Abstract
Objectives
The efficiency of local anesthetics (LAs) in the treatment of peripheral pain is commonly attributed to their capacity to block the axon conduction of sensory nerves. LAs even in non-blocking concentration suppress oscillations of the resting membrane potential. Spiking in sensory neurons is triggered by subthreshold membrane potential oscillations (SMPOs), which reach threshold and is maintained by depolarizing impulse after oscillations. The suppression of these oscillations abolishes sustained afferent discharge in sensory nerves without blocking the axon conduction. In a retrospective observational study, we examined if LAs in low concentration and very small quantities could reduce peripheral pain in patients.
Design
During a period of 2 years, a total of 127 consecutive patients, 43 with cervico-brachial, 12 with intercostal and 72 with lumbo-sciatic pain received an identical treatment, which consisted of LAs applied in 4–8 sessions on average to a fixed set of epidermal, epithelial and periosteal locations. The primary outcome was relief of symptoms measured by verbal analog scales at the end of therapy.
Results
At the end of therapy, 53 (41.7%) of all patients (127) had a complete remission (reduction of pain 100%). Twenty-three patients (18.1%) had a partial remission with >90% reduction of pain and 50 patients (39.4%) had a pain reduction of 30%–90%. One patient did not respond.
Conclusion
LAs in low concentration and small quantities proved to be highly efficient in the treatment of peripheral pain. An almost complete remission could be obtained in a majority of patients. Given the extent of pain reduction achieved, the method of application seems to be of major importance.
Introduction
It is well known that local anesthetics (LAs) in the treatment of pain are not confined to their capacity to block axon conduction in sensory nerves.Citation1–Citation3 Under normal conditions, action potentials in a sensory neuron are generated from the peripheral nerve ending and conducted axonally to the dorsal cord/CNS. However, injury or inflamation enhances subthreshold membrane potential oscillations (SMPOs) in the peripheral nerve or dorsal root ganglion neuron, which triggers discharge upon reaching threshold. In the laboratory, only sensory neurons of dorsal root ganglia with SMPOs were capable of generating sustained afferent discharge and hence neuropathic paresthesias and pain.
LAs in a concentration much lower than required to block axon conduction are able to suppress these SMPOs and ectopic firing. But these results were gained mainly under laboratory conditions in vitro and in vivo in animals. Our study aimed to examine if a treatment with LAs in lowest concentration and smallest quantities could reduce peripheral pain in patients under the conditions of an outpatient practice.
Patients and methods
Consecutive patients presenting to an outpatient office with neuralgias of peripheral nerves with and without sensory impairment were enrolled into the study. All patients gave consent to be treated with LAs for their symptoms. Exclusion criteria were allergies to LAs, neuralgia with impairment of motor functions or due to a disturbed metabolism (diabetes, nephropathy), to medications, to infections (herpes zoster, HIV), to a spinal cord injury or related to cancer. The sensory and motor function within the treated location of each patient was examined before and after each therapeutic session.
Patients were divided into three groups according to their complaints:
Group 1: patients with cervico-brachial pain (C3-8) spreading to the shoulder and/or arm, ICD10, 2016: M53.1;
Group 2: patients with intercostal pain along the spinal nerves (Th1-10), ICD10: G58.9;
Group 3: patients with lumbo-sciatic pain (Th11-S3), spreading to the lower abdominal wall, the inguinal region and/or into the leg, ICD10: M51.1–2, M54.3–5, G57.1+9.
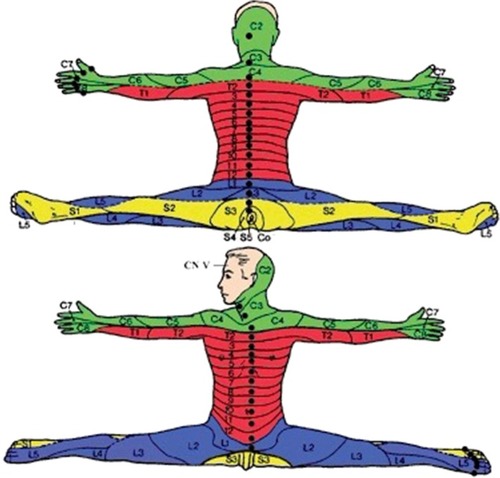
All groups were differentiated in regard to acute pain (<2 weeks) and chronic pain (≥2 weeks). The dermatomes of the spinal nerves involved and the adjacent dermatomes were treated with injections of LAs in low concentration and small quantities to avoid blocking of axon conduction.
