Abstract
Epidural analgesia is an extremely effective and popular treatment for labor pain. In this review, we trace the history of the use of epidural analgesia and its refinements. We then outline the goals of treatment and methods used to attain those goals. The use of low concentrations of local anesthetics, combined with lipid-soluble opioids, does not impede the progress of labor or depress the newborn. The incidence of side effects is low. Maintenance of analgesia that allows patient control enhances patient satisfaction.
Introduction
Epidural analgesia is an extremely effective and popular treatment for labor pain. In Canada, the epidural rate varies between the provinces from 30% to 69%.Citation1 The use of epidural analgesia in the US has tripled between 1981 and 2001, with 60% of women using this technique in large hospitals.Citation2 In this review, we will outline a brief history of the use of epidural analgesia and examine the current techniques of initiation and maintenance of pain relief. We will also discuss the main complications and contraindications for this method of analgesia.
In this review, epidural analgesia refers to local anesthetics and adjuvants injected into the epidural space. Spinal anesthesia refers to local anesthetic, with or without adjuvants, injected into the subarachnoid space. Combined spinal–epidural analgesia includes analgesia initiated with an intrathecal injection and placement of an epidural catheter to provide a route for additional drug. Neuraxial analgesia includes spinal, epidural, and combined spinal–epidural analgesia.
History
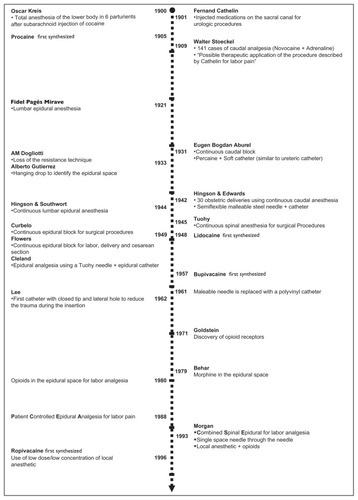
An outline of the history of neuraxial analgesia is shown in . The introduction of neuraxial analgesia into obstetric practice took place at the end of the 19th century, one year after August Bier, a German surgeon, described six lower extremity operations rendered painless by means of “cocainization of the spinal cord”. Oskar Kreis, a Swiss obstetrician, described total anesthesia of the lower body in six laboring parturients after subarachnoid injection of cocaine. He injected 0.01 g of cocaine intrathecally at the L4–5 interspace and observed complete pain relief within 5–10 minutes. Like Bier, Kreis observed no serious complications, but severe vomiting and headache occurred frequently. Postdural puncture headache would prove to be one of the main limitations associated with subarachnoid block for labor analgesia.
In 1909 Walter Stoeckel, a German obstetrician, reported his experience in 141 cases of caudal epidural analgesia for labor pain. He studied healthy parturients of mixed parity. The injections were done at the end of the first stage or during the second stage of labor. His success rate was about 50%, with 16 patients experiencing “very little pain”. This technique did not require puncture of the dura mater, and caused severe headache much less frequently than subarachnoid block. He used procaine (novocaine), which had been synthesized in 1905 and was much less toxic than cocaine. This added to the safety of the technique.Citation3
The use of a catheter placed into the caudal epidural space was first described by Eugen Bogdan Aburel in 1931. Aburel introduced a needle at the caudal level, then a soft catheter was advanced through the needle, after which the needle was removed, leaving the catheter in situ.Citation4 This allowed repeated injections throughout labor without the need to repeat the procedure.
Dissatisfaction with neuraxial analgesia occurred because of poor reliability, safety concerns, and the feeling of lower limb paralysis patients experienced when given large doses of local anesthetic. In the early 1960s, the lumbar epidural replaced caudal analgesia as the preferred technique. Compared with the caudal route, lumbar epidural analgesia is more comfortable for the patient and easier to perform. The technique required less local anesthetic. Motor function of the lower extremities and abdominal muscles can be maintained. The extent of sympathectomy can be better controlled, resulting in less maternal hypotension. The block can be extended and used for cesarean section if necessary. During this period of time, bupivacaine was synthesized and became the drug of choice in obstetrics because of its long duration of action and absence of tachyphylaxis. The use of lumbar epidural catheters in the 1970s permitted administration of pain relief early in labor, rather than only at the time of delivery.
Several improvements in epidural analgesia occurred in the 1970s and 1980s. Continuous infusions replaced clinician boluses, leading to enhanced patient safety and satisfaction.Citation5 During this period of time, epidural infusion pumps became more compact and reliable. In 1988, Gambling et al described “patient-controlled epidural analgesia” for pain control during labor.Citation6 This technique allowed the patient to titrate the amount of drug required to her own needs. Originally, the technique consisted of patient-initiated boluses only, but soon most clinicians included a continuous background infusion in addition to patient-initiated doses. The discovery of opioid receptors in the spinal cord led to the use of opioid/local anesthetic mixtures that further reduced maternal motor block and reduced the risk of local anesthetic toxicity.
