Abstract
Electromagnetic navigation bronchoscopy (ENB) is one of several technological advances which have broadened the indications for bronchoscopy in the diagnostic workup of lung cancer. The technique facilitates bronchoscopic sampling of peripheral pulmonary nodules as well as mediastinal lymph nodes, although wide availability and expertise in endobronchial ultrasonography has limited its application in routine clinical practice to the former. ENB in this setting is quite versatile and may be considered an established alternative to more invasive techniques, especially in selected patients with underlying pulmonary disease or comorbidities at high risk for complications from computer topography-guided fine needle aspiration or surgical resection. Nodule sampling may be performed with a variety of instruments, including forceps, cytology brushes, and transbronchial needles. Although samples are generally small, they are often suitable for molecular analysis.
Introduction
Solitary pulmonary nodules (SPNs) are often found incidentally on chest radiographs or computed tomography (CT) scans and are quite common. As many as 150,000 pulmonary nodules are diagnosed every year in the US.Citation1 The advent of lung cancer screening with low-dose CT implies that clinicians will face a virtual avalanche of nodules in need of workup, albeit few of them will merit invasive interventions.Citation2 Management of the SPNs generally depends on the clinical suspicion of cancer. While most small nodules can be followed by imaging alone, those ranging in size between 10 mm and 30 mm in diameter are often considered too large for watchful waiting.
The choice of diagnostic procedure depends on patient preference and practitioner expertise, available resources, as well as local biases. Surgery is often recommended for low- to intermediate-risk patients with a high clinical suspicion of cancer in order to avoid the pitfalls of minimally invasive diagnostic tests.Citation3 Many practitioners feel strongly that opting for immediate surgical resection may be the best choice for many patients with resectable nodules because the negative predictive value of a benign diagnosis obtained by minimally invasive techniques is not acceptable in this setting. However, although surgical mortality is low for patients with SPNs and the procedure is diagnostic for the vast majority of cases, only 55%–68% of surgically resected nodules turn out to be malignant, reflecting the shortcomings of a “straight to surgery” approach.Citation4,Citation5 An aggressive management strategy may lead to many invasive procedures for benign nodules. Furthermore, patients with suspicious nodules are often older and heavy smokers with underlying COPD, cardiovascular disease, or other tobacco-related comorbidities, and surgery in these patients is never straightforward.
CT-guided transthoracic needle aspiration (TTNA) and core needle biopsy are the minimally invasive alternatives to bronchoscopy or surgery. Percutaneous biopsy is the gold standard for the minimally invasive diagnosis of peripheral lesions, and remains the most common approach to the intermediate-sized pulmonary nodule with a diagnostic accuracy of 90%.Citation6 The sensitivity of TTNA for lung cancer is 80%–95% and the specificity is 50%–88%.Citation1 However, diagnostic yields reported by individual studies vary from 62% to 99%.Citation7 The biggest drawback is the risk of pneumothorax, with a recent study of more than 15,000 procedures reporting a 15% pneumothorax rate and a 1% incidence of significant bleeding.Citation8 Several factors including needle and nodule size, a history of smoking, and patient age have been linked to the procedural risk of pneumothorax.Citation9 This risk is 11 times greater if the nodule measures less than 20 mm and increases with an increase in the distance from the nodule to the pleural surface. Another drawback of TTNA, common to all minimally invasive techniques, is a modest negative predictive value which often leads to surgery despite a benign diagnosis.Citation10 As many as 68% of patients undergoing surgery with a benign diagnosis obtained by TTNA have cancer.Citation11
The role of conventional bronchoscopy in lung cancer
Recent advances in endobronchial ultrasonography have catapulted bronchoscopy to its current preeminent role in the diagnosis and staging of patients with advanced lung cancer. Conventional bronchoscopy, however, faces many limitations in the workup of pulmonary nodules. The technique often relies on blind or fluoroscopically guided techniques such as bronchoalveolar lavage and use of cytology brushes, needle aspiration, or forceps in order to biopsy a given nodule. Flexible bronchoscopy is invaluable in the diagnosis of endoscopically visible or diffuse disease, including lymphangitic spread of tumors in the lung, but successful sampling of pulmonary nodules depends on the size and location of the nodule and local expertise. Furthermore, diagnostic bronchoscopes have small caliber working channels, so diagnostic samples are often small. Crush artifacts and the lack of rapid onsite sample evaluation at many centers can also limit the diagnostic yields.
