCLC depending on their neuroendocrine differentiation and gene expression profile.Citation5 SCLC-Y is driven by the expression of YAP1, a key regulator of the Hippo pathway. TEAD transcription factors mediate YAP1 activity to promote cell proliferation and growth. In SCLC, the YAP1 subtype is associated with a better prognosis compared to the other subtypes.Citation77 Several molecules, including verteporfin, decursin, amlexanox, and latrunculin, are known to inhibit YAP1-mediated transcription. Recent work in prostate cancer cells indicated that blocking TEAD activity potentiated the effect of arginine deprivation thus expanding these studies to SCLC are warranted.Citation78
PI3K/AKT/mTOR (PAM) Pathway
The PAM pathway is frequently dysregulated and active in SCLC in line with several other cancers. This pathway promotes cell growth and proliferation (via mTORC1) and cell cycle progression (via CDK4) while inhibiting apoptosis (via caspase-3). Various strategies are under development to target the recurrent alterations of this pathway in cancer, including inhibitors of the PAM pathway in combination with arginine deprivation, with clear applicability to SCLC.Citation79
Glycolysis and Warburg Effect
In SCLC, MYC-driven cancers display upregulated expression of glycolysis genes, correlating with a robust Warburg effect whereby the MYC-driven cells are less reliant on oxidative metabolism compared to cells with low MYC expression.Citation80 The use of glycolysis inhibitors such as PFK158 showed a significant decrease in glucose uptake, ATP production, and lactate for the MYC-driven cells, and controlled tumor growth in vivo.Citation80 ADI-PEG20 inhibited oxidative phosphorylation and aerobic glycolysis, therefore effectively inhibiting the Warburg effect in melanoma and sarcoma cell lines. Hence, further testing of ADI-PEG20 with inhibitors of glycolysis may provide a novel metabolic combination strategy for MYC-driven SCLC.
Immunotherapy Combinations
SCLC is a highly mutated cancer with a history of heavy tobacco use in 98% of patients, the highest for any malignancy. Consequently, SCLC is immunologically tractable with recent studies defining a role for programmed death-ligand 1 (PD-L1) antagonists in combination with chemotherapy.Citation1 However, despite the high tumor mutation burden, the benefits of immune checkpoint therapy have been modest, implying a role for extensive tumor-induced immunosuppression. Recent single cell transcriptome data have revealed marked T cell dysfunction – a low ratio of CD8+ effector to Treg cells – and enrichment of profibrotic macrophages linked to exhausted CD8+ T cells as major drivers of the immune cold TME.Citation81
An anticancer immune response is dependent on multiple factors, with arginine playing pivotal roles especially in maintaining T cell receptor function, proliferation and memory.Citation82 Critically, nitric oxide synthase (NOS) via the generation of NO, and arginase (1 and 2) via the production of ornithine, are major arginine catabolizing enzymes involved in orchestrating cellular crosstalk and thereby immune and inflammatory responses.Citation83,Citation84 Recently, low plasma levels of arginine have been linked to poor outcomes in patients receiving immune checkpoint inhibitors, whereas high circulating arginine was linked to improved disease control, further emphasizing the potential of arginase inhibitors as cancer therapeutics.Citation85 Nonetheless, preclinically, there is also a good rationale for combining immune checkpoint immunotherapy with arginine deprivation as shown in . ADI-PEG20 increases tumoral PD-L1 expression and T cell infiltration and reduces Treg cell accumulation with potentiation of PD-1/PD-L1 inhibitors in arginine auxotrophic syngeneic tumor murine models.Citation86 Similarly, Co-Arg1-PEG has shown enhanced tumor control in vivo in combination with PD-1/PD-L1 inhibitors and anti-OX40 antibodies.Citation87 Moreover, analysis of urea cycle dysfunctional cancer predicts for gene signatures linked to immune responsiveness.Citation88 Additionally, targeted antigens for chimeric antigen receptor (CAR) T-cell-based therapy in preclinical and clinical studies of SCLC, namely DLL-3, CD55 and GPC3, may be enhanced through metabolic engineering of urea cycle enzymes, ASS1 and OTC, thereby maintaining T cell immunocompetency.Citation89 Additionally, citrulline the byproduct of ADI-PEG20, may be recycled by T cells to regenerate arginine thereby maintaining immune cell function, a critical consideration with immune checkpoint inhibitors.Citation90
Figure 2 Potential immunometabolic combinations incorporating arginine deprivation for the management of SCLC.