More recently, combined spinal–epidural analgesia has become popular. The spinal component provides rapid analgesia with very little motor block of the lower extremities. An epidural catheter is then placed to ensure analgesia is available throughout labor.
Goals of therapy
Childbirth has been recognized as among the most painful experiences known. Numerous strategies, both pharmacologic and nonpharmacologic, have been used as treatment. However, childbirth is a multidimensional experience and when considering treatment, one must balance between pain relief and other aspects, such as physical, emotional, psychological, sociologic, and sometimes religious considerations. In other words, pain relief may not be enough to make childbirth a fulfilling and satisfactory experience. In this section we will consider the goals of therapy for labor pain and how neuraxial analgesia helps to accomplish these goals.
During the 1930s and 1940s, regional block was rarely used for labor analgesia. Instead, women often received high doses of morphine and scopolamine. This was sometimes supplemented with inhalational analgesia with ether, chloroform, nitrous oxide, or trichloroethylene. These medications were often accompanied by complete loss of consciousness with the accompanying dangers of maternal aspiration and neonatal depression. In addition, amnesia and the inability to participate at the time of delivery resulted in poor patient satisfaction. As a result, better methods of providing pain relief were sought. lists some of the characteristics of an “ideal” labor analgesic. The main goal of neuraxial analgesia is to have as many of these characteristics as possible.
Table 1 Characteristics of ideal labor analgesia
Effective pain relief
Neuraxial analgesia fulfils many of these characteristics. While parenteral opioids may provide sedation, relaxation, and comfort, there is strong evidence to suggest that morphine and meperidine do not decrease pain intensity.Citation7 Epidural analgesia provides significantly more analgesia, as measured by visual analog scale in both the first and second stage of labor than parenteral opioid.Citation8
Safety
While side effects can occur, the incidence of permanent maternal injury is low. Neuraxial analgesia results in less neonatal depression than parenteral opioids.Citation8
Good progress and outcome of labor
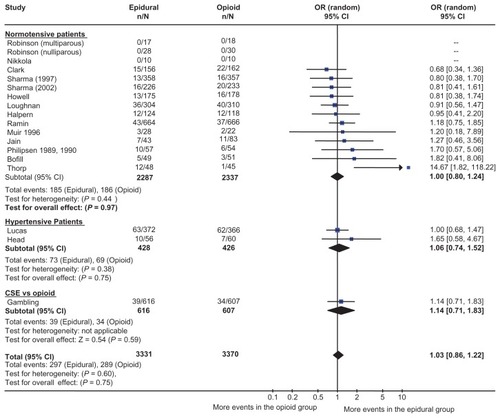
There have been numerous randomized controlled trials comparing neuraxial analgesia with parenteral opioid. A meta-analysis combined 18 studies comprised of over 6600 patients.Citation9 shows the odds ratio (OR) and 95% confidence interval (CI) for the studies in the meta-analysis. The incidence of cesarean section was almost identical in the two groups (OR 1.03; 95% CI: 0.86–1.22). Neuraxial analgesia was associated with a statistically significant increase in the incidence of operative vaginal delivery, but this may have been due to changes in behavior. One of the authors explicitly noted the use of forceps for resident training was facilitated in parturients who received epidural analgesia.Citation10 There was no difference in the length of the first stage of labor, although the second stage was a few minutes longer.
Figure 2 Epidural analgesia versus parenteral opioid analgesia and incidence of cesarean section. The number of patients who had a cesarean section, odds ratio, and 95% confidence interval are shown for each study. The size of the box is proportional to the weight of the study in the meta-analysis. The scale is logarithmic. For studies with no cesarean sections, the odds ratio could not be calculated. Copyright © 2005, Blackwell Publishing. Reproduced with permission from Leighton BL, Halpern SH. Epidural analgesia and the progress of labor. In: Halpern SH, Douglas MJ, editors. Evidence-based Obstetric Anesthesia. Oxford, UK: Blackwell Publishing; 2005.
Minimal effects on fetus and newborn
While all medications cross the placenta and may be measured in the newborn, local anesthetics do not cause neonatal depression. Compared with parenteral opioids, neuraxial analgesia is associated with better Apgar scores at one minute and a reduced need for administration of neonatal naloxone.Citation8
Minimal maternal side effects
For the last three decades, research into labor analgesia has been focused on minimizing the maternal side effects of neuraxial analgesia. Several strategies have been explored. These will be discussed in detail in the following sections.