Per site of injection, the LA (mepivacaine 0.5%) was applied in a quantity of 0.1–0.2 mL to the following locations:
Intraepidermal () as superficial wheals of 5–10 mm in diameter to the affected side: a) paravertebral, 1–2 cm lateral of the spinous process, as a proximal access to a dermatome; b) paramedian of the ventral body midline, as a distal access to the dermatomes of the trunk and/or c) midline of interdigital skin folds to the dorsal side, as a distal access to the dermatomes of the extremities.
The therapeutic efficiency was increased when – in addition to a proximal access to a dermatome – a distal access to its periphery was used.
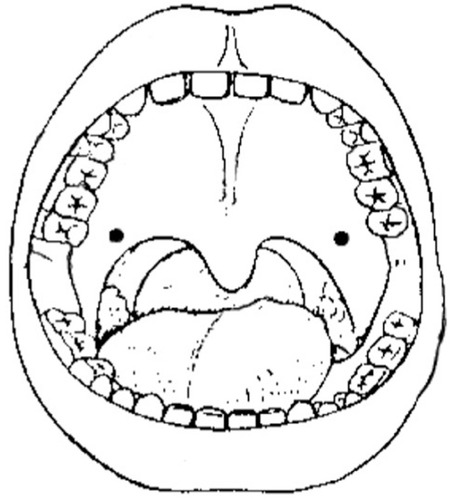
Intraepithelial () as a superficial wheal to the palatal velum on both sides.
Periosteal lateral to the spinous process through the epidermal wheal already applied down to the base of the vertebral body perceiving the right depth by bone resistance.
Epidermal scars (in nonresponders) distributed in the midline of the scar over the whole extension and directly under the surface.
Figure 1 Dorsal site of intraepidermal and periosteal injections and ventral site of intraepidermal injections within the segments concerned for the left side of the body.
In addition to the described procedure in intercostal pain, the LA was applied also directly periosteal to the involved ribs at the site of their highest sensitivity to pressure.
In cases of acute pain, 1–3 sessions/week were performed.
In chronic pain, 2 sessions/week were applied in the beginning followed by increasing phases of remission and corresponding longer intervals between the sessions.
Before therapy, patients classified the strength of their pain as “medium”, “strong” or “very strong”.
The primary endpoint was the change in pain level from prior to first treatment to the end of treatment. The pain level was measured by verbal analog scalesCitation4 during and at the end of therapy with 0% meaning no reduction of pain up to 100% meaning a total reduction. To reduce individual/psychological changes of complaints, we used 4 categories of pain reduction only: 1) 100%, 2) >90%, 3) 90%–30%, 4) <30%.
The Wilcoxon rank sum test was used to compare the pain reduction between patients with acute and chronic pain. As the number of patients with acute and chronic pain was not equally distributed between the three pain groups “cervico-brachial pain”, “intercostal pain” and “lumbo-sciatic pain”, the Kruskal–Wallis test to compare the pain reduction between patients of different pain groups was performed stratified for acute/chronic pain. Moreover, inverse probability weight for acute/chronic pain was computed to perform weighted two-sample rank tests.
Results
One hundred and twenty-seven consecutive outpatients (57 males, 70 females; age range 29–88, mean ± SD=63.17±12.75 years) were enrolled into the study. A total of 43 patients had cervico-brachial pain (group 1; 19 acute; 24 chronic), 12 patients had intercostal pain along the spinal nerves (group 2; 11 acute; 1 chronic) and 72 patients were affected by lumbo-sciatic pain (group 3; 36 acute; 36 chronic).