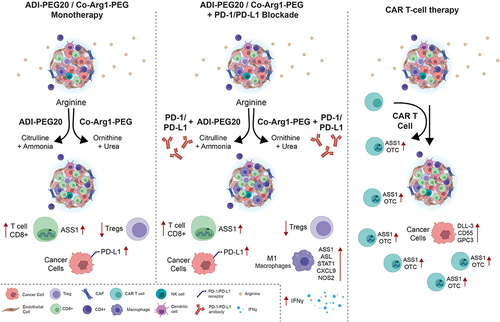
Clinically, ADI-PEG20 was tested with pembrolizumab in a small phase 1b study of patients with advanced treatment-refractory cancers, mostly aerodigestive in origin, and none with lung cancer.Citation91 The combination was safe and active, documenting a partial response rate of 24% and a grade 3–4 neutropenia rate of 40%, which is notable in that anti-PD-1 inhibitors rarely cause neutropenia and this occurs infrequently with ADI-PEG20 monotherapy.Citation29,Citation41,Citation42,Citation59 Repeat biopsies on ADI-PEG20 – and prior to commencement of pembrolizumab – revealed infiltration by CD3+ T cells consistent with the pre-clinical data. Results are also expected from a phase I/II study of pegzilarginase combined with pembrolizumab which included a SCLC dose-expansion cohort (ClinicalTrials.gov Identifier: NCT03371979).
Current Therapy for Small Cell Lung Cancer and Outcomes
Whilst in general SCLC responds robustly to initial platinum-based chemotherapy, the majority of patients subsequently relapse.Citation92,Citation93 The chance of responding to second-line treatment is approximately 10% and resistance emerges rapidly in nearly all patients.Citation94 Clinically, the terms “limited disease”, and “extensive disease” are used to define the extent of SCLC burden. Limited disease is defined as the tumor being confined to one hemithorax and regional lymph nodes and treatment options include surgical resection in a minority aiming for R0 resection, concurrent chemotherapy (with platinum-based chemotherapy in combination with the topoisomerase II inhibitor, etoposide) and thoracic radiotherapy. Prophylactic cranial irradiation (PCI) serves to decrease the risk of symptomatic brain metastases and increases overall survival in patients who are Performance status 0–1 and experience a complete remission.Citation95 Despite this, the median OS of limited-stage SCLC is approximately 17 months.
Patients with “extensive disease” are treated with platinum-based chemotherapy usually combined with etoposide with a resultant median overall survival (OS) of 9–10 months, progression free survival (PFS) of 5–6 months and 1 year OS of ~35%.Citation96 A modest advance in the last 40 years in the first-line treatment setting of SCLC has been the advent of chemoimmunotherapy with the addition of an anti-programmed death-ligand 1 (PD-L1) antibody based on the IMpower133 and CASPIAN studies.Citation97–99 The IMpower 133 trial tested the addition of the anti-PD-L1 antibody, atezolizumab to carboplatin and etoposide for 4 cycles followed by atezolizumab maintenance versus placebo and carboplatin and etoposide. Patients in the atezolizumab arm achieved a median OS of 12.3 months compared to 10.3 months in the placebo arm (HR = 0.70; 95% CI 0.54 to 0.91 p = 0.007) and with almost 2 years of median follow-up, patients in the atezolizumab arm continued to display benefit with ~22% of patients alive at 24 months in the atezolizumab arm, compared with ~14% in the placebo arm.Citation97
The CASPIAN trial enrolled 805 patients to durvalumab with or without the anti-cytotoxic T-cell lymphocyte-4 blocking antibody, tremelimumab, plus platinum-etoposide followed by durvalumab maintenance or platinum-etoposide only. The combination of platinum-etoposide plus durvalumab significantly extended OS, compared with platinum-etoposide alone (HR = 0.73) 95% CI, CI 0.59 to 0.91; p = 0.0047. Furthermore, patients in the durvalumab/platinum/etoposide arm had a sustained OS benefit; an additional 8% of patients were alive at 24 months in the experimental arm (22.2%) compared with the platinum/etoposide arm (14.4%). The addition of tremelimumab provided no additional survival benefit.Citation98 While KEYNOTE-604, which tested the role of pembrolizumab with platinum-etoposide, significantly improved PFS, the pre-specified endpoint for OS was not met.Citation100 Despite these recent therapeutic developments with the addition of anti-PD-L1-based immunotherapy to standard chemotherapy for SCLC, the prognosis remains poor and new strategies are clearly required to go beyond the current therapeutic plateau.