Choice of local anesthetics
In North America, bupivacaine and ropivacaine are commonly used for labor analgesia. Although there is some experience with levobupivacaine, primarily in the UK, this drug does not seem to have any advantages when compared with the others. Bupivacaine was superior to the older local anesthetics, such as lidocaine, because of its increased duration of action, reduced incidence of tachyphylaxis, and reduced intensity of lower limb motor block. Ropivacaine was synthesized in order to reduce the cardiotoxicity associated with bupivacaine and to reduce motor block further.Citation11
The use of bupivacaine and ropivacaine in labor has recently been reviewed.Citation12 Considering the low doses used for labor, toxicity is rarely associated with either drug. Both are effective analgesics, with little or no difference in maternal satisfaction or effect on labor. There is some evidence to suggest that ropivacaine may produce less motor block in prolonged labors, but the difference may be attributable to differences in drug potency.Citation13
Choice of concentration of local anesthetic
Traditional epidural analgesia was initiated with 0.25%– 0.5% bupivacaine and maintained with intermittent bolus doses of similar anesthetic solutions. Dense motor block of the lower extremities resulted in dissatisfaction with the technique. Collis et alCitation14 conducted a randomized controlled trial that compared bupivacaine 0.25% to 0.1% with fentanyl for maintenance of labor analgesia. Using post-partum questionnaires, they found that women who received 0.1% bupivacaine felt that they had better self-control (P = 0.001), less lower limb weakness, and more mobility than the control group. It is also possible that drug concentration may affect mode of delivery. While the cesarean section rate is not affected, COMET (Comparative Obstetric Mobile Epidural Trial) investigators in the UK found an increase in operative vaginal delivery rate in women assigned to maintenance of analgesia using 0.1% bupivacaine compared with those maintained with 0.25%.Citation15 In both of these trials, there was no difference in the quality of analgesia. These large randomized trials provide sufficient evidence to suggest that low concentrations of local anesthetics provide excellent analgesia and superior maternal satisfaction compared with higher concentrations.
Maintenance of analgesia
The use of continuous epidural catheters allows maintenance of labor analgesia for prolonged periods of time. Intermittent boluses (by physician or midwife) can provide satisfactory analgesia, but require constant availability of a clinician capable of providing analgesia. Continuous infusions of low concentrations of local anesthetic result in less variability in the quality of analgesia, and require clinician boluses only for breakthrough pain. More recently patient-controlled epidural analgesia has become the preferred technique for maintenance of labor analgesia. This technique has proven to be safe and effective when used with dilute solutions of local anesthetics, with or without a lipid-soluble opioid, such as fentanyl or sufentanil. Clinicians set the bolus dose and lockout interval, and may choose a continuous infusion rate. Compared with continuous infusion alone, patients who receive patient-controlled epidural analgesia require fewer clinician interventions, a reduced dose of local anesthetic, and have less motor block of the lower extremities.Citation16 Patient-controlled epidural analgesia superimposed on a continuous infusion further reduces the need for clinician interventions without increasing the incidence of motor block.Citation17
There are a wide range of patient-controlled epidural analgesia settings that result in excellent analgesia with minimal motor block. The bolus dose can be set between 4 and 12 mL, with the most common settings between 5 and 8 mL. The lockout interval can be varied, bearing in mind it takes about 10 minutes for the patient to experience pain relief. There is also a wide range of appropriate settings for the background infusion. Low background rates result in more control by the parturient. A recent review discusses these issues in detail.Citation17
Combined spinal–epidural analgesia
The combined spinal–epidural technique results in rapid analgesia with minimal or no impairment of ambulation. Typically, 1 or 2 mg of bupivacaine are combined with 5–15 μg of fentanyl and given intrathecally. An epidural catheter is placed for immediate or later activation using patient-controlled epidural analgesia. This results in profound analgesia that takes effect more quickly than other low-dose neuraxial techniques. There is no difference in obstetric outcomes, such as the incidence of emergency cesarean section or duration of labor.Citation18 There is no difference in the incidence of instrumental vaginal delivery when combined spinal-epidural analgesia is compared with low concentrations of local anesthetic.
Norris et al enrolled 2183 patients in a study that compared combined spinal–epidural versus epidural analgesia for labor. No difference was found in the incidence of emergency cesarean section or duration of the first or second stages of labor for both techniques.Citation18
The COMET study enrolled 1054 patients in three groups, ie, epidural with high-concentration bupivacaine (0.25%), epidural with a low concentration of bupivacaine (0.125% bupivacaine + fentanyl 2 μg/mL) and combined spinal-epidural analgesia.Citation15,Citation19 The investigators found no difference in the incidence of cesarean section, fetal distress, or length of the first or second stages of labor. Patients who received combined spinal–epidural and low-dose epidural analgesia had a similar incidence of operative vaginal delivery that was lower than those who received 0.25% bupivacaine.