Before therapy, the strength of pain was classified by ten (7.9%) patients as “very strong”, by 63 (49.6%) patients as “strong” and by 54 (42.5%) as “medium”.
The mean duration of therapy was 9.1 days with 3.8 sessions on average in the acute cases and 25.6 days with 7.1 sessions in the chronic cases.
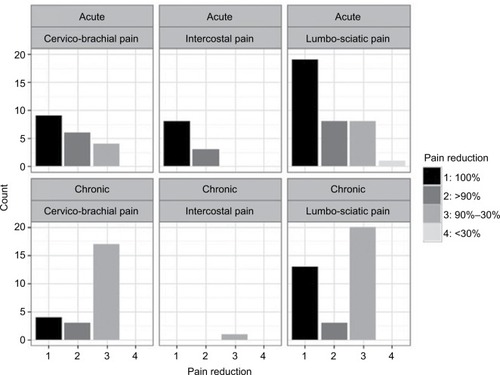
The primary endpoint, the change in pain level, showed an obvious reduction at the end of treatment. Thus, 53 (41.7%) patients had a complete remission. 23 (18.1%) patients had a partial remission with >90% reduction of pain and 50 (39.4%) patients showed a partial remission with a pain reduction of 30%–90%. One patient did not respond ().
Table 1 Absolute and relative frequencies of pain reduction by pain group and acute/chronic pain
Patients with acute pain experienced a higher pain reduction than patients with chronic pain (). The difference between their pain reduction was highly significant (P<0.001).
There was no significant difference of pain reduction between the three pain groups with an acute onset (P=0.263), or between the three pain groups with a chronic onset (P=0.283). Likewise, the weighted analysis did not indicate a significant difference between the pain groups (acute+ chronic), neither between patients with cervico-brachial and intercostal pain (P=0.997) nor between patients with cervico-brachial and lumbo-sciatic pain (P=0.332) or between patients with intercostal and lumbo-sciatic pain (P=0.720).
Among the complete remissions, there were three cases of intercostal neuralgia of the abdominal wall = anterior cutaneous nerve entrapment syndrome (Carnett’s test positive),Citation5–Citation8 one case with a displacement of the intervertebral disc (L4/5) and 11 cases with pain projection merely to the knee.
The application of LAs into epidermal scars for non-responders was performed in 33 (25.9%) cases leading to complete remissions in 20 patients.
Motor impairments after a therapeutic session as possible results of an unintended conduction block were never observed.
Discussion
The results of our study could not have been achieved by pure blockages of the axon conduction of sensory nerves which last only for hours. Furthermore, the very low concentration and quantity of the LA applied, the missing of motor impairments after the application as well as the locations of application make a conduction block as the underlying mechanism of these results unlikely; whereas the ability of LAs to suppress SMPOs even in lowest concentration could explain them, especially the always observed suddenness of pain improvement. So far, these suppressions were provable only under laboratory conditions in animals and not in patients. The results presented show the possible importance of these supressions of SMPOs for the treatment of peripheral pain with LAs.
The achieved extent of pain reduction and its long duration are also attributed to the special mode of LAs application. It aimed to address not only the sensory afferent limb of pain signals from the periphery to the spinal cord but simultaneously also the sympathetic efferent one from the spinal cord back to the periphery. Strategies for treatment must recognize and aim to eliminate both those factors that are responsible for initiating the vicious circle of maladaptive plasticity and those which perpetuate it.Citation9
The presented therapy is characterized by three unusual features.
LAs are applied in low concentration and small quantities. They are administered distal to the structures affected and finally within the dermatomes involved and in the neighboring dermatomes.