Thus, in view of the modest gains obtained by incorporating anti-PD-L1 blockade to the carboplatin-etoposide chemotherapy backbone, there is clear scope for further improving survival outcomes for patients with SCLC. Arginine deprivation is a particularly attractive anti-metabolite option that on current evidence is safe in triplet chemotherapy combinations and as a doublet with immune checkpoint blockade. Indeed, in view of the preclinical data described above, there are potential synergies to explore for both the chemotherapy and immunotherapy components as a quadruplet chemo-immunometabolic therapy with arginine depletion.
Perspectives for Arginine Deprivation in SCLC
ATOMIC-meso is the first front-line triplet chemotherapy phase III study of arginine deprivation using ADI-PEG20 combined with platinum and pemetrexed in cancer, specifically non-epithelioid mesothelioma, and is expected to report by the summer of 2022. Based on the premise that ADI-PEG20 sensitises ASS1-deficient cancers to antifolates by exploiting a critical dependency on nucleotide synthesis, ATOMIC-meso, if positive, will herald the advent of arginine deprivation as a novel antimetabolite therapy for cancer.
As alluded to earlier, there is significant potential to leverage arginine auxotrophic SCLC with arginine deprivation in combination with chemoimmunotherapy, namely platinum plus etoposide and an anti-PD-L1 agent which would be a rational pathway to development. Other combinations may have merit particularly replacing etoposide for pemetrexed in patients with reduced performance status. Efforts are also underway with a phase II trial investigating ADI-PEG20 in combination with gemcitabine and docetaxel, that includes a small cohort of patients with SCLC. (NCT03449901). Additional agents that warrant investigation in relapsed SCLC based on a study in progress in glioblastoma multiforme, include ADI-PEG20 with temozolomide with or without radiation (NCT04587830).
Conclusions
There are significant opportunities ahead to develop our understanding of the role of arginine deprivation as part of combined multimodality care for patients with SCLC. More broadly, arginine depletion as an antimetabolite strategy should be reinterpreted both in cancer treatment and in reshaping the tumor microenvironment, with a specific focus on tumor-type. Ultimately, a strong translational base will be needed to decipher which SCLC subgroups benefit the most from incorporating arginine depletion to the standard of care.
Disclosure
Dr Szlosarek reports grant research support from Polaris Group. The remaining authors report no other conflicts of interest.
References
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. doi:10.1038/s41572-020-00235-0
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725–737. doi:10.1038/nrc.2017.87
- Schwendenwein A, Megyesfalvi Z, Barany N, et al. Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics. 2021;20:470–483. doi:10.1016/j.omto.2021.02.004
- Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther. 2017;180:16–23. doi:10.1016/j.pharmthera.2017.06.002
- Baine MK, Hsieh MS, Lai WV, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15(12):1823–1835. doi:10.1016/j.jtho.2020.09.009
- Morris SM Jr. Arginine: beyond protein. Am J Clin Nutr. 2006;83(2):508S–12S. doi:10.1093/ajcn/83.2.508S
- Morris SM Jr. Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137(6Suppl 2):1602S–9S. doi:10.1093/jn/137.6.1602S
- Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270(9):1887–1899. doi:10.1046/j.1432-1033.2003.03559.x
- Boger RH, Bode-Boger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. 2001;41:79–99. doi:10.1146/annurev.pharmtox.41.1.79
- Misel ML, Gish RG, Patton H, Mendler M. Sodium benzoate for treatment of hepatic encephalopathy. Gastroenterol Hepatol. 2013;9(4):219–227.