An increased incidence of fetal bradycardia may be associated with the use of combined spinal–epidural analgesia. Mardirosoff conducted a systematic review and concluded that the incidence of fetal bradycardia was higher in patients who received a combined spinal–epidural (8.3% versus 4.7%).Citation20 However, there was no increase in the rate of cesarean section, operative deliveries, oxytocin usage, or babies with low Apgar score.
The mechanisms through which intrathecal opioids cause bradycardia is not well understood, but it may be related to a rapid decrease in circulating catecholamines, mainly β-sympathomimetics, secondary to rapid pain relief. The sudden drop in circulating levels of catecholamines, which are known to reduce uterine tone, could increase uterine activity and tone, in turn, can cause a decrease in amount of oxygen delivery to the fetus, leading to fetal bradycardia. An increase in uterine hypertonus has been associated with the use of combined spinal–epidural analgesia.Citation21 Therefore, there may be an additional risk of fetal bradycardia, but this does not impact on obstetric or neonatal outcomes.
Side effects and complications
While neuraxial analgesia is usually safe, complications can occur. Some may be directly attributable to drugs or technique, and these are discussed here and shown in . Others, such as chronic back painCitation22 and cesarean section,Citation8 are attributed to neuraxial block but are not caused by the technique. Finally, the cause of some complications, such as intrapartum fever and breastfeeding difficulties, is unclear.
Table 2 Complications of neuraxial analgesia
Hypotension
Hypotension is often defined as a 20%–30% drop in systolic blood pressure (compared with baseline) or a systolic blood pressure less than 100 mmHg. Because uterine blood flow and fetal oxygenation is directly related to maternal arterial pressure, hypotension is an important side effect that must be treated rapidly. The incidence of hypotension after the initiation of neuraxial analgesia during labor is estimated to be about 10%. This incidence is similar between combined spinal–epidurals and low-concentration epidurals.Citation23 The incidence of hypotension is lower in laboring women than in nonlaboring women. Hypotensive episodes are easily treated with complete uterine displacement, additional intravenous fluids, and in some occasions, addition of vasopressors. The treatment should be more aggressive if there is a concerning fetal heart rate pattern or if the mother is symptomatic.
Pruritus
Pruritus is the most common side effect of neuraxial analgesia. Citation24 The incidence and severity is dependent on the opioid dose, and is more frequent with intrathecal opioids than with epidural opioids (58% versus 30%).Citation20 The cause of pruritus is not well understood, but it is unlikely to be related to histamine release. Antihistamines, often prescribed to treat pruritus after neuraxial opioids, are usually ineffective. There is increasing evidence that neuraxial opioid-induced pruritus is mediated through central μ-opioid receptors.Citation25 Opioid antagonists (eg, naloxone) or partial agonist-antagonists (eg, nalbuphine) are effective in relieving pruritus.
Inadequate analgesia
According to the definition of “neuraxial analgesia failure” employed by researchers, the incidence varies widely among different studies. In a retrospective quality assurance study, Pan et alCitation26 investigated the failure rate with 12,590 neuraxial procedures for labor analgesia in a teaching institution. Failure was defined as epidural or combined spinal–epidural procedures resulting in inadequate analgesia or no sensory block after adequate dosing at any time after initial placement, inadvertent dural puncture by the epidural needle or catheter, intravenous epidural catheter, or any technique requiring replacement or alternative management. The overall failure rate was 12%, being significantly lower after combined spinal–epidural than after epidural analgesia (10% versus 14%; P < 0.001). In that study, 5.6% of the epidural catheters that were initially functioning had to be replaced during the course of labor. Inadequate analgesia with the epidural catheter was reported in 8.4% of the epidural group and in 4.2% of the combined spinal–epidural group.
Accidental dural puncture and postdural puncture headache
Accidental dural puncture is an uncommon complication of epidural block. When it occurs, it can produce severe morbidity, although usually of limited duration. The postdural headache that results from accidental dural puncture can severely limit a new mother’s ability to care for her newborn. Therefore, these headaches are often treated soon after diagnosis. Epidural blood patch (a sample of the patient’s own blood, aseptically drawn and injected into the epidural space) is the most effective treatment of this complication.Citation27
Choi et alCitation28 performed a meta-analysis that involved more than 30,000 obstetric patients. They determined that the risk of accidental dural puncture during epidural insertion was 1.5%. Of those patients who had accidental dural puncture, approximately 52% will result in postdural puncture headache.
van de Velde et alCitation29 reported a retrospective review of more than 17,000 obstetric neuraxial blocks. The overall incidences of accidental dural puncture and postdural puncture headache in this population were 0.32% and 0.38%, respectively. However if more than one attempt was required to identify the epidural space, the accidental dural puncture rate increased to 0.91%. Fifty-six percent of patients with witnessed accidental dural puncture developed postdural puncture headache. The postdural puncture headache rate was similar between the epidural group (0.21%) and the combined spinal–epidural group (0.20%). When compared with epidural techniques, the combined spinal–epidural did not protect against accidental dural puncture. The size of spinal needle (27-gauge or 29-gauge pencil-point needle) did not affect the incidence of postdural puncture headache in the combined spinal–epidural group. In this study, 84% of patients who had postdural puncture headache needed a blood patch, and 15% needed a second blood patch.
