Abstract
Background and aims
A popliteal block is effective in managing postoperative pain for foot surgery, but since the duration of analgesia is limited following a single-shot popliteal fossa block technique, methods to prolong effective postoperative analgesia are mandatory. The aim of this study was to assess the effect of adjuvants to ropivacaine on the duration of sensory and motor block.
Methods
In this double-blind randomized placebo-controlled study, we evaluated the analgesic effect of clonidine or dexamethasone (DXM) when added to ropivacaine for hallux valgus surgery. After obtaining institutional ethics research board approval and written informed consent, a total of 72 patients were randomly allocated. Fifty-seven of these patients were statistically analyzed. All patients received an ultrasound-guided single-shot popliteal fossa block with 30 mL of ropivacaine 0.75%, supplemented with saline, clonidine 100 µg, or DXM 5 mg. The primary end point was time to first pain sensation. Secondary end points were time to complete sensory and motor block regression.
Results
Compared to saline, duration to first pain sensation was prolonged by 9 hours (mean ± standard deviation: 31±9 hours) (42%) in the DXM group (P=0.024) and by 6 hours (28±10 hours) (27%) in the clonidine group (P=0.024). Compared to saline, DXM prolonged both complete sensory and motor blockade by 12 hours (25±7 hours) (46%) and 13 hours (36±6 hours) (55%), respectively, while clonidine prolonged complete sensory and motor blockade by 7 hours (30±7 hours) (27%) and 2 hours (22±5 hours) (10%), respectively. DXM prolonged sensory block regression time by 6 hours (21±7 hours) (41%) and clonidine by 2 hours (17±6 hours) (13%) compared to the control group (P=0.006). Similarly, DXM prolonged motor block regression by 7 hours (25±7 hours) (46%) and clonidine by 4 hours (21±4 hours) (19%) (P<0.0001).
Conclusion
Addition of DXM and clonidine to ropivacaine significantly prolonged the duration of postoperative sensory and motor block.
Introduction
Foot surgery is associated with severe pain that can extend significantly up to 48 hours and often requires large amounts of parenteral opioids.Citation1,Citation2 Sciatic nerve blockade reduces postoperative pain after major foot and ankle surgery with minimal side effects;Citation3 however, the maximum duration of effective analgesia with long-acting local anesthetics after a single injection technique is only 8–24 hours.Citation4 Therefore, perineural catheters or local anesthetics in combination with adjuvants are often used in attempts to prolong the duration of a single-shot popliteal fossa block (SSPFB).Citation5,Citation6 Pharmacodynamics and pharmacokinetics of dexamethasone (DXM) and clonidine administered in regional nerve blocks are not yet fully understood; however, quite some literature has been published concerning the prolonged effect of DXM and clonidine on block characteristics.
Clonidine is a frequently used additive to local anestheticsCitation7–Citation9 and is used for neuraxial blocks (eg, caudal blocks in childrenCitation10) and for peripheral nerve blocks.Citation8 In particular, clonidine is known to prolong the duration of analgesia for brachial plexus and popliteal fossa blocks.Citation11 However, some studies have reported no change in onset time or block duration.Citation11–Citation14 This lack of effect has been attributed to methodological issues, eg, difficulties in data interpretation, lack of systematic controls, or to the specific intermediate- and short-acting local anesthetics that were studied.Citation7 Additionally, many studies were performed prior to the widespread use of ultrasound-guided nerve blockade, which may have limited the accuracy of local anesthetic delivery.Citation1–Citation3,Citation18
DXM has also shown some promise as an adjuvant that prolongs analgesia in brachial plexus blocks.Citation15–Citation18 Recently, Cummings et alCitation5 reported prolonged analgesia when DXM was combined with a long-acting local anesthetic during interscalene block. Desmet et al and Cummings et al found that DXM (8 mg) as an adjuvant to ropivacaine or bupivacaine almost doubled block duration (up to 24 hours) when used as an additive for the interscalene block.Citation5,Citation18
In this double-blind randomized placebo-controlled study, we evaluated the addition of DXM or clonidine to an ultrasound-guided SSPFB with 30 mL of ropivacaine 0.75%. The primary end point was time to first pain sensation visual analog scale ([VAS] score >3). We hypothesized that, compared to saline, DXM and clonidine prolong the time to first pain sensation.