- Riess C, Shokraie F, Classen CF, et al. Arginine-depleting enzymes - an increasingly recognized treatment strategy for therapy-refractory malignancies. Cell Physiol Biochem. 2018;51(2):854–870. doi:10.1159/000495382
- Synakiewicz A, Stachowicz-Stencel T, Adamkiewicz-Drozynska E. The role of arginine and the modified arginine deiminase enzyme ADI-PEG 20 in cancer therapy with special emphasis on phase I/II clinical trials. Expert Opin Investig Drugs. 2014;23(11):1517–1529. doi:10.1517/13543784.2014.934808
- Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012;303(10):E1177–89. doi:10.1152/ajpendo.00284.2012
- Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762–2772. doi:10.1002/ijc.25202
- Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62(19):5443–5450.
- Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12(23):7126–7131. doi:10.1158/1078-0432.CCR-06-1101
- Przystal JM, Hajji N, Khozoie C, et al. Efficacy of arginine depletion by ADI-PEG20 in an intracranial model of GBM. Cell Death Dis. 2018;9(12):1192. doi:10.1038/s41419-018-1195-4
- Huang HY, Wu WR, Wang YH, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861–2872. doi:10.1158/1078-0432.CCR-12-2641
- Delage B, Luong P, Maharaj L, et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012;3:e342. doi:10.1038/cddis.2012.83
- Szlosarek PW, Grimshaw MJ, Wilbanks GD, et al. Aberrant regulation of argininosuccinate synthetase by TNF-alpha in human epithelial ovarian cancer. Int J Cancer. 2007;121(1):6–11. doi:10.1002/ijc.22666
- Kelly MP, Jungbluth AA, Wu BW, Bomalaski J, Old LJ, Ritter G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer. 2012;106(2):324–332. doi:10.1038/bjc.2011.524
- Long Y, Tsai WB, Wangpaichitr M, et al. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther. 2013;12(11):2581–2590. doi:10.1158/1535-7163.MCT-13-0302
- Szlosarek PW, Luong P, Phillips MM, et al. Metabolic response to pegylated arginine deiminase in mesothelioma with promoter methylation of argininosuccinate synthetase. J Clin Oncol. 2013;31(7):e111–3. doi:10.1200/JCO.2012.42.1784
- Locke M, Ghazaly E, Freitas MO, et al. Inhibition of the polyamine synthesis pathway is synthetically lethal with loss of argininosuccinate synthase 1. Cell Rep. 2016;16(6):1604–1613. doi:10.1016/j.celrep.2016.06.097
- Chalishazar MD, Wait SJ, Huang F, et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin Cancer Res. 2019;25(16):5107–5121. doi:10.1158/1078-0432.CCR-18-4140
- Hajaj E, Sciacovelli M, Frezza C, Erez A. The context-specific roles of urea cycle enzymes in tumorigenesis. Mol Cell. 2021;81(18):3749–3759. doi:10.1016/j.molcel.2021.08.005
- Kremer JC, Prudner BC, Lange SES, et al. Arginine deprivation inhibits the Warburg effect and upregulates glutamine anaplerosis and serine biosynthesis in ASS1-deficient cancers. Cell Rep. 2017;18(4):991–1004. doi:10.1016/j.celrep.2016.12.077
- Nicholson LJ, Smith PR, Hiller L, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125(6):1454–1463. doi:10.1002/ijc.24546
- Szlosarek PW, Steele JP, Nolan L, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3(1):58–66. doi:10.1001/jamaoncol.2016.3049
- Szlosarek PW, Wimalasingham AG, Phillips MM, et al. Phase 1, pharmacogenomic, dose-expansion study of pegargiminase plus pemetrexed and cisplatin in patients with ASS1-deficient non-squamous non-small cell lung cancer. Cancer Med. 2021;10(19):6642–6652. doi:10.1002/cam4.4196
- Allen MD, Luong P, Hudson C, et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74(3):896–907. doi:10.1158/0008-5472.CAN-13-1702