Breastfeeding
Whether or not neuraxial analgesia may impact breastfeeding initiation and duration is controversial. Observational studies give conflicting results.Citation30,Citation31 Beilin et al reported a randomized controlled trial using three different doses of fentanyl for maintenance of epidural analgesia. While there was no effect on breastfeeding initiation, they reported a reduction in breastfeeding frequency at six weeks in the high-dose fentanyl group.Citation32 No biologically plausible explanation could be provided. The largest randomized controlled trial on breastfeeding initiation and duration was published by Wilson et al.Citation33 In this secondary analysis of the COMET study cited earlier,Citation19 1054 nulliparous patients were randomly assigned to a control epidural (bupivacaine, no fentanyl), combined spinal–epidural, or low-dose epidural (both with bupivacaine/fentanyl at initiation and during maintenance). A fourth group comprising matched controls received parenteral pethidine for labor analgesia. There was no difference in the incidence of initiation of breastfeeding among the three groups (63%–66%). This rate was higher than the pethidine control group (56%). The duration of breastfeeding was also similar among the three groups (mean 14–15 weeks). This was similar to patients who received parenteral pethidine (14 weeks). The authors concluded that neither epidural analgesia alone or epidural analgesia with fentanyl had any adverse effect on the initiation or duration of breastfeeding.
Nerve damage
Epidural catheters may injure nerve roots either because they are inappropriately rigid or because they are threaded too deeply and may compress a root,Citation34 although a flexible catheter is unlikely to do lasting damage to a nerve root in the epidural space.
In 2009, the Royal College of Anaesthetists in the UK published the third National Audit Project of Major Complications of Central Neuraxial Block.Citation35 In total, 320,425 obstetric procedures were analyzed. The incidence of permanent harm after spinal anesthesia was 1.5 in 100,000, 0.6 in 100,000 after epidural, and 3.9 in 100,000 after a combined spinal–epidural procedure. Overall, in this series, the incidence of permanent harm following neuraxial block was 1.2 in 100,000. Of note, the incidence of nerve damage from obstetric causes unrelated to neuraxial analgesia is almost 1%.Citation36
Infection
Epidural abscess and meningitis are infrequent complications of neuraxial techniques. Reynolds et alCitation37 reported a combination of the findings of 10 surveys. The incidence of epidural abscess after obstetric epidural procedures was 3/100,000. The incidence of meningitis after spinal and combined spinal–epidural anesthesia was 1/39,000.
Abscess formation complicates 0.2–3.7 per 100,000 obstetric epidurals. Bacterial meningitis after neuraxial block had a projected incidence of 0–3.5 in 100,000 (95% CI). It is more frequent after spinal and combined spinal–epidural techniques than after an epidural.Citation35
Epidural hematoma
In spite of the engorgement of epidural veins during pregnancy, epidural hematoma causing neurologic deficits is very rare in the obstetric population, and perhaps the hyper-coagulable state of pregnancy acts as a protective factor. In a report of six surveys that together involved more than 1,220,000 obstetric epidural procedures, one case of epidural hematoma was found.Citation34
Ruppen et alCitation38 summarized the results of 27 studies involving 1,370,000 women who had received neuraxial blocks. The risk of epidural hematoma was 1/168,000 overall.