Most important for the therapeutic results is the ability of LAs even in non-blocking concentration to suppress resting membrane potential oscillations of nerve cells. Only sensory neurons of dorsal root ganglia with SMPOs are capable of generating sustained afferent discharge and hence neuropathic paresthesias and pain.Citation10–Citation12 Spiking in sensory neurons is started by oscillations, which reach the threshold necessary to trigger an action potential and is maintained by depolarizing impulse after oscillations.Citation13–Citation15 These oscillations are highly sensitive to LAs even in lowest non-blocking concentration (also to gabapentin) by which they are suppressed and in consequence ectopic firing, paresthesias and pain.Citation10,Citation16–Citation18 The suppression of SMPOs by LAs and the pain reduction starts immediately and lasts much longer (for days or even longer) than after the blockage of nerve conduction by LAs (for hours only).
Regrettably in our study, a late recording of complaints was performed only in seven cases, 11–31 months (on average 17 months) after the end of therapy. All of them had a chronic onset and showed a complete remission, which still lasted.
The SMPOs are also sensitive to epinephrine and norepinephrine, which are able to evoke and increase oscillations and repetitive firing.Citation12,Citation19 This seems to be the underlying mechanism of the peripheral sensitization of nociceptors in the skin/epidermis as well as in nerve end neuromas in epidermal scars by sympathetic efferent activity, which is of special importance for the treatment of sensitized scars in nonresponders discussed later.
When applying LAs distal to the structures affected, it should be realized that intraepidermal receptors of pain-mediating afferents are likely to become generators of spontaneous activity after nerve lesions proximal to them.Citation20,Citation21
This increased spontaneous activity distal to the side of the nerve lesion has been proven not only in the affected dermatomes but also in the unaffected neighboring ones.Citation22,Citation23 For this reason, not only the dermatomes involved were treated in their periphery but also the adjacent ones.
The LAs were applied to four different structures (epidermal, epithelial, periosteal and into epidermal scars), which seemed to be particularly recommendable by their anatomical characteristics.
The epidermal application in form of superficial wheals was used as an access to afferent sensitive and efferent sympathetic activity.
Afferent sensitive nerve fibers are found in the epidermis in dense distribution and reach all three epidermal layers as free nerve endings frequently branching in bush-like clumps.Citation20,Citation24,Citation25 This high density of very thin afferent nerve fibers in the outer layers of the skin presents a sensitive access for intraepidermal injections of LAs to afferent sensitive activity.
The same application renders simultaneously an access to efferent sympathetic activity arriving from the spinal cord in the periphery.
The augmented activity of epidermal nociceptors after nerve lesions is accompanied by an increased expression of α1-adrenoceptors and consequently also by an abnormal excitability of nociceptive afferents to adrenergic agents which are able to induce and increase SMPOs in those afferents (see above).Citation20,Citation26–Citation28 In addition, the density of α1-adrenoceptors is significantly greater in the hyperalgesic skin of patients than in the skin of normal individuals.Citation31 This is of interest in the development of sympathetically maintained pain in conditions such as cutaneous neuromas, amputation stump pain, complex regional pain syndrome and postherpetic neuralgiaCitation26,Citation29,Citation30 since these nociceptors are highly susceptible to LAs and in the form of superficial wheals.Citation27
The epithelial application was used as an access to efferent sympathetic activity. The epithelium of the palatal velum represents one of the most dense innervations of the oral mucosa.Citation25 The palatal epithelium served as a site of LA injections because of its immediate connection to the upper cervical ganglion and thereby to the sympathetic chain as proven by retrograde tracer methods.Citation32,Citation33 Finally, this location was assumed as an access to sympathetic efferents by the often experienced observation of impressive improvements especially of lumbo-sciatic pains immediately following an application of LAs to the palatal velum.
The periosteal application was used as an access to efferent sympathetic activity. The periosteum, with its sensory and sympathetic nerve fibers, is the most densely innervated tissue of all bone structures.Citation34 The periosteal sympathetic fibers are derived from and stand in direct connection to the sympathetic chain.Citation35,Citation36 The LAs were applied to the periosteum of the vertebral column as a further access to the sympathetic chain.