Methods
Population and study design
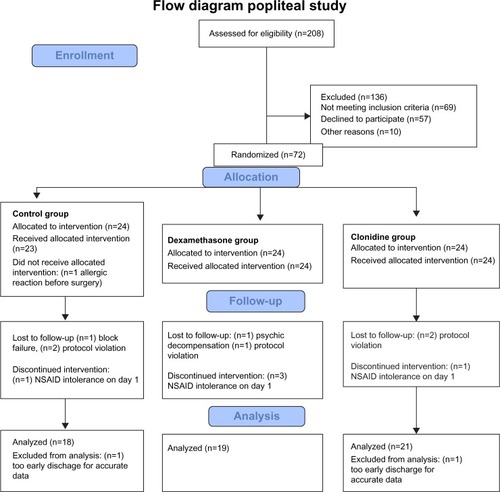
This trial is registered at the Community Clinical Trial System (EudraCT number: 2012-005573-31). Hospital ethics committee approval (EC approval no 3734, Commissie voor Medische Ethiek – Ziekenhuisnetwerk Antwerpen, December 01, 2011) was received. All patients scheduled for elective foot surgery were screened. Exclusion criteria were refusal of the patient, protocol violations, Nonsteroidal anti-inflammatory drug (NSAID) intolerance, diabetic patients, allergy for local anesthetics, peripheral neuropathy, coagulation disorders, and patients with an impaired kidney function. Patients were asked to participate when no contraindications to study participation were identified. We asked 208 patients to give consent, but 57 refused. A total of 72 patients were randomly allocated by a computer-generated model to one of three study groups (clonidine, DXM, or saline). Fifty-seven of these patients were statistically analyzed (). An SSPFB sciatic nerve block was performed 60 minutes before surgery, and block onset times were recorded.
Procedure
Following establishment of intravenous (IV) access, an ultrasound-guided SSPFB was performed with the patient in the prone position using the posterior approach. The probe was placed in the popliteal fossa (short axis view of the nerves) to detect the popliteal artery and vein, and the biceps femoris muscle. The tibial nerve was identified usually lateral and more superficial to the popliteal artery. Following visualization of these structures, the probe was moved proximally to search for the bifurcation of the tibial nerve with the common peroneal nerve (4–10 cm proximal to the popliteal crease). Correct needle placement was confirmed using a nerve stimulator. Muscle twitches of the foot (plantar flexion, dorsiflexion or inversion of the ankle) evoked at a current of 0.5 mA served as an indicator of correct needle placement. When motor stimulation was accomplished with ≤0.2 mA, or excessive resistance (pressure) to injection was felt, the needle was repositioned.
Ropivacaine was the local anesthetic of choice because of its favorable clinicalCitation19,Citation20 and toxicity profile.Citation21 Of the available concentrations, the 0.75% concentration was selected based on previous studies.Citation19,Citation22 Ropivacaine 0.75% (30 mL) was mixed with either 1 mL of saline (control group), 1 mL of DXM (5 mg), or 1 mL of clonidine (100 µg) according to randomized assignment that was prepared by the pharmacist and blinded to the blinded block performer. In total, 31 mL of local anesthetic mixture was injected.
“Donut” signs around the posterior tibial nerve and the common peroneal nerve indicated successful distribution of the study solution. Two anesthesiologists with extensive experience with the popliteal block performed all blocks.