Technique
Patient position
Correct patient positioning is probably the most important factor leading to a successful block. First, it is important to align the vertebrae so that the needle can be inserted between the spinous processes into the epidural space. Twisting of the back will result in the needle contacting the lamina. Second, a position that maximizes the distance between the spinous processes is preferable. Because most parturients at term have a lordotic lumbar spine, maneuvers that flatten or reverse the curvature are advantageous. For example, the “hamstring stretch position” (sitting position with maximum knee extension, hip adduction, and forward lean) has been described to accomplish this. Finally, it is important to be able to identify the midline. In most patients, this can be done by palpating the spinous processes. However, in some patients, the spinous processes are not palpable because of excess adipose tissue or well developed paraspinous muscles. In the sitting position, the midline can be found by drawing a straight line between the vertebra of C7 (palpable in most patients) and the coccygeal cleft. Ultrasound identification of the midline may be useful to locate the midline, determine the approximate depth to the epidural space, and to determine the level of puncture.Citation39,Citation40
Epidural block can be performed in the lateral or sitting position, and the decision is usually based on anesthesiologist and patient preferences. When the spinous processes are not easily palpable, the sitting position is preferred. In patients with easily identifiable landmarks, Vincent and ChestnutCitation41 found that neither the lateral nor the sitting position was clearly superior with regard to patient comfort, but heavier patients preferred the sitting position. In some patients, the sitting position may be associated with orthostatic hypotension and syncope. For this reason, it is important for an assistant to provide continuous support to the patient during the procedure. Maternal cardiac output can be reduced in the left lateral position, if held too tightly in position.Citation42
Identification of epidural space
Early methods to find the epidural space relied on identification of negative pressure in the epidural space (eg, hanging drop, Macintosh balloon). The two most common methods used rely on loss of resistance to injection of saline or air as the needle advances through the ligamentum flavum and enters the epidural space. Each technique has its own benefits and drawbacks. Compared with air, loss of resistance to saline has the advantage of providing a more obvious tactile endpoint when the needle enters the epidural space. However, because saline is a clear fluid, it may be confused with cerebrospinal fluid and dural puncture may be masked. A large volume of air or saline should not be injected when confirming needle placement. Large volumes of air may result in inadequate analgesia or patchy block.Citation43 Large volumes of saline may result in inadequate analgesia because of dilution.Citation44
Schier et alCitation45 identified four studies that enrolled obstetric patients who had epidural analgesia and performed a metaanalysis on the results. In these randomized, controlled trials, epidural needle placement was confirmed with loss of resistance to either air or liquid. The outcomes included the incidence of difficulty passing the epidural catheter, intravascular cannulation, paresthesia, dural puncture, postdural puncture headache, and partial block. The authors concluded that the use of air or fluid to identify the epidural space did not change the incidence of any of these outcomes. Recently, a large randomized controlled trial confirmed these results.Citation46 This suggests that either method is suitable for epidural placement in labor.
Aseptic technique
In order to avoid the risk associated with infectious complications of neuraxial analgesia, meticulous aseptic technique should be observed. Because complications such as meningitis and epidural abscess are rare, there are few clinical trials that demonstrate whether or not a particular intervention is useful in preventing infection. Colony counts are often reported as surrogate outcomes instead. Recently the American Society of Anesthesiologists published clinical practice guidelines to prevent infectious complications from neuraxial blocks.Citation47 While the guidelines recognize limitations in the data, experts in the field and members of the American Society of Anesthesiologists suggest that the precautions listed in be employed.
Table 3 Recommended aseptic technique for neuraxial analgesiaTable Footnote*
Contraindications
While neuraxial analgesia is versatile and safe, there are contraindications to the technique. Absolute contraindications include patient refusal, lack of adequate equipment, lack of expertise or supervisory staff, severe coagulopathy, and infection at the site of puncture. Some patients may be technically challenging because of previous back surgery, such as lumbar fusions and Harrington rods. Relative contraindications are listed in .
Table 4 Relative contraindications for neuraxial block
Patients with a low platelet count may have a neuraxial block provided they do not have abnormal bleeding. The exact “safe number” of platelets is unknown, but most anesthesiologists would offer neuraxial analgesia if the platelet count was more than 80,000/mm3 and platelet function is normal. Most anesthesiologists consider a platelet count of less than 80,000/mm3 as a relative contraindication to neuraxial anesthesia.Citation47
Fever is not a contraindication to neuraxial block. However, if septicemia is suspected, it should be treated with antibiotics before proceeding with neuraxial analgesia. The most common reason for septicemia in the obstetric patient is chorioamnionitis.
Progressive neurologic disease, such as multiple sclerosis, presents a challenge to the anesthesiologist. The disease may progress unpredictably and, if a relapse occurs, neuraxial analgesia may be implicated as a cause. Therefore, it is prudent to document any pre-existing neurologic deficits and have a full discussion of the risks and benefits of neuraxial analgesia, preferably before labor starts in order to inform the patient.
Raised intracranial pressure from a supratentorial space-occupying lesion is an absolute contraindication to lumbar puncture because the brain may shift down, causing coning and compression of the medulla. In most cases, it is also prudent to avoid epidural analgesia because accidental dural puncture may occur.
Neuraxial analgesia causes a reduction in sympathetic tone, resulting in increased venous pooling in the legs and reduced systemic vascular resistance. This may cause severe hypotension in patients with severe hypovolemia or critical aortic stenosis.
