Abstract
Emphysema is an incurable and underdiagnosed disease with obstructive ventilatory impairment of lung function. Despite decades of research, medical treatments available so far did not significantly improve the survival benefits. Different bronchoscopic methods for lung volume reduction (LVR) in emphysema were used in the past 2 decades aiming to close the airways serving the hyperinflated lung regions and to allow the gas in the more distal bullas to be absorbed. Sealants and adhesives can be natural/biological, synthetic and semisynthetic. In lung surgery, lung sealants are used to treat prolonged air leak, which is the most common complication. Sealants can also be applied in bronchoscopic lung volume reduction (BLVR) as they administer into the peripheral airways where they polymerize and act as tissue glue on the surface of the lung to seal the target area to cause durable permanent absorption atelectasis. Initial studies analyzed the efficacy of bronchoscopic instillation of a fibrinogen–thrombin complex solution in advanced emphysema. Future studies will analyze the effects of adding chondroitin sulfate and poly-L-lysine to thrombin–fibrinogen complex thus promoting fibroblast attachment, proliferation and scarring, causing bronchial fibrostenosis and preventing ventilation of the affected part of the lung. Modifications of these methods were later developed, and the efficacy of BLVR with other sealants was analyzed in clinical studies. Results from current studies using this treatment method are promising showing that it is effective in improving exercise tolerance and quality of life in patients with advanced emphysema. It seems that subjective benefits in dyspnea scores and quality of life are more marked than improvements in lung function tests. The safety profile of sealant techniques in BLVR was mostly acceptable in clinical studies. The definite conclusions about the effectiveness of sealant in BLVR could be difficult because only a small population was involved in the current studies. More randomized large controlled studies are needed in establishing the definite role of biological BLVR in the bronchoscopic treatment of emphysema.
Keywords:
Introduction to current management strategies for patients with emphysema
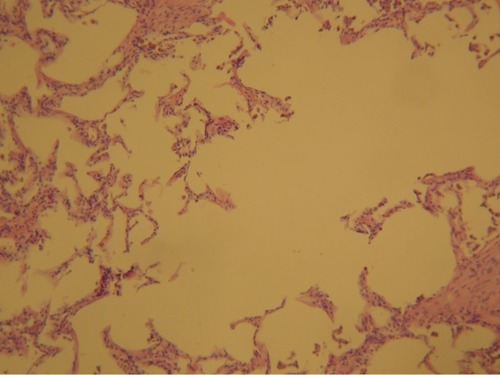
Emphysema is an incurable and underdiagnosed disease with irreversible enlargement of alveolar spaces distal to the terminal bronchiole. The number and size of alveolar fenestrae are increased with eventual destruction of alveolar septa (). Centriacinar emphysema affects the respiratory bronchioles, panacinar emphysema affects central and peripheral portions of acinus, and periacinar/paraseptal emphysema is characterized with distention of alveolar spaces adjacent to septal and pleural surfaces.Citation1 Alveoli and alveolar ducts can be enlarged in people older than 50 years with senile emphysema. Destruction of alveoli results in cysts or bullae that increase physiological dead space and compress the surrounding lung tissue. Hogg et alCitation1 measured the extent of emphysema, the number of terminal bronchioles and the minimum diameters and cross-sectional areas of terminal bronchioles in isolated lungs removed from patients with COPD who underwent lung transplantation and in donor (control) lungs using multidetector computed tomography (CT). Their results suggested that narrowing and disappearance of small conducting airways before the onset of emphysematous destruction can explain the increased peripheral airway resistance reported in COPD.Citation1
Figure 1 Photomicrographs of emphysematous lung parenchyma showing hyperdistension of alveolar ducts, increased number and size of alveolar fenestrae with marked destruction of alveolar septa (H&E) ×100 increased (by courtesy of Dr Jelena Stojsic).