- Kobayashi E, Masuda M, Nakayama R, et al. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010;9(3):535–544. doi:10.1158/1535-7163.MCT-09-0774
- Tsai CY, Chi HC, Chi LM, et al. Argininosuccinate synthetase 1 contributes to gastric cancer invasion and progression by modulating autophagy. FASEB J. 2018;32(5):2601–2614. doi:10.1096/fj.201700094r
- Phillips MM, Sheaff MT, Szlosarek PW. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat. 2013;45(4):251–262. doi:10.4143/crt.2013.45.4.251
- Xu S, Lam SK, Cheng PN, Ho JC. Recombinant human arginase induces apoptosis through oxidative stress and cell cycle arrest in small cell lung cancer. Cancer Sci. 2018;109(11):3471–3482. doi:10.1111/cas.13782
- Chan SL, Cheng PNM, Liu AM, et al. A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Invest New Drugs. 2021;39(5):1375–1382. doi:10.1007/s10637-021-01111-8
- Cheng PNM, Liu AM, Bessudo A, Mussai F. Safety, PK/PD and preliminary anti-tumor activities of pegylated recombinant human arginase 1 (BCT-100) in patients with advanced arginine auxotrophic tumors. Invest New Drugs. 2021;39(6):1633–1640. doi:10.1007/s10637-021-01149-8
- De Santo C, Cheng P, Beggs A, Egan S, Bessudo A, Mussai F. Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J Hematol Oncol. 2018;11(1):68. doi:10.1186/s13045-018-0612-6
- Yau T, Cheng PN, Chan P, et al. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33(2):496–504. doi:10.1007/s10637-014-0200-8
- Agnello G, Alters SE, Rowlinson SW. Preclinical safety and antitumor activity of the arginine-degrading therapeutic enzyme pegzilarginase, a PEGylated, cobalt-substituted recombinant human arginase 1. Transl Res. 2020;217:11–22. doi:10.1016/j.trsl.2019.12.005
- Izzo F, Marra P, Beneduce G, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22(10):1815–1822. doi:10.1200/JCO.2004.11.120
- Ascierto PA, Scala S, Castello G, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23(30):7660–7668. doi:10.1200/JCO.2005.02.0933
- Hall PE, Ready N, Johnston A, et al. Phase II study of arginine deprivation therapy with pegargiminase in patients with relapsed sensitive or refractory small-cell lung cancer. Clin Lung Cancer. 2020;21(6):527–533. doi:10.1016/j.cllc.2020.07.012
- He M, Chi X, Shi X, et al. Value of pretreatment serum lactate dehydrogenase as a prognostic and predictive factor for small-cell lung cancer patients treated with first-line platinum-containing chemotherapy. Thorac Cancer. 2021;12(23):3101–3109. doi:10.1111/1759-7714.13581
- Chen CL, Hsu SC, Ann DK, Yen Y, Kung HJ. Arginine Signaling and Cancer Metabolism. Cancers. 2021;13(14):3541.
- Keshet R, Erez A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis Model Mech. 2018;11(8). doi:10.1242/dmm.033332
- Schenk MW, Humphrey S, Hossain A, et al. Soluble guanylate cyclase signalling mediates etoposide resistance in progressing small cell lung cancer. Nat Commun. 2021;12(1):6652. doi:10.1038/s41467-021-26823-6
- Grzywa TM, Sosnowska A, Matryba P, et al. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938. doi:10.3389/fimmu.2020.00938
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi:10.1038/nri1668
- Miret JJ, Kirschmeier P, Koyama S, et al. Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity. J Immunother Cancer. 2019;7(1):32. doi:10.1186/s40425-019-0504-5
- Cembrzyńska-Nowak M, Bieńkowska M, Szklarz E. Exogenous interleukin 2 regulates interleukin 6 and nitric oxide but not interferon gamma and tumor necrosis factor alpha production in bronchoalveolar leukocytes from patients with small cell lung cancer. Arch Immunol Ther Exp. 1998;46:367–374.