Future directions
Technologic advances may change the way analgesia is maintained. Recently, Wong et al reported a computer-integrated method that adjusts the background infusion to the number of patient-controlled demands.Citation48 Compared with traditional patient-controlled epidural analgesia, this system increased maternal analgesic satisfaction. More recently, intermittent mandatory boluses have been added to patient-controlled epidural analgesia. When compared with a basal infusion, there was a reduction in the amount of local anesthetic used and an increase in patient satisfaction.Citation49 Currently, neither of these technologies is available commercially.
Summary
Neuraxial analgesia is commonly performed to relieve labor pain. Compared with other techniques, it is the most effective form of analgesia. Recent innovations in drug combinations and delivery systems have resulted in a flexible technique that meets the needs of most parturients in a safe and effective manner. The use of low concentrations of local anesthetics, combined with lipid-soluble opioids does not impede the progress of labor or depress the newborn. The addition of patient-controlled epidural analgesia and innovations using new technologies enhance patient satisfaction.
Disclosure
The authors report no conflicts of interest in this work.
References
- Canadian Institute of Health InformationHighlights of 2008–2009: Selected indicators describing the birthing process in Canada Available from: http://www.cihi.caAccessed 2010 Sep 3
- BucklinBAHawkinsJLAndersonJRUllrichFAObstetric anesthesia workforce survey: Twenty-year updateAnesthesiology200510364565316129992
- DoughtyAWalter Stoeckel (1871–1961): A pioneer of regional analgesia in obstetricsAnaesthesia1990454684712200301
- CurelaruISanduLEugen Bogdan Aburel (1899–1975). The pioneer of regional analgesia for pain relief in childbirthAnaesthesia1982376636696178307
- EvansKRCarrieLEContinuous epidural infusion of bupivacaine in labour: A simple methodAnaesthesia197934310315453503
- GamblingDRYuPColeCMcMorlandGHPalmerLA comparative study of patient controlled epidural analgesia (PCEA) and continuous infusion epidural analgesia (CIEA) during labourCan J Anaesth1988352492543289769
- OlofssonCEkblomAEkman-OrdebergGHjelmAIrestedtLLack of analgesic effect of systemically administered morphine or pethidine on labour painBr J Obstet Gynaecol19961039689728863693
- LeightonBLHalpernSHThe effects of epidural analgesia on labor, maternal, and neonatal outcomes: A systematic reviewAm J Obstet Gynecol2002186S69S7712011873
- LeightonBLHalpernSHEpidural analgesia and the progress of laborHalpernSHDouglasMJEvidence-based Obstetric AnesthesiaOxford, UKBlackwell Publishing2005
- BofillJAVincentRDRossELNulliparous active labor, epidural analgesia, and cesarean delivery for dystociaAm J Obstet Gynecol1997177146514709423752
- AlbrightGACardiac arrest following regional anesthesia with etidocaine or bupivacaineAnesthesiology197951285287484889
- BeilinYHalpernSFocused review: Ropivacaine versus bupivacaine for epidural labor analgesiaAnesth Analg201011148248720529986
- HalpernSHBreenTWCampbellDCA multicenter, randomized, controlled trial comparing bupivacaine with ropivacaine for labor analgesiaAnesthesiology2003981431143512766654
- CollisREDaviesDWAvelingWRandomised comparison of combined spinal-epidural and standard epidural analgesia in labourLancet1995345141314167760614
- Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UKEffect of low-dose mobile versus traditional epidural techniques on mode of delivery: A randomised controlled trialLancet2001358192311454372
- van der VyverMHalpernSJosephGPatient-controlled epidural analgesia versus continuous infusion for labour analgesia: A metaanalysisBr J Anaesth20028945946512402726
- HalpernSHCarvalhoBPatient-controlled epidural analgesia for laborAnesth Analg200910892192819224805
- NorrisMCFogelSTConway-LongCCombined spinal-epidural versus epidural labor analgesiaAnesthesiology20019591392011605932
- WilsonMJCooperGMacArthurCShennanARandomized controlled trial comparing traditional with two “mobile” epidural techniques: Anesthetic and analgesic efficacyAnesthesiology2002971567157512459686
- MardirosoffCDumontLBoulvainMTramerMRFetal bradycardia due to intrathecal opioids for labour analgesia: A systematic reviewBJOG200210927428111950182
- AbraoKCFranciscoRPMiyadahiraSCicarelliDDZugaibMElevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: A randomized controlled trialObstet Gynecol2009113414719104358
- MacarthurAJMacarthurCWeeksSKIs epidural anesthesia in labor associated with chronic low back pain? A prospective cohort studyAnesth Analg199785106610709356101