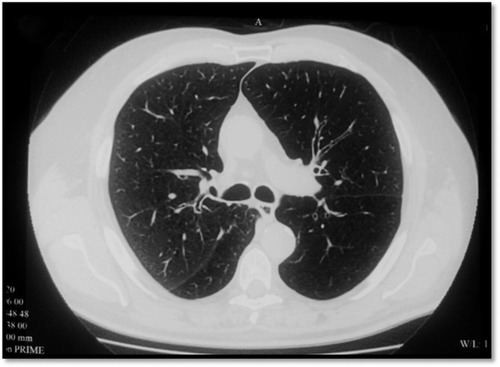
The destruction of lung tissue can be visualized on high-resolution CT (HRCT; ).
Figure 2 CT of a patient with emphysema with giant bulla.
Abbreviations: CT, computed tomography; HRCT, high-resolution CT; GOLD, Global Initiative on Obstructive Lung Disease.
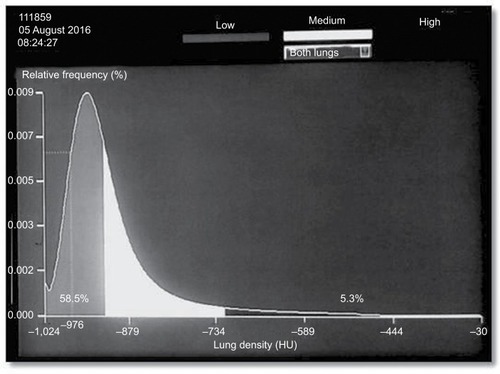
Emphysema is recognized as a phenotype of COPD, and it is found in most patients with COPD. The main symptom is progressive dyspnea during exercise. Patients with emphysema tend to be very symptomatic with shortness of breath even on mild exertion. Dyspnea is largely owing to hyperinflation in emphysema.Citation2,Citation3 Lung function in patients with emphysema is abnormal with obstructive ventilatory impairment. Destruction of alveoli with cysts or bullae does not contribute to gas exchange. Lung density can be assessed using CT analyzing attenuation of the pulmonary tissue which reflects the degree of structural lung abnormalities ().Citation4
Figure 3 Lung density histogram in 71-year-old man with stable COPD (GOLD III).
Abbreviation: GOLD, Global Initiative on Obstructive Lung Disease.
Despite decades of research, medical treatments available so far have helped improve quality of life but did not significantly improve the survival benefits. Unfortunately, the drug that can repair lung tissue is not yet available. Nonpharmacological treatment such as smoking cessation and respiratory rehabilitation has beneficial effects on patient’s lung function, exercise tolerance, quality of life and overall survival. Bronchodilator therapy is mostly ineffective because the cysts and bullae tend to increase in size and number over time and occupy space and press the surrounding lung tissue.
Surgical (nonbronchoscopic) lung volume resection is performed in patients with emphysema to achieve expansion of healthy lung tissue that surrounds giant bullae. Bullectomy can be performed using two surgical approaches such as thoracotomy/sternotomy and video-assisted thoracic surgery (VATS). Removing the giant bulla improves the chest mechanics and remodels the thorax and diaphragm, with symptomatic and functional improvement. VATS was transpired as a valuable surgical option that allows quicker recovery and is associated with less pain and with lower morbidity and mortality than open thoracotomy.Citation5,Citation6 Nevertheless, the overall mortality over 29-month follow-up was same in the lung volume reduction surgery (LVRS) and medically treated groups, and the overall risk of death in the first 3 months was higher in the LVRS group than the medical group.Citation7 Upper lobe emphysema has been associated with short-term improvement in pulmonary function after LVRS, and by NETT trial, and is predictive of improved survival in patients who also have low maximal exercise capacity. It further implies that upper lobe predominance, as compared to other patterns, may result in clearer and more accessible areas for excision, or that lungs in other areas are healthier.Citation8
The common LVRS-related morbidity has led to the development of bronchoscopic lung volume reduction (BLVR). BLVR refers to several bronchoscopic techniques for treating severe emphysema such as bronchial valves, bronchial plugs/occluders/blockers, biological BLVR (Bio-BLVR), bronchoscopic thermal vapor ablation, endobronchial coils, bronchial fenestration and airway bypass and some techniques that are analyzed in clinical experimentation.Citation9–Citation14
Several ways of closing the airways that served the hyperinflated regions have been attempted with the primary aim to allow the gas in the more distal bullae to be reabsorbed over the next days and weeks. Lung sealant technologies include instillation of biologically active reagents as tissue sealants that lead to replacement of diseased emphysematous tissue by a contracted organized scar thus making it essentially irreversible procedure. Lung sealant is injected into the peripheral airways where it polymerizes and acts as tissue glue on the surface of the lung to seal the target area to cause durable permanent absorption atelectasis.Citation15