Major advantages of conventional bronchoscopy in the workup of patients with suspected lung cancer include its excellent safety profile and wide availability. Serious complications are rare and generally do not exceed 1%. In a recent study of more than 20,000 procedures, only three deaths were reported.Citation12 Pneumothorax rates are much lower than those reported following TTNA, often well below 5%, with only a handful of patients requiring chest tube placement.Citation13 That notwithstanding, patients with suspected peripheral lung cancer must undergo transbronchial biopsies, a technique which is responsible for 73% of all serious complications related to the procedure.Citation14
Unfortunately, despite its safety profile, conventional bronchoscopy has a poor diagnostic yield for small peripheral lesions. Nodule size and location are key factors, but since bronchoscopy mandates an endobronchial approach, the relationship of the lesion to the bronchial tree is particularly important. Current American College of Chest Physicians (ACCP) guidelines do not recommend conventional bronchoscopy for the evaluation of small pulmonary nodules unless a bronchus sign is clearly present.Citation15 Since a benign diagnosis is often nonspecific and unreliable, the prevalence of cancer in a given practice setting is also paramount and quite variable. Not surprisingly, reported diagnostic yields range from 18% to 62%.Citation16,Citation17 Large malignant nodules located close to the hilum are more amenable to the conventional bronchoscopic approach.Citation18,Citation19 Yields may improve with the use of fluoroscopy, but lesions <2 cm in diameter are often missed. Furthermore, fluoroscopy is not only cumbersome, but requires specific radiation safety measures and training, more space than is often available in a standard bronchoscopy suite, and prolongs the procedure.
Conventional bronchoscopy may be reserved for the perioperative evaluation of the patient with limited-stage peripheral lung cancer. Spanish thoracic society guidelines recommend conventional bronchoscopy be performed in a selected group of patients with suspicious pulmonary nodules who are good surgical candidates.Citation20 A prospective European study found that preoperative bronchoscopy in this setting has an overall diagnostic yield of 41%, and may contribute to changes in surgical strategy in a significant number of cases.Citation21 However, a recent retrospective study reported that little can be expected from preoperative bronchoscopy as fewer than 8% of the procedures lead to unexpected findings,Citation22 particularly in patients with ground-glass opacities or small non-solid nodules.Citation23
Finally, lung cancer screening poses a new challenge to the conventional bronchoscope, since lung cancers detected in this setting are often less than 2 cm in size. van’t Westeinde et al recently reported yields of flexible bronchoscopy in the evaluation of pulmonary nodules detected in the context of lung cancer screening.Citation24 Mean nodule size in that study did not exceed 15 mm and the overall sensitivity of bronchoscopy was a disappointing 13.5%. More importantly, surgical resection was necessary in 95% of the patients, a strong argument in favor of a “straight to surgery” strategy ().
Table 1 Relative yields, advantages, and shortcomings of currently available diagnostic techniques in the clincal workup of lung cancer
Electromagnetic navigation bronchoscopy
Electromagnetic navigation bronchoscopy (ENB) was developed in order to overcome the shortcomings of conventional bronchoscopy in the periphery of the lung. ENB is indicated in the diagnostic workup of peripheral pulmonary nodules and mediastinal adenopathy, although widespread use of endobronchial ultrasound has rendered its use in the central airways anecdotal. The procedure is more time consuming than conventional bronchoscopy, including preprocedural planning and simulation as well as the actual intervention, but probably safer, limiting the risk of pneumothorax and bleeding. Current users of this technology rely on ENB for high-risk patients with small- to intermediate-size nodules as an alternative to surgery or CT-guided TTNA. Contraindications to ENB are similar to those described for conventional bronchoscopy in centers relying on conscious sedation. Some centers perform ENB under general anesthesia and must therefore take this into account when selecting high-risk patients for the procedure.