- SimmonsSWCynaAMDennisATHughesDCombined spinal-epidural versus epidural analgesia in labourCochrane Database Syst Rev20073CD00340117636721
- HermanNLChoiKCAffleckPJAnalgesia, pruritus, and ventilation exhibit a dose-response relationship in parturients receiving intrathecal fentanyl during laborAnesth Analg19998937838310439751
- GaneshAMaxwellLGPathophysiology and management of opioid-induced pruritusDrugs2007672323233317983254
- PanPHBogardTDOwenMDIncidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: A retrospective analysis of 19,259 deliveriesInt J Obstet Anesth20041322723315477051
- MacarthurAPostpartum headacheChestnutDHPolleyLSTsenLCWongCAChestnut’s Obstetric Anesthesia: Principles and PracticePhiladelphia, PAMosby2009
- ChoiPTGalinskiSETakeuchiLLucasSTamayoCJadadARPDPH is a common complication of neuraxial blockade in parturients: A meta-analysis of obstetrical studiesCan J Anaesth20035046046912734154
- van de VeldeMSchepersRBerendsNVandermeerschEde BuckFTen years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia departmentInt J Obstet Anesth20081732933518691871
- TorvaldsenSRobertsCLSimpsonJMThompsonJFEllwoodDAIntrapartum epidural analgesia and breastfeeding: A prospective cohort studyInt Breastfeed J200612417134489
- HalpernSHLevineTWilsonDBMacDonellJKatsirisSELeightonBLEffect of labor analgesia on breastfeeding successBirth199926838810687571
- BeilinYBodianCAWeiserJEffect of labor epidural analgesia with and without fentanyl on infant breast-feeding: A prospective, randomized, double-blind studyAnesthesiology20051031211121716306734
- WilsonMJMacArthurCCooperGMBickDMoorePAShennanAEpidural analgesia and breastfeeding: A randomised controlled trial of epidural techniques with and without fentanyl and a non-epidural comparison groupAnaesthesia20106514515319912160
- ReynoldsFNeurologic complications of pregnancy and neuroaxial anesthesiaChestnutDHPolleyLSTsenLCWongCAChestnut’s Obstetric Anesthesia: Principles and PracticePhiladelphia, PAMosby2009
- CookTMCounsellDWildsmithJAMajor complications of central neuraxial block: Report on the Third National Audit Project of the Royal College of AnaesthetistsBr J Anaesth200910217919019139027
- WongCAScavoneBMDuganSIncidence of postpartum lumbosacral spine and lower extremity nerve injuriesObstet Gynecol200310127928812576251
- ReynoldsFNeurological infections after neuraxial anesthesiaAnesthesiol Clin2008262352v18319178
- RuppenWDerrySMcQuayHMooreRAIncidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesiaAnesthesiology200610539439916871074
- BalkiMLeeYHalpernSCarvalhoJCUltrasound imaging of the lumbar spine in the transverse plane: The correlation between estimated and actual depth to the epidural space in obese parturientsAnesth Analg20091081876188119448216
- ArzolaCDaviesSRofaeelACarvalhoJCUltrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epiduralsAnesth Analg20071041188118917456672
- VincentRDChestnutDHWhich position is more comfortable for the parturient during identification of the epidural space?Int J Obstet Anesth1991191115636789
- AndrewsPJAckermanWEJunejaMMAortocaval compression in the sitting and lateral decubitus positions during extradural catheter placement in the parturientCan J Anaesth1993403203248485791
- ShenoudaPECunninghamBJAssessing the superiority of saline versus air for use in the epidural loss of resistance technique: A literature reviewReg Anesth Pain Med200328485312567344
- OkutomiTHokaSEpidural saline solution prior to local anaesthetic produces differential nerve blockCan J Anaesth1998451091109310021958
- SchierRGuerraDAguilarJEpidural space identification: A meta-analysis of complications after air versus liquid as the medium for loss of resistanceAnesth Analg20091092012202119923534
- GrondinLSNelsonKRossVAponteOLeeSPanPHSuccess of spinal and epidural labor analgesia: Comparison of loss of resistance technique using air versus saline in combined spinal-epidural labor analgesia techniqueAnesthesiology200911116517219512882
- American Society of Anesthesiologists Task Force on infectious complications associated with neuraxial techniquesPractice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniquesAnesthesiology201011253054520051824
- WongCARatliffJTSullivanJTScavoneBMToledoPMcCarthyRJA randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesiaAnesth Analg200610290490916492849
- LeoSOcampoCLimYSiaAA randomized comparison of automated intermittent mandatory boluses with a basal infusion in combination with patient-controlled epidural analgesia for labor and deliveryInt J Obstet Anesth20101935736420832282
- MardirosoffCTramerMRIntrathecal opioids in labor – do they increase the risk of fetal bradycardiaHalpernSHDouglasMJEvidence-based Obstetric AnesthesiaOxford, UKBlackwell Publishing2005