- Dora D, Rivard C, Yu H, et al. Characterization of tumor-associated macrophages and the immune microenvironment in limited-stage neuroendocrine-high and -low small cell lung cancer. Biology. 2021;10(6):502. doi:10.3390/biology10060502
- Rotondo R, Mastracci L, Piazza T, et al. Arginase 2 is expressed by human lung cancer, but it neither induces immune suppression, nor affects disease progression. Int J Cancer. 2008;123(5):1108–1116. doi:10.1002/ijc.23437
- Simon MS, Eckenrode J, Natale RB. Phase II trial of methylglyoxal bis-guanylhydrazone (MGBG) in refractory small cell lung cancer. Invest New Drugs. 1990;8(Suppl 1):S79–81. doi:10.1007/BF00171989
- Tsai HJ, Jiang SS, Hung WC, et al. A phase ii study of arginine deiminase (ADI-PEG20) in relapsed/refractory or poor-risk acute myeloid leukemia patients. Sci Rep. 2017;7(1):11253. doi:10.1038/s41598-017-10542-4
- Uy GL, Savona MR, Tomlinson BK, et al. Phase 1 trial of pegzilarginase in patients (pts) with relapsed/refractory (R/R) AML or MDS refractory to hypomethylating agents (HMAs). J Clin Oncol. 2018;36:7031. doi:10.1200/JCO.2018.36.15_suppl.7031
- Szlosarek PW, Phillips MM, Pavlyk I, et al. Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Rep. 2020;1(4):100093. doi:10.1016/j.jtocrr.2020.100093
- Manca A, Sini MC, Izzo F, et al. Induction of arginosuccinate synthetase (ASS) expression affects the antiproliferative activity of arginine deiminase (ADI) in melanoma cells. Oncol Rep. 2011;25(6):1495–1502. doi:10.3892/or.2011.1220
- Feun LG, Marini A, Walker G, et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012;106(9):1481–1485. doi:10.1038/bjc.2012.106
- Xu S, Lam SK, Cheng PN, Ho JC. Contactin 1 modulates pegylated arginase resistance in small cell lung cancer through induction of epithelial-mesenchymal transition. Sci Rep. 2019;9(1):12030. doi:10.1038/s41598-019-48476-8
- Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126(7):2610–2620. doi:10.1172/JCI81603
- Shen W, Zhang X, Fu X, et al. A novel and promising therapeutic approach for NSCLC: recombinant human arginase alone or combined with autophagy inhibitor. Cell Death Dis. 2017;8(3):e2720. doi:10.1038/cddis.2017.137
- Hall PE, Lewis R, Syed N, et al. A phase I study of pegylated arginine deiminase (pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma. Clin Cancer Res. 2019;25(9):2708–2716. doi:10.1158/1078-0432.CCR-18-3729
- Golden EB, Cho HY, Hofman FM, Louie SG, Schonthal AH, Chen TC. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. doi:10.3171/2014.12.FOCUS14748
- Van Den Borg R, Leonetti A, Tiseo M, Giovannetti E, Peters GJ. Novel targeted strategies to overcome resistance in small-cell lung cancer: focus on PARP inhibitors and rovalpituzumab tesirine. Expert Rev Anticancer Ther. 2019;19(6):461–471. doi:10.1080/14737140.2019.1624530
- Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379–383. doi:10.1038/nature15529
- Cheng PN, Lam TL, Lam WM, et al. Pegylated recombinant human arginase (rhArg-peg5000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67(1):309–317. doi:10.1158/0008-5472.CAN-06-1945
- Kim SS, Xu S, Cui J, et al. Histone deacetylase inhibition is synthetically lethal with arginine deprivation in pancreatic cancers with low argininosuccinate synthetase 1 expression. Theranostics. 2020;10(2):829–840. doi:10.7150/thno.40195
- Prudner BC, Rathore R, Robinson AM, et al. Arginine starvation and docetaxel induce c-Myc-Driven hENT1 surface expression to overcome gemcitabine resistance in ASS1-negative tumors. Clin Cancer Res. 2019;25(16):5122–5134. doi:10.1158/1078-0432.CCR-19-0206
- Crisanti MC, Wallace AF, Kapoor V, et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8(8):2221–2231. doi:10.1158/1535-7163.MCT-09-0138
- De Marinis F, Atmaca A, Tiseo M, et al. A phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in pretreated patients with small-cell lung cancer. J Thorac Oncol. 2013;8(8):1091–1094. doi:10.1097/JTO.0b013e318293d88c