Efficacy studies including relevant randomized and case studies of sealants in respiratory medicine
Review of clinical utility of sealants in thoracic surgery
Sealants can be used for sutures to establish tissue continuity instead of traditional surgical closure. In the past decade, tissue defects were treated with sutures, wires and staples, and bleeding was controlled with cautery. Nowadays, adhesives and sealants are finding their place in surgery with different uses along with different advantages and disadvantages. Sealants and adhesives can be natural/biological (polypeptide/protein-based and polysaccharide-based sealants), synthetic and semisynthetic. Concerning the indication field, the surgical sealants are used for hemostasis, tissue sealing and tissue engineering and are applicable in several body tissues. Challenge of sealant application differ among the different body structures, since some of the organs are more complex due to their consistency and biological characteristics.
In lung surgery, lung tissues can be sealed using mechanical methods such as sutures, staples or the implantation of surgical meshes. These methods are inevitably associated with lung tissue damage caused by deep piercing, ischemia and prolonged air leaks.Citation16 Prolonged air leak represents the most common complication after lung surgery and could lead to extended chest tube drainage time and development of infections, and broncho-pleural fistulae.Citation5,Citation17,Citation18
In addition, lung tissues can be sealed biologically via lung sealants/adhesives. Lung sealants must demonstrate specific characteristics due to the morphologic, functional and immunologic organ complexity. Respiratory movements pose additional stress to the reparation site; therefore, strong adhesiveness to the tissue is desirable. However, to preserve elastic recoil of the lung, sealant/adhesive should be highly elastic and flexible, as well.Citation19 Nonsterile and potentially septic environment in lungs can predispose wound infection.Citation20 Lungs are very immunogenic, so sealant should be biocompatible and biodegradable to avoid inflammation.Citation21 Potential air or liquid leakages during lung surgery necessitate sealants that tolerate high pressure and wet environment.Citation22 Concerning the complex vascularity of the lungs, sealant/adhesive should also have the function of hemostasis. Limited space sometimes complicates suturing, so sealant/adhesive can be delivered through applicators; in addition, endoscopic procedures can be a possible method to deliver sealant.
Review of clinical utility of sealants in bronchoscopic volume reduction methods
Biological lung volume reduction (Bio-LVR) uses direct application of a sealant or remodeling substances aimed at obtaining atelectasis and fibrosis of the lung parenchyma, thus preventing ventilation of these parts. Initial human studies were using bronchoscopic instillation of a fibrinogen–thrombin complex solution.Citation23,Citation24 Modification of that method, called Bio-LVR, was developed, adding chondroitin sulfate and poly-L-lysine to thrombin–fibrinogen complex. The idea was to form a hydrogel which can promote fibroblast attachment, proliferation and scarring, cause bronchial fibrostenosis and prevent ventilation of the affected part of the lung. Gel polymerization occurred in situ, which produced localized inflammatory reaction and resorptive atelectasis and lung region collapse over 4–6 weeks.Citation24,Citation25 Hydrogel was also used to seal interalveolar and bronchiole-alveolar pores which was supposed to result in disabling collateral ventilation