ENB is based on a Global Positioning System-like framework, but instead of using a satellite signal, it employs an electromagnetic field in order to pinpoint the precise location of a steerable probe inside the patient’s chest wall. The ENB system consists of four basic components: the aforementioned electromagnetic field and steerable probe, also known as the locatable guide, an extended working channel (EWC), and licensed software. The procedure may be performed under conscious sedation or general anesthesia, but seldom in a fully conscious patient because constant motion, rapid breathing, and coughing can preclude adequate navigation and/or sampling, while the length of the procedure will otherwise put patient tolerance to the test. We generally perform ENB under conscious sedation with a combination of midazolam and fentanyl at Fundación Jiménez Díaz University Hospital.
Procedure planning
All ENB procedures require careful planning. Data from a recent chest CT scan with specific slice thickness and intervals are saved on a disc and introduced in the planning software module of a laptop computer. During planning, the software generates a virtual bronchoscopic reconstruction of the patient’s airways and axial, coronal, and sagittal images of the patient’s chest which the operator must navigate in order to generate a number of reference points and targets. Once planning is complete, a file containing all necessary information is saved on a USB stick which is then inserted in the bronchoscopy suite’s hardware in order to download its contents and perform the ENB procedure. Virtual reference points selected during planning will be registered either manually or automatically during ENB by advancing the steerable probe (). Generally, well-known reference points such as the main and lobar carinas are chosen and although the risk of pneumothorax is quite low, usually only targets in one lung are selected for a given procedure. Mediastinal lymph nodes may also be selected, and these may be located on either side of the mediastinum.
Figure 1 Virtual reconstruction of the endobronchial route to a lesion of interest showing an obvious bronchus sign leading to the tumor.
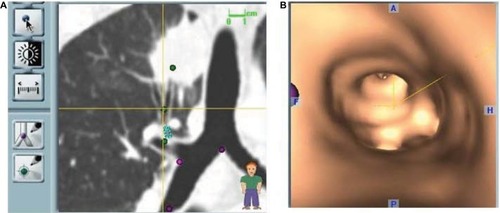
Planning allows for the operator to study the airways leading to a given target and advance through them in a virtual simulation in order to prepare for the procedure. The planning software may be used even if the procedure is planned as a conventional bronchoscopy, since the cost of ENB should discourage its use when a lesion is accessible using standard techniques.
ENB procedure
The ENB procedure, including topical airway anesthesia and conscious sedation, begins as a routine bronchoscopy. Careful monitoring of vital signs, telemetry, and oxygen saturation is of paramount importance. Since the operator’s attention will be devoted almost entirely to navigation, it is imperative that one or more assistants be in charge of patient monitoring and sample processing during ENB. The ENB procedure begins following routine airway inspection by inserting the steerable probe through the EWC of the bronchoscope. The EWC is a small flexible catheter which can reach the periphery of the lung and is designed to accommodate the steerable probe and sampling instruments. We generally wait to deploy the probe until a conventional inspection is complete, because patient tolerance of the procedure can be gauged in this way and incidental findings may alter procedure objectives. Furthermore, once the probe is connected to the hardware, it is permanently assigned to the patient in the room and cannot be cleaned or reused, so making sure the patient will tolerate the procedure is just common sense.
Once the steerable probe is inserted and advanced, registration begins. While current software upgrades rely on automatic registration, which is accomplished by advancing the steerable probe gently past preselected virtual reference points, manual registration remains an option. The latter alerts the operator to the so-called divergence error, also known as average fiducial target registration error (AFTRE), which estimates the divergence between virtual and actual targets and registration points. AFTREs of 4 mm are considered optimal, although ENB can be performed irrespective of the AFTRE, as long as the potential for missing the target is taken into account. Although automatic registration does not provide an estimate of registration error, the ENB software will not allow the procedure to continue unless the AFTRE is low enough for ENB to be performed safely and accurately.