- Lee SM, James LE, Qian W, et al. Comparison of gemcitabine and carboplatin versus cisplatin and etoposide for patients with poor-prognosis small cell lung cancer. Thorax. 2009;64(1):75–80. doi:10.1136/thx.2007.093872
- Yao S, Peng L, Elakad O, et al. One carbon metabolism in human lung cancer. Transl Lung Cancer Res. 2021;10(6):2523–2538. doi:10.21037/tlcr-20-1039
- Yeon A, You S, Kim M, et al. Rewiring of cisplatin-resistant bladder cancer cells through epigenetic regulation of genes involved in amino acid metabolism. Theranostics. 2018;8(16):4520–4534. doi:10.7150/thno.25130
- Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18(4):1138–1145. doi:10.1158/1078-0432.CCR-11-2059
- Kodama M, Oshikawa K, Shimizu H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun. 2020;11(1):1320. doi:10.1038/s41467-020-15136-9
- Owonikoko TK, Dwivedi B, Chen Z, et al. YAP1 expression in SCLC defines a distinct subtype with T-cell-inflamed phenotype. J Thorac Oncol. 2021;16(3):464–476. doi:10.1016/j.jtho.2020.11.006
- Chen CL, Hsu SC, Chung TY, et al. Arginine is an epigenetic regulator targeting TEAD4 to modulate OXPHOS in prostate cancer cells. Nat Commun. 2021;12(1):2398. doi:10.1038/s41467-021-22652-9
- Krencz I, Sztankovics D, Danko T, Sebestyen A, Khoor A. Progression and metastasis of small cell lung carcinoma: the role of the PI3K/Akt/mTOR pathway and metabolic alterations. Cancer Metastasis Rev. 2021;40(4):1141–1157. doi:10.1007/s10555-021-10012-4
- Cargill KR, Stewart CA, Park EM, et al. Targeting MYC-enhanced glycolysis for the treatment of small cell lung cancer. Cancer Metab. 2021;9(1):33. doi:10.1186/s40170-021-00270-9
- Chan JM, Quintanal-Villalonga A, Gao VR, et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell. 2021;39(11):1479–1496. doi:10.1016/j.ccell.2021.09.008
- Popovic PJ, Zeh HJ 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137(6Suppl 2):1681S–6S. doi:10.1093/jn/137.6.1681S
- Mondanelli G, Ugel S, Grohmann U, Bronte V. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr Opin Pharmacol. 2017;35:30–39. doi:10.1016/j.coph.2017.05.002
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi:10.1038/ni1001-907
- Peyraud F, Guegan JP, Bodet D, et al. Circulating L-Arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann Oncol. 2022. doi:10.1016/j.annonc.2022.07.001
- Brin E, Wu K, Lu HT, He Y, Dai Z, He W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget. 2017;8(35):58948–58963. doi:10.18632/oncotarget.19564
- Badeaux MD, Rolig AS, Agnello G, et al. Arginase therapy combines effectively with immune checkpoint blockade or agonist anti-OX40 immunotherapy to control tumor growth. Cancer Immunol Res. 2021;9(4):415–429. doi:10.1158/2326-6066.CIR-20-0317
- Lee JS, Adler L, Karathia H, et al. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. 2018;174(6):1559–70 e22. doi:10.1016/j.cell.2018.07.019
- Fultang L, Booth S, Yogev O, et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood. 2020;136(10):1155–1160. doi:10.1182/blood.2019004500
- Werner A, Koschke M, Leuchtner N, et al. Reconstitution of T cell proliferation under arginine limitation: activated human T cells take up citrulline via L-type amino acid transporter 1 and use it to regenerate arginine after induction of argininosuccinate synthase expression. Front Immunol. 2017;8:864. doi:10.3389/fimmu.2017.00864
- Chang KY, Chiang NJ, Wu SY, et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG 20) plus Pembrolizumab in advanced solid cancers. Oncoimmunology. 2021;10(1):1943253. doi:10.1080/2162402X.2021.1943253
- Dowell JE, Palmer BF. Small cell lung cancer: are we making progress? Am J Med Sci. 2010;339(1):68–76. doi:10.1097/MAJ.0b013e3181bccef5
- Zugazagoitia J, Paz-Ares L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J Clin Oncol. 2022;40:671–680.