In intercostal neuralgias, the application of LAs directly on the rib at the site of its highest sensitivity to pressure used the periosteal access as well and had by far better results than a conduction blockage of the intercostal nerve with the risk of a pneumothorax.Citation5
LAs into epidermal scars were applied in cases of nonresponders. Cases of partial or complete failures could be due to epidermal scars within dermatomes involved and/or even in neighboring or contralateral dermatomes.Citation37 In the superficial area of scars, there are countless cut epidermal afferent nerve endings with a potentially afferent hyperexcitability due to the demyelination at the cut nerve end and hence a Na+ channel protein accumulation.Citation38–Citation41 Even after years of silence, these nerve end neuromas can be sensitized by efferent sympathetic activity from the spinal cord to the scar induced by afferent action potentials from the periphery to the spinal cord due to a fresh pain event.Citation37 Those sensitized neuromas develop the same oscillatory behavior with the same sensitivity to LAs as the peripheral nociceptors do in peripheral sensitization.Citation1,Citation10,Citation42 Normally these scars are without obvious signs of irritation. The sensitization of a scar can be recognized when the pain attributed to such a sensitization is actually present. It will immediately vanish or considerably improve after a superficial injection of LAs in and along the scar.Citation37,Citation43 In therapeutic failures, it is recommended to also check for scars when they are old and have been silent for years or decades. They could have been sensitized by a single fresh event of pain, the accompanying afferent action potentials and the following efferent sympathetic activity. They could be responsible even if they are found in considerable distance from and contralateral to the pain affected dermatomes due to possible cross excitations and contralateral spreading of pain.Citation23,Citation44–Citation49 Especially scars of the dermatomes involved and those of the extremities should be checked. Often this simple desensitization of one or more scars, single or repeated, proved to be decisive in the treatment of chronic or neuropathic pain.
Each of these four different locations proved its own individual efficiency; their combination however improved the results.
The stastistical evaluation showed no significant therapeutic differences between the pain groups. This could indicate that the therapeutic efficiency is independent of the affected location.
The demonstrated results are mainly attributed to the suppression of sensitive afferent action potentials from peripheral nociceptors in the skin to neurons in the spinal cord and the simultaneous suppression of efferent sympathetic activity from these neurons back to the peripheral nociceptors in the skin. These processes were demonstrated under laboratory conditions with animals in vitro and in vivo only. To our knowledge, there are no studies so far to show the efficiency of LAs in very low concentration and quantity in the treatment of peripheral pain in patients. It is not surprising therefore that many questions remain open. For instance, the long duration of the suppression of membrane potential oscillations, which last much longer than an axon conduction block due to LAsCitation5,Citation22,Citation37,Citation44,Citation50–Citation52; the rapid action of nonblocking LAs also in dissipating central sensitization and allodynia within minutesCitation16 and after their repeated application the increasing length of remissions.Citation43–Citation45,Citation50,Citation53
Conclusion
The presented results demonstrate a high efficiency of low-dose LAs in the reduction of peripheral pain most likely due to a suppression of SMPOs in neurons blocking sensitive afferent activity from the periphery to the spinal cord and simultaneously efferent sympathetic activity from the spinal cord back to the periphery. The possible contribution of activated nerve end neuromas in scars to this maladaptive circle of plasticity is emphasized and it is shown how to inactivate it. Due to the complexity of the mechanisms of peripheral pain, the theory and method of this treatment leave many questions open.
The strength of these data is that they were observed under routine conditions of a normal outpatient practice. A shortcoming is the lack of a control group. However, the results of this therapy with its simplicity of application, negligible side effects, very low costs and often fast improvements even in neuropathic pain conditions, where alternatives rarely exist, are so encouraging that a clarification of the remaining questions by further laboratory and clinical studies seems to be a worthwhile recommendation.
Acknowledgments
This study was approved by the ethics committee Ärztekammer Nordrhein, Düsseldorf, with a waiver of patients consent as all data were anonymized.
Disclosure
The authors report no conflicts of interest in this work.