Once registration is complete, the EWC and the probe are simultaneously guided through the airways following a computer-generated pathway complete with individual way points. Advancing the probe can also be a trial and error process in which the operator relies on virtual sagittal, coronal, and axial views together with a screen indicating the distance to the target and an icon representing the probe’s position. The ENB hardware relies on the selected reference points and information from three external sensors placed on the patient’s chest wall in order to triangulate the location of the steerable probe and make recommendations regarding navigation to the selected target(s). The appropriate turns are made using a hand-held, telescopic device and an EWC with a curved tip catheter design similar to those used in coronary angiography procedures. Feedback from the software is constant and in real time, including information regarding the probe’s pitch, roll, and direction. Once the target is reached, the EWC is fixed in place and the probe removed in order to begin sampling ().
Figure 2 ENB procedure screen capture showing that an adenocarcinoma has been reached (green dot); (A–C) Sagittal, coronal, and axial views. The nodule is quite central and difficult to reach. (D) Indicates distance to the target and probe’s position. The patient required a preoperative diagnosis in order to be eligible for a clinical trial.
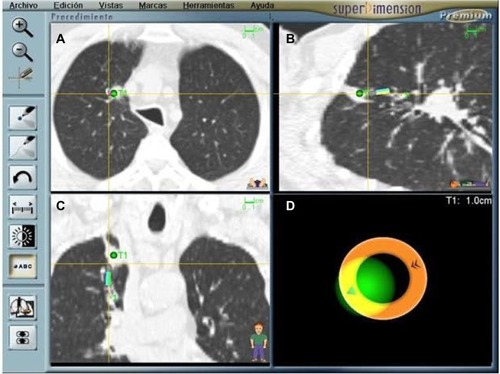
The ENB EWC is large enough to accommodate most conventional sampling instruments, including forceps, cytology brushes, and needles. A protocolized sampling procedure was routinely followed in performing catheter aspiration first, followed by forceps biopsies, brushing and/or needle sampling, and finally a mini-lavage using the EWC and a 10 mL aliquot of sterile saline. Needle aspiration can be challenging in some locations because of the resistance met by the needle as it negotiates the turns made by the EWC en route to a given target. However, the advantage of the needle is evident, as it can penetrate the airway and reach beyond it in order to sample a nodule which does not invade the bronchi in its vicinity.
Since the information provided by the software is only reliable insofar as it was obtained from static images, any significant change in the position of the target and/or registration points resulting from coughing, rapid breathing, or a pneumothorax may lead to misinformation regarding the probe’s location and therefore limit diagnostic yield. Breathing can be a challenge when a lesion is close to the diaphragm or fissures leading to constant target excursion and uncertainty regarding the exact location of the probe, since the ENB’s software cannot account for respiratory movement. It is common practice to check the sampling site by periodically reintroducing the probe in order to verify that the EWC has not been displaced or that a sudden unexpected inconsistency in the probe’s location has taken place. The latter can be an ominous warning that a pneumothorax has occurred and may be a reason to terminate the procedure, especially if the patient complains of chest discomfort.
ENB in lung cancer
Current ACCP guidelines recommend ENB for lesions which are hard to reach with the conventional bronchoscope in order to avoid unnecessary invasive tests if the equipment and expertise are available.Citation7 Cost is an issue with ENB because the locatable guide and EWC are single-use only, and start-up costs are considerable. A recent cost-analysis study comparing ENB with TTNA found that ENB increased average costs by US$3,719 per patient.Citation25 However, ENB is safer, avoiding chest tube placement in 6% of patients and minimizing other complications such as major bleeding and respiratory failure.Citation25 The major advantage of ultrasound-guided bronchoscopy is that the ultrasound probe can be reused ().
ENB yields are highly dependent on the presence of a bronchus sign as evidenced by a landmark prospective study.Citation26 The bronchus sign is more common in malignancies which tend to be spiculated and larger than benign nodules (>30 mm).Citation27,Citation28 A positive bronchus sign has also been found to be a strong predictor of diagnostic success for ultrasound-guided bronchoscopic sampling of peripheral lung nodules.Citation29 Nodule location and size may also be important for ENB success.Citation30–Citation32
ENB is a safe procedure. The risk of pneumothorax is very low and chest tube placement is rare. A recent meta-analysis of more than 1,000 procedures found that ENB caused only 32 pneumothoraces and less than 20 patients required chest tube placement.Citation33 Pneumothorax following TTNA is almost 10 times more common. Other complications such as major bleeding, arrhythmias, and death have not been reported or are exceedingly rare. Unfortunately, safety comes at a cost, since malignancy cannot be entirely excluded due to the low negative predictive value of the procedure (52.1%).Citation33 The latter remains low when a benign diagnosis such as chronic or granulomatous inflammation is obtained mirroring the accrued experience with other minimally invasive diagnostic techniques such as TTNA. This shortcoming of ENB is clinically relevant precisely because ENB is often challenged to rule out cancer in a high-risk patient with a suspicious nodule ().