A research assistant blinded to group allocation evaluated sensory and motor block every 5 minutes after injection until start of surgery and every 10 minutes after surgery until discharge from the postanesthesia care unit. Sensory block was tested using a big toe pinprick and rated as 2= normal, 1= loss of sensation to pinprick, or 0= loss of sensation to light touch. Motor block was tested and rated as 2= normal movement, 1= partial paresis of ankle or toe movement, or 0 = absent movement of the ankle and toes. Block success was defined as loss of sensation to pinprick (sensory score ≤1) 45 minutes after injection of the local anesthetic mixture. Onset time was defined as the interval between the end of injection and loss of sensation to pinprick.Citation23–Citation28
All patients received general anesthesia induced with propofol (2.5 mg/kg) and sufentanil (0.1 µg/kg). A laryngeal mask airway was introduced following loss of consciousness. Sevoflurane 1–1.5 minimum alveolar concentration (MAC) was administered through a laryngeal mask airway to maintain anesthesia. Inspiratory oxygen fraction was 40%. IV antibiotics were given before tourniquet insufflation. Standard anesthesia monitoring included pulse oximetry, capnography, noninvasive blood pressure, and a three-lead electrocardiogram. At the end of surgery, the tourniquet was deflated, and paracetamol 1 g was administered intravenously. Total tourniquet time was recorded from insufflation to deflation. No skin or wound traction was applied at the end of surgery. The same surgeon performed all interventions.
Paracetamol 1 g was administered every 6 hours, and ketorolac (Taradyl®) 20 mg was administered every 8 hours for the first 24 hours. The leg was elevated, and early postoperative mobilization was applied in the postanesthesia care unit and in the orthopedic ward.
After 24 hours, the analgesic regimen consisted of oral paracetamol 1 g qid and naproxen 550 mg bid. Patients who developed stomachache on the first postoperative day were excluded from the study because NSAIDs were stopped and tramadol sublingual was then given to manage pain.
A blinded study nurse performed all postoperative recordings. The primary outcome variable was the time between start of block and the first pain reported by the patient. Patient-reported pain scores were recorded using the VAS ranging from 0 (no pain) to 10 (worst pain imaginable). Patients were considered to have pain if the VAS score exceeded 3. Sensory and motor block regression times were self-reported by the patients at different times. They were asked to report VAS scores every 2 hours until complete sensory and motor block regression and to report supplementary scores when VAS scores exceeded 3 out of 10. Complications such as sedation, nausea, vomiting, and itching were noted. Onset of sensory block regression was defined as the first moment any feeling returned in the toes, typically a tingling sensation; complete sensory regression was defined as the return of normal sensation to touch of the toes. Onset of motor block regression was the first time the patient was able to move toes, and complete motor regression was the subjective feeling of normal motor function of the lower leg. Patients were discharged after full recovery of sensory and motor block and with a VAS score of 3 or less. After discharge, they were asked to keep a diary of VAS scores for 2 days (at least four times a day).
Statistical analysis
Sample size calculations were performed, and 18 patients per group were deemed necessary to achieve a power of 90% with a significance level of 0.05 to detect a difference of 6 hours in postoperative analgesia with estimated group standard deviations of 4.5 hours.Citation18,Citation29 A two-sided Student’s t-test was performed.
Block regression duration was modeled using Kaplan–Meier curves, and a log-rank survival analysis was used to evaluate the differences among the study groups. Differences in age, tourniquet time, and motor and sensory block onset times were evaluated by Kruskal–Wallis analysis.
P-values <0.05 were considered statistically significant. The Statistical Package for the Social Sciences (SPSS for Windows, version 17.0; SPSS Inc., Chicago, IL, USA) was used for these analyses.