References
- DevorMWallPDCatalanNSystemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conductionPain19924822612681589245
- AmirRArgoffCEBennettGJThe role of sodium channels in chronic inflammatory and neuropathic painJ Pain200675 Suppl 3S1S29
- DochertyRJGinsbergLJadoonSOrrellRWBhattacharjeeATRPA1 insensitivity of human sural nerve axons after exposure to lidocainePain201315491569157723707266
- WrisleyDMSpartoPJWhitneySLFurmanJMCervicogenic dizziness: a review of diagnosis and treatmentJ Orthop Sports Phys Ther2000301275576611153554
- CarnettJBBatesWThe treatment of intercostal neuralgia of the abdominal wallAnn Surg193398582082917867078
- van AssenTBrounsJAScheltingaMRRoumenRMIncidence of abdominal pain due to the anterior cutaneous nerve entrapment syndrome in an emergency departmentScand J Trauma Resusc Emerg Med2015231925887961
- BoelensOBScheltingaMRHoutermanSRoumenRMManagement of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patientsAnn Surg201125461054105821881494
- LindsetmoROStulbergJChronic abdominal wall pain--a diagnostic challenge for the surgeonAm J Surg2009198112913419555786
- WoolfCJRecent advances in the pathophysiology of acute painBr J Anaesth19896321391462669905
- AmirRMichaelisMDevorMMembrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic painJ Neurosci199919198589859610493758
- LiuCNMichaelisMAmirRDevorMSpinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic painJ Neurophysiol200084120521510899197
- AmirRMichaelisMDevorMBurst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentialsJ Neurosci20022231187119811826148
- RaymondSASubblocking concentrations of local anesthetics: effects on impulse generation and conduction in single myelinated sciatic nerve axons in frogAnesth Analg19927569069211443710
- AmirRKocsisJDDevorMMultiple interacting sites of ectopic spike electrogenesis in primary sensory neuronsJ Neurosci200525102576258515758167
- KovalskyYAmirRDevorMSubthreshold oscillations facilitate neuropathic spike discharge by overcoming membrane accommodationExp Neurol2008210119420618162184
- SukhotinskyIBen-DorERaberPDevorMKey role of the dorsal root ganglion in neuropathic tactile hypersensibilityEur J Pain20048213514314987623
- YangRHXingJLDuanJHHuSJEffects of gabapentin on spontaneous discharges and subthreshold membrane potential oscillation of type a neurons in injured DRGPain2005116318719315935557
- YangRHWangWTChenJYXieRGHuSJGabapentin selectively reduces persistent sodium current in injured type-A dorsal root ganglion neuronsPain20091431–2485519269740
- XingJLHuSJJianZDuanJHSubthreshold membrane potential oscillation mediates the excitatory effect of norepinephrine in chronically compressed dorsal root ganglion neurons in the ratPain20031051–217718314499434
- AliZRingkampMHartkeTVUninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkeyJ Neurophysiol199981245546610036297
- WangTHurwitzOShimadaSGChronic compression of the dorsal root ganglion enhances mechanically evoked pain behavior and the activity of cutaneous nociceptors in micePLoS One2015109e013751226356638
- YoonYWNaHSChungJMContributions of injured and intact afferents to neuropathic pain in an experimental rat modelPain199664127368867245
- MaCShuYZhengZSimilar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neuronsJ Neurophysiol20038931588160212612024
- HilligesMWangLJohanssonOUltrastructural evidence for nerve fibers within all vital layers of the human epidermisJ Invest Dermatol199510411341377798631
- HilligesMAstbäckJWangLArvidsonKJohanssonOProtein gene product 9.5-immunoreactive nerves and cells in human oral mucosaAnat Rec199624546216328837720
- DrummondPDNeuronal changes resulting in up-regulation of alpha-1 adrenoceptors after peripheral nerve injuryNeural Regen Res20149141337134025221588