Figure 3 Spiculated nodule in a high-risk patient. ENB results were consistent with aspergilloma. Long-term follow-up has confirmed the benign diagnosis which spared this patient (with limited lung function) a risky surgical resection.
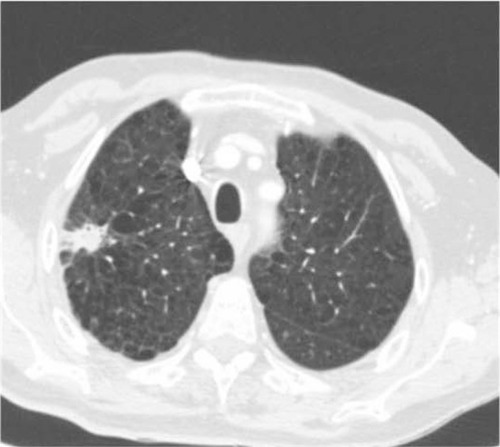
The performance of ENB is clearly inferior to the gold standard of surgical resection. ENB’s pooled sensitivity for malignancy has been reported as 71% and accuracy for malignancy does not exceed 80%.Citation33 Direct comparison of ENB with conventional or alternative navigational techniques is limited to a single trial assessing the yield of ENB compared to radial ultrasound probes.Citation30 In that randomized trial, diagnostic yields of 59%, 69%, and 87.5% were reported for ENB, radial ultrasound probe, and the combination of both techniques, respectively. The combined procedure was statistically superior to either ENB or radial probe ultrasound as stand-alone interventions. Trials comparing conventional bronchoscopy with ultrasound probe-guided sampling of peripheral pulmonary nodules have shown the latter to be more sensitive, even for small lesions.Citation34,Citation35 Probe location and lesion size determine diagnostic yields with this technique, which faces many of the limitations ENB grapples with.Citation36,Citation37 A recent meta-analysis found that ultrasound-guided bronchoscopy has a pooled sensitivity and specificity of 73% and 100%, respectively, for the detection of lung cancer in peripheral nodules.Citation38 The procedure is as safe as ENB with pneumothorax rates as low as 1%.Citation34
ENB yields may improve using general anesthesia and rapid onsite evaluation.Citation33,Citation39 Better ENB yields have also been reported in recent studies suggesting that learning curves may have underestimated outcomes, although results from a multicenter registry found disappointing results with ENB in the diagnosis of peripheral lesions.Citation40,Citation41
Finally, it should be noted that ENB’s applications in lung cancer go beyond diagnosis. The procedure has been used for the accurate placement of fiducial markers in order to facilitate stereotactic radiation therapy treatments in selected patients with lung cancer.Citation42 It has also been employed as a guide for endoscopic brachytherapy treatment of peripheral lesions in inoperable patients,Citation43 and may be useful in order to mark small pulmonary nodules earmarked for resection using pleural dye.Citation44 Furthermore, ENB has been shown to be an effective means of obtaining tissue samples for molecular analysis, including EGFR mutations and EML4-ALK trans-locations in lung tumors, proving its versatility and clinical value.Citation45 Novel applications include placement of ablation probes in a target tumor and guidance of endobronchial ablation catheters for the minimally invasive treatment of lung cancer.Citation46,Citation47
Conclusion
The clinical utility of ENB in the diagnosis of lung cancer is well established, although it continues to be the subject of ongoing clinical studies. Patient and target selection continue to be the key variables influencing ENB yields and outcomes. Future applications of ENB in lung cancer go beyond diagnostics, with ongoing studies investigating its role in therapeutics, either as an aid to radiation therapy or as a guide for novel ablation catheters. Alternative guided bronchoscopic techniques such as ultrasound-guided bronchoscopy currently compete with ENB, although they face many of its shortcomings with the possible exception of cost and availability.