- Neal JW, Gubens MA, Wakelee HA. Current management of small cell lung cancer. Clin Chest Med. 2011;32(4):853–863. doi:10.1016/j.ccm.2011.07.002
- Dingemans AC, Fruh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(). Ann Oncol. 2021;32(7):839–853. doi:10.1016/j.annonc.2021.03.207
- Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer. 2000;30(1):23–36.
- Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–630. doi:10.1200/JCO.20.01055
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
- Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi:10.1200/JCO.20.00793
- Tao X, Zuo Q, Ruan H, et al. Argininosuccinate synthase 1 suppresses cancer cell invasion by inhibiting STAT3 pathway in hepatocellular carcinoma. Acta Biochim Biophys Sin. 2019;51(3):263–276. doi:10.1093/abbs/gmz005
- Ohshima K, Nojima S, Tahara S, et al. Argininosuccinate synthase 1-deficiency enhances the cell sensitivity to arginine through decreased DEPTOR expression in endometrial cancer. Sci Rep. 2017;7:45504. doi:10.1038/srep45504
- Bateman LA, Ku WM, Heslin MJ, Contreras CM, Skibola CF, Nomura DK. Argininosuccinate synthase 1 is a metabolic regulator of colorectal cancer pathogenicity. ACS Chem Biol. 2017;12(4):905–911. doi:10.1021/acschembio.6b01158
- Moren L, Perryman R, Crook T, et al. Metabolomic profiling identifies distinct phenotypes for ASS1 positive and negative GBM. BMC Cancer. 2018;18(1):167. doi:10.1186/s12885-018-4040-3
- Kim S, Lee M, Song Y, et al. Argininosuccinate synthase 1 suppresses tumor progression through activation of PERK/eIF2alpha/ATF4/CHOP axis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2021;40(1):127. doi:10.1186/s13046-021-01912-y
- Gong H, Zolzer F, Von Recklinghausen G, et al. Arginine deiminase inhibits cell proliferation by arresting cell cycle and inducing apoptosis. Biochem Biophys Res Commun. 1999;261(1):10–14. doi:10.1006/bbrc.1999.1004
- Thomas JB, Holtsberg FW, Ensor CM, Bomalaski JS, Clark MA. Enzymic degradation of plasma arginine using arginine deiminase inhibits nitric oxide production and protects mice from the lethal effects of tumor necrosis factor alpha and endotoxin. Biochem J. 2002;363(Pt 3):581–587. doi:10.1042/bj3630581
- Savaraj N, Wu C, Kuo MT, et al. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target Insights. 2007;2:119–128. doi:10.1177/117739280700200016
- Bowles TL, Kim R, Galante J, et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123(8):1950–1955. doi:10.1002/ijc.23723
- Kim RH, Bold RJ, Kung HJ. ADI, autophagy and apoptosis: metabolic stress as a therapeutic option for prostate cancer. Autophagy. 2009;5(4):567–568. doi:10.4161/auto.5.4.8252
- You M, Savaraj N, Wangpaichitr M, et al. The combination of ADI-PEG20 and TRAIL effectively increases cell death in melanoma cell lines. Biochem Biophys Res Commun. 2010;394(3):760–766. doi:10.1016/j.bbrc.2010.03.066
- Huang CC, Tsai ST, Kuo CC, et al. Arginine deprivation as a new treatment strategy for head and neck cancer. Oral Oncol. 2012;48(12):1227–1235. doi:10.1016/j.oraloncology.2012.06.004
- Tsai WB, Aiba I, Long Y, et al. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012;72(10):2622–2633. doi:10.1158/0008-5472.CAN-11-3605
- Maletzki C, Rosche Y, Riess C, et al. Deciphering molecular mechanisms of arginine deiminase-based therapy - Comparative response analysis in paired human primary and recurrent glioblastomas. Chem Biol Interact. 2017;278:179–188. doi:10.1016/j.cbi.2017.10.007