Results
We screened 208 patients. Sixty-nine were excluded from participation and 57 refused to participate. Finally, 72 patients were enrolled, of whom 15 were excluded. In the control group, six people were excluded. One was excluded because of an allergic reaction to antibiotics prior to incision, one because of block failure, and one because of suspected NSAID intolerance on day 1 (gastric pain). In this group, there were two protocol violations on the surgical ward, and one patient was sent home early for adequate data assessment. In the DXM group, there were five excluded patients. One patient turned out to be mentally instable for adequate data assessment. There was one protocol violation and three suspected NSAID intolerance. In the clonidine group, four people were excluded, two due to protocol violations, one with suspected NSAID intolerance, and one due to early discharge.
The groups did not differ in age, tourniquet time, and block onset times (sensory or motor) as shown by Kruskal–Wallis analysis ().
Table 1 Age, tourniquet time, and onset times of 57 subjects undergoing popliteal blockade
After obtaining institutional ethics research board approval and written informed consent from eligible patients, 57 American Society of Anesthesiologists classification 1 and 2 patients were studied. All participants were female and the groups did not differ in other demographic characteristics. Onset of sensory and motor block did not differ among groups ( and ).
Table 2 Primary and secondary end point results of 57 subjects undergoing popliteal blockade
Table 3 Pairwise analysis of block survival times
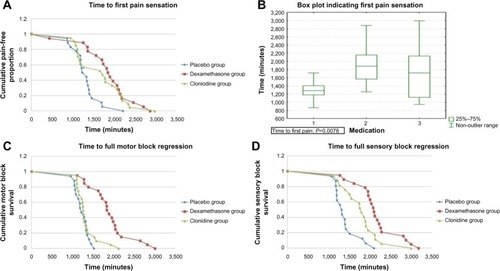
Compared to saline, duration to first pain sensation was prolonged by 9 hours (42%) with the addition of DXM (P=0.024) and by 6 hours (27%) with the addition of clonidine (P=0.024). Compared to saline, DXM prolonged both complete sensory and motor blockade by 12 and 13 hours (55%), respectively, while clonidine prolonged complete sensory and motor blockade by 7 hours (27%) and 2 hours (10%), respectively ().
DXM prolonged sensory block regression time by 6 hours (41%) and clonidine by 2 hours (13%). Log-rank survival analysis showed that these findings are statistically significant (P=0.006). Similarly, DXM prolonged motor block regression by 7 hours (46%) and clonidine by 4 hours (19%) (P<0.0001). No significant side effects were recorded ().
Discussion
Corticosteroids have a long history of use in neuraxial blocks and chronic pain treatment, for instance, in carpal tunnel syndrome, and they are acknowledged to be an effective and safe therapy.Citation30 Desmet et alCitation18 and Cummings et alCitation5 have found no evidence that the off-label use of perineural DXM is harmful. When studying the effect of ropivacaine and different adjuvants on isolated sensory neuron cell bodies of rats, Williams et alCitation31,Citation32 found that, unlike ropivacaine, DXM did not increase cell death after 24 hours of exposure. Moreover, Ma et alCitation33 reported a protective effect for DXM on bupivacaine-induced neuronal injury in rats. Thus, the short-term use of DXM appears to be safeCitation34 and without risk for steroid-induced hyperglycemia.Citation35
The perineural use of DXM has been widely published.Citation31 Both Cummings et alCitation18 and Desmet et alCitation5 found that when DXM was added to an interscalene block, DXM prolonged the interscalene nerve block nearly twofold. These authors used 8 and 10 mg DXM, while we used only 5 mg. This perhaps explains why in the present study the effect of DXM was less pronounced and a dose–response effect might occur. However, alternatively in the present study for the first time, DXM was added to a popliteal block, which also might explain the different results. Further difference between the present study and those of Cummings et al and Desmet et al is that we used ultrasound guidance to place the block.