Disclosure
LS served as a paid consultant for Covidien, owner of Superdimension in 2013. The author reports no other conflicts of interest in this work.
References
- OstDFeinAMFeinsilverSHClinical practice. The solitary pulmonary noduleN Engl J Med2003348252535254212815140
- The National Lung Screening Trial Research TeamReduced lung cancer mortality with low-dose computed tomographic screeningN Engl J Med2011365539540921714641
- GambhirSSShepherdJEShahBDAnalytical decision model for the cost-effective management of solitary pulmonary nodulesJ Clin Oncol1998166211321259626211
- BernardAResection of pulmonary nodules using video-assisted thoracic surgery. The Thorax GroupAnn Thorac Surg19966112022048561553
- Congregado LoscertalesMGirón ArjonaJCJiménez MerchánRUsefulness of video-assisted thoracoscopy for the diagnosis of solitary pulmonary nodulesArch Bronconeumol200238941542012237012
- SchreiberGMcCroryDCPerformance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidenceChest2003123Suppl 1115S128S12527571
- RiveraMPMehtaACWahidiMMEstablishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelinesChest20131435e142Se165S23649436
- WeinerRSSchwartzLMWoloshinSWelchHGPopulation-based risk for complications after transthoracic needle biopsy of a pulmonary nodule: an analysis of discharge recordsAnn Intern Med2011155313714421810706
- CoveyAMGandhiRBrodyLAGetrajdmanGThalerHTBrownKTFactors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patientsJ Vasc Interv Radiol200415547948315126658
- ZarboRJFenoglio-PreiserCMInterinstitutional database for comparison of performance in lung fine-needle aspiration cytology. A College of American Pathologists Q-Probe Study of 5264 cases with histologic correlationArch Pathol Lab Med199211654634701580748
- MitrukaS1LandreneauRJMackMJDiagnosing the indeterminate pulmonary nodule: percutaneous biopsy versus thoracoscopySurgery199511846766847570322
- JinFMuDChuDFuEXieYLiuTSevere complications of bronchoscopyRespiration200876442943318716395
- HuangCTRuanSYLiaoWYRisk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesionsPLoS One2012711e4912523145094
- ReinosoMALechinAVaronJWadeLComplications from flexible bronchoscopy in a training programJ Bronchology Interv Pulmonol19963177181
- RiveraMPMehtaACAmerican College of Chest PhysiciansInitial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition)Chest2007132Suppl 3131S148S17873165
- TorringtonKCKernJDThe utility of fiberoptic bronchoscopy in the evaluation of the solitary pulmonary noduleChest19931044102110248404158
- StringfieldJTMarkowitzDJBentzRRWelchMHWegJGThe effect of tumor size and location on diagnosis by fiberoptic bronchoscopyChest1977724474476908215
- ChechaniVBronchoscopic diagnosis of solitary pulmonary nodules and lung masses in the absence of endobronchial abnormalityChest199610936206258617067
- BaakliniWAReinosoMAGorinABSharafkanehAManianPDiagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodulesChest200011741049105410767238
- ÁlvarezCJBastarrikaGDisdierCManejo del nódulo pulmonar solitario [Guideline on management of solitary pulmonary nodule]Arch Bronconeumol201450285293 Spanish24630316
- SchwarzCSchonfeldNBittnerRCValue of flexible bronchoscopy in the pre-operative work-up of solitary pulmonary nodulesEur Respir J201341117718222496316
- ZhangYZhangYChenSIs bronchoscopy necessary in the preoperative workup of a solitary pulmonary nodule?J Thorac Cardiovasc Surg20151501364025841658
- JhunBWUmSWSuhGYPreoperative flexible bronchoscopy in patients with persistent ground-glass nodulePLoS One2015103e012125025803430
- van ’t WesteindeSCHorewegNVernhoutRMThe role of conventional bronchoscopy in the workup of suspicious CT scan screen-detected pulmonary nodulesChest2012142237738422302298