- NamTSYeonDSLeemJWPaikKSAdrenergic sensitivity of uninjured C-fiber nociceptors in neuropathic ratsYonsei Med J200041225225710817027
- DawsonLFPhillipsJKFinchPMInglisJJDrummondPDExpression of α1-adrenoceptors on peripheral nociceptive neuronsNeuroscience201117530031421182905
- FinchPMDrummondESDawsonLFPhillipsJKDrummondPDUp-regulation of cutaneous α1 -adrenoceptors in complex regional pain syndrome type IPain Med201415111945195625220453
- DrummondPDDrummondESDawsonLFUpregulation of α1-adrenoceptors on cutaneous nerve fibres after partial sciatic nerve ligation and in complex regional pain syndrome type IIPain2014155360661624342464
- DrummondPDSkipworthSFinchPMalpha 1-adrenoceptors in normal and hyperalgesic human skinClin Sci199691173778774263
- OyagiSItoJHonjoIThe autonomic innervation in the pharynx. A study by the horseradish peroxidase tracer methodArch Otolaryngol Head Neck Surg198911511135813612803718
- SatodaTTakahashiOMurakamiCUchidaTMizunoNThe sites of origin and termination of afferent and efferent components in the lingual and pharyngeal branches of the glossopharyngeal nerve in the Japanese monkey (Macaca fuscata)Neurosci Res19962443853928861108
- MachDBRogersSDSabinoMCOrigins of skeletal pain: sensory and sympathetic innervation of the mouse femurNeuroscience2002113115516612123694
- AsmusSEParsonsSLandisSCDevelopmental changes in the transmitter properties of sympathetic neurons that innervate the periosteumJ Neurosci20002041495150410662839
- CherruauMMorvanFOSchirarASaffarJLChemical sympathectomy-induced changes in TH-, VIP-, and CGRP-immunoreactive fibers in the rat mandible periosteum: influence on bone resorptionJ Cell Physiol2003194334134812548553
- NathanPWImprovement in cutaneous sensibility associated with relief of painJ Neurol Neurosurg Psychiatry19602320220613727925
- DevorMGovrin-LippmannRAngelidesKNa+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formationJ Neurosci1993135197619927683047
- EnglandJDGamboniFFergusonMALevinsonSRSodium channels accumulate at the tips of injured axonsMuscle Nerve19941765935988196701
- EnglandJDHappelLTKlineDGSodium channel accumulation in humans with painful neuromasNeurology19964712722768710095
- KapoorRLiYGSmithKJSlow sodium-dependent potential oscillations contribute to ectopic firing in mammalian demyelinated axonsBrain1997120Pt 46476529153126
- MatznerODevorMHyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channelsJ Neurophysiol19947213493597965019
- GracelyRHLynchSABennettGJPainful neuropathy: altered central processing maintained dynamically by peripheral inputPain19925121751941484715
- LohLNathanPWPainful peripheral states and sympathetic blocksJ Neurol Neurosurg Psychiatry1978417664671690645
- NathanPWThe sympathetic system and painFunct Neurol1989411115
- WoolfCJEvidence for a central component of post-injury pain hypersensitivityNature198330659446866886656869
- DevorMWallPDCross-excitation in dorsal root ganglia of nerve-injured and intact ratsJ Neurophysiol1990646173317462074461
- AmirRDevorMChemically mediated cross-excitation in rat dorsal root gangliaJ Neurosci19961615473347418764660
- VierckCJLightARAllodynia and hyperalgesia within dermatomes caudal to a spinal cord injury in primates and rodentsProg Brain Res200012941142811098708
- ArnérSLindblomUMeyersonBAMolanderCProlonged relief of neuralgia after regional anesthetic blocks. A call for further experimental and systematic clinical studiesPain19904332872971705693
- BarnsleyLBogdukNMedial branch blocks are specific for the diagnosis of cervical zygapophyseal joint painReg Anesth19931863433508117629
- PriceDDLongSWilseyBRafiiAAnalysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patientsClin J Pain19981432162269758071
- ChenSMChenJTKuanTSHongCZMyofascial trigger points in intercostal muscles secondary to herpes zoster infection of the intercostal nerveArch Phys Med Rehabil19987933363389523788