Although the pharmacodynamics and pharmacokinetics of DXM and clonidine administered in regional nerve blocks are not yet fully understood, there is increasing evidence that DXM prolongs the duration of postoperative analgesia.Citation5,Citation18,Citation36
Desmet et alCitation18 also had an IV DXM group and concluded that IV DXM is as good as perineural DXM in terms of pain relief. It was previously already demonstrated that systemic steroids improve postoperative pain sensation and reduce postoperative nausea and vomiting.Citation37–Citation39 Several hypotheses have been proposed to explain the analgesic effect of DXM. Steroids may induce vasoconstriction, leading to substantial reduction in the absorption of local anesthetic solution.Citation15,Citation40,Citation41 Alternatively, DXM might block nociceptive impulse transmission along unmyelinated C-fibers through its anti-inflammatory and/or immunosuppressive effect.Citation5,Citation42–Citation44 After intracellular uptake, glucocorticoids activate cytoplasmatic receptors that bind to their response elements in DNA. This decreases the production of inflammatory proteins (eg, cyclooxygenase-2, inducible nitric oxide synthase, cytoplasmatic phospholipase A2, interleukins, and inflammatory chemokines) and increases the production of anti-inflammatory proteins (lipocortin-1 receptor antagonist).Citation18,Citation45
Although clonidine is the most frequently used perineural additive to local anesthetics,Citation46,Citation47 its role as adjuvant in peripheral nerve blocks remains controversial, and side effects seem to increase at doses above 150 µg.Citation46 YaDeau et alCitation7 showed that a popliteal nerve block duration was prolonged when 100 µg of clonidine was added to bupivacaine. Nonetheless, the peripheral analgesic effect of clonidine remains unclear, and further investigation examining the mechanisms of action is required.
The present study has significant limitations. Since we did not use IV patient-controlled analgesia morphine as rescue analgesia, we could not conclude that opioid sparing occurs with DXM or clonidine. However, to our knowledge, this is the first study to look at not only the analgesic effect but also in combination with sensory and motor block characteristics. Furthermore, we used a relatively large amount of local anesthetic volume (30 mL). It can be argued that given the fact that ultrasound was used for block placement, lower volumes should have been injected. However, since previous studies used high volumes, we decided to use similar volumes in the present study. Further study is required to evaluate if DXM can effectively reduce the dose of local anesthetic without affecting the duration of analgesia.
Conclusion
Our results demonstrate that adjuvants with ropivacaine 0.75% prolong the duration of an SSPFB. This is the first study to use a limited dose (5 mg) of DXM for SSPFB. Perineural clonidine (limited to 100 µg) also prolonged block duration but to a lesser extent than DXM. Future dose–response studies are needed to evaluate if the positive effects of DXM are also present in lower doses. Additionally, neuronal toxicity of DXM and its effects on glucose metabolism need further study. Furthermore, combining clonidine and DXM might produce additive or synergistic effects.
Author contributions
Van de Velde and Hadzic contributed to the conception and design of this study. Data collection was done by Engelen and Roofthooft. Vermeylen mainly did the writing of the article, but the critical evaluation and final approval were done by all co-authors, in particular, Van de Velde. De Puydt performed the statistical analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- OngCKLirkPSeymourRAJenkinsBJThe efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysisAnesth Analg20051003757773 table of contents15728066
- NeedoffMRadfordPCostiganPLocal anesthesia for postoperative pain relief after foot surgery: a prospective clinical trialFoot Ankle Int199516111137697147