- DaleCRMadtesDKFanVSGordenJAVeenstraDLNavigational bronchoscopy with biopsy versus computed tomography-guided biopsy for the diagnosis of a solitary pulmonary nodule: a cost-consequences analysisJ Bronchology Interv Pulmonol201219429430323207529
- SeijoLMde TorresJPLozanoMDDiagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective studyChest201013861316132120435658
- BilaçerogluSKumcuogluZAlperHCT bronchus sign-guided bronchoscopic multiple diagnostic procedures in carcinomatous solitary pulmonary nodules and massesRespiration199865149559523368
- Haro EstarriolMRubio GodayMVizcaya SánchezMBaldó PadróXCasamitjá SotMTSebastian QuetglásFBiopsia pulmonar broncoscópica con fluoroscopia en lesiones pulmonares localizadas. Estudio de 164 casos [Bronchoscopic lung biopsy with fluoroscopy to study 164 localized pulmonary lesions]Arch Bronconeumol200440483488 Spanish15530339
- MinezawaTOkamuraTYatsuyaHBronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational studyBMC Med Imaging2015152126092497
- EberhardtRAnanthamDErnstAFeller-KopmanDHerthFMultimodality bronchoscopic diagnosis of peripheral lung lesions: a randomised controlled trialAm J Respir Crit Care Med20071761364117379850
- JensenKWHsiaDWSeijoLMMulticenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodulesJ Bronchology Interv Pulmonol201219319519923207460
- RiveraPGonzalezJRabinesAPredictors of diagnostic yield and complications of electromagnetic navigation bronchoscopy in peripheral pulmonary lesions. ATS International Conference AbstractsATS Journal2015 abstract 3731
- GexGPralongJACombescureCSeijoLRochatTSoccalPMDiagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysisRespiration201487216517624401166
- Wang MemoliJSNietertPJSilvestriGAMeta-analysis of guided bronchoscopy for the evaluation of the pulmonary noduleChest2012142238539321980059
- PaoneGNicastriELucantoniGEndobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesionsChest200512853551355716304312
- YamadaNYamazakiKKurimotoNFactors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesionsChest2007132260360817573504
- HuangCTHoCCTsaiYJYangPCFactors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesionsRespirology200914685986419703067
- SteinfortDPKhorYHManserRLIrvingLBRadial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysisEur Respir J201137490291020693253
- BrownbackKRQuijanoFLathamHESimpsonSQElectromagnetic navigational bronchoscopy in the diagnosis of lung lesionsJ Bronchology Interv Pulmonol2012192919723207349
- LamprechtBPorschPWegleitnerBStrasserGKaiserBStudnickaMElectromagnetic navigation bronchoscopy (ENB): increasing diagnostic yieldRespir Med2012106571071522391437
- OstDEErnstALeiXDiagnostic yield and complications of bronchoscopy for peripheral lung lesions: results of the AQuIRE registryAm J Respir Crit Care Med20161931687726367186
- AnanthamDFeller-KopmanDShanmughamLNElectromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility studyChest2007132393093517646225
- HarmsWKrempienRGrehnCHensleyFDebusJBeckerHDElectromagnetically navigated brachytherapy as a new treatment option for peripheral pulmonary tumorsStrahlenther Onkol2006182210811116447018
- KrimskyWSMinnichDJCattaneoSMThoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye markingJ Community Hosp Intern Med Perspect20144123084
- HaDChoiHAlmeidaFAHistologic and molecular characterization of lung cancer with tissue obtained by electromagnetic navigation bronchoscopyJ Bronchology Interv Pulmonol2013201101523328135
- NarsuleCKSales Dos SantosRGuptaAThe efficacy of electromagnetic navigation to assist with computed tomography-guided percutaneous thermal ablation of lung tumorsInnovations (Phila)20127318719022885459
- EberhardtRKahnNHerthF‘Heat and Destroy’: bronchoscopic-guided therapy of peripheral lung lesionsRespiration201079426527320203535