- di BenedettoPCasatiABertiniLFanelliGChellyJEPostoperative analgesia with continuous sciatic nerve block after foot surgery: a prospective, randomized comparison between the popliteal and subgluteal approachesAnesth Analg20029449961000 table of contents11916811
- CappelleriGAldegheriGRuggieriFMamoDFanelliGCasatiAMinimum effective anesthetic concentration (MEAC) for sciatic nerve block: subgluteus and popliteal approachesCan J Anaesth200754428328917400980
- CummingsKC3rdNapierkowskiDEParra-SanchezIEffect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaineBr J Anaesth2011107344645321676892
- PaqueronXNarchiPMazoitJXSingelynFBenichouAMacairePA randomized, observer-blinded determination of the median effective volume of local anesthetic required to anesthetize the sciatic nerve in the popliteal fossa for stimulating and nonstimulating perineural cathetersReg Anesth Pain Med200934429029519585697
- YaDeauJTLaSalaVRParoliLClonidine and analgesic duration after popliteal fossa nerve blockade: randomized, double-blind, placebo-controlled studyAnesth Analg200810661916192018499632
- CasatiAMagistrisLFanelliGSmall-dose clonidine prolongs postoperative analgesia after sciatic-femoral nerve block with 0.75% ropivacaine for foot surgeryAnesth Analg200091238839210910854
- CasatiAVinciguerraFCappelleriGAdding clonidine to the induction bolus and postoperative infusion during continuous femoral nerve block delays recovery of motor function after total knee arthroplastyAnesth Analg20051003866872 table of contents15728080
- ConstantIGallOGouyetLChauvinMMuratIAddition of clonidine or fentanyl to local anaesthetics prolongs the duration of surgical analgesia after single shot caudal block in childrenBr J Anaesth19988032942989623426
- BernardJMMacairePDose-range effects of clonidine added to lidocaine for brachial plexus blockAnesthesiology19978722772849286891
- DumaAUrbanekBSitzwohlCKreigerAZimpferMKapralSClonidine as an adjuvant to local anaesthetic axillary brachial plexus block: a randomized, controlled studyBr J Anaesth200594111211615516351
- CulebrasXVan GesselEHoffmeyerPGamulinZClonidine combined with a long acting local anesthetic does not prolong postoperative analgesia after brachial plexus block but does induce hemodynamic changesAnesth Analg200192119920411133627
- MurphyDBMcCartneyCJChanVWNovel analgesic adjuncts for brachial plexus block: a systematic reviewAnesth Analg20009051122112810781465
- MovafeghARazazianMHajimaohamadiFMeysamieADexamethasone added to lidocaine prolongs axillary brachial plexus blockadeAnesth Analg2006102126326716368840
- ParringtonSJO’DonnellDChanVWDexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockadeReg Anesth Pain Med201035542242620814282
- ShresthaBRMaharjanSKTabedarSSupraclavicular brachial plexus block with and without dexamethasone – a comparative studyKathmandu Univ Med J (KUMJ)20031315816016388222
- DesmetMBraemsHReynvoetMI.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled studyBr J Anaesth2013111344545223587875
- CasatiAFanelliGBorghiBTorriGRopivacaine or 2% mepivacaine for lower limb peripheral nerve blocks. Study Group on Orthopedic Anesthesia of the Italian Society of Anesthesia, Analgesia, and Intensive CareAnesthesiology19999041047105210201676
- FanelliGCasatiABeccariaPA double-blind comparison of ropivacaine, bupivacaine, and mepivacaine during sciatic and femoral nerve blockadeAnesth Analg19988735976009728836
- DonyPDewindeVVanderickBThe comparative toxicity of ropivacaine and bupivacaine at equipotent doses in ratsAnesth Analg20009161489149211094006
- CasatiAFanelliGCappelleriGA clinical comparison of ropivacaine 0.75%, ropivacaine 1% or bupivacaine 0.5% for interscalene brachial plexus anaesthesiaEur J Anaesthesiol1999161178478910713873
- BuysMJArndtCDVaghFHoardAGersteinNUltrasound-guided sciatic nerve block in the popliteal fossa using a lateral approach: onset time comparing separate tibial and common peroneal nerve injections versus injecting proximal to the bifurcationAnesth Analg2010110263563719996137
- DufourEQuennessonPVan RobaisALCombined ultrasound and neurostimulation guidance for popliteal sciatic nerve block: a prospective, randomized comparison with neurostimulation aloneAnesth Analg2008106515531558 table of contents18420875
- PrasadAPerlasARamloganRBrullRChanVUltrasound-guided popliteal block distal to sciatic nerve bifurcation shortens onset time: a prospective randomized double-blind studyReg Anesth Pain Med201035326727120921838
- Tran deQHDuganiSPhamKAl-ShaafiAFinlaysonRJA randomized comparison between subepineural and conventional ultrasound-guided popliteal sciatic nerve blockReg Anesth Pain Med201136654855222005661
- AbdallahFWBrullRMaking sense of block “success” in ambulatory anesthesia practiceInt Anesthesiol Clin20114931921697666
- RancourtMPAlbertNTCoteMLetourneauDRBernardPMPosterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaineAnesth Analg2012115495896222826530
- BinghamAEFuRHornJLAbrahamsMSContinuous peripheral nerve block compared with single-injection peripheral nerve block: a systematic review and meta-analysis of randomized controlled trialsReg Anesth Pain Med201237658359423080349
- MarshallSTardifGAshworthNLocal corticosteroid injection for carpal tunnel syndromeCochrane Database Syst Rev20072CD00155417443508
- WilliamsBAMurinsonBBGrableBROrebaughSLFuture considerations for pharmacologic adjuvants in single-injection peripheral nerve blocks for patients with diabetes mellitusReg Anesth Pain Med200934544545719920420
- WilliamsBAHoughKATsuiBYIbinsonJWGoldMSGebhartGFNeurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaineReg Anesth Pain Med201136322523021519308
- MaRWangXLuCDexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanismNeuroscience2010167232934220038443
- TanPHLiuKPengCHYangLCLinCRLuCYThe effect of dexamethasone on postoperative pain and emesis after intrathecal neostigmineAnesth Analg200192122823211133633
- PasternakJJMcGregorDGLanierWLEffect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomyJ Neurosurg Anesthesiol200416212212515021280
- ShresthaBRMaharjanSKShresthaSComparative study between tramadol and dexamethasone as an admixture to bupivacaine in supraclavicular brachial plexus blockJNMA J Nepal Med Assoc20074616815816418340366
- AasboeVRaederJCGroegaardBBetamethasone reduces postoperative pain and nausea after ambulatory surgeryAnesth Analg19988723193239706923
- NagelschmidtMFuZXSaadSDimmelerSNeugebauerEPreoperative high dose methylprednisolone improves patients outcome after abdominal surgeryEur J Surg19991651097197810574107
- BisgaardTKlarskovBKehletHRosenbergJPreoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trialAnn Surg2003238565166014578725
- WangPHTsaiCLLeeJSWuKCChengKIJouIMEffects of topical corticosteroids on the sciatic nerve: an experimental study to adduce the safety in treating carpal tunnel syndromeJ Hand Surg Eur Vol201136323624321282223
- ShishidoHKikuchiSHeckmanHMyersRRDexamethasone decreases blood flow in normal nerves and dorsal root gangliaSpine200227658158611884905
- BrullRMacfarlaneAJParringtonSJKoshkinAChanVWIs circumferential injection advantageous for ultrasound-guided popliteal sciatic nerve block?: a proof-of-concept studyReg Anesth Pain Med201136326627021490520
- McCormackKThe spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effectsDrugs199447Suppl 52845 discussion 46–477525183
- AhlgrenSCWangJFLevineJDC-fiber mechanical stimulus-response functions are different in inflammatory versus neuropathic hyperalgesia in the ratNeuroscience19977612852908971778
- BarnesPJAnti-inflammatory actions of glucocorticoids: molecular mechanismsClin Sci (Lond)19989465575729854452
- McCartneyCJDugganEApatuEShould we add clonidine to local anesthetic for peripheral nerve blockade? A qualitative systematic review of the literatureReg Anesth Pain Med200732433033817720118
- EisenachJCDe KockMKlimschaWAlpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995)Anesthesiology19968536556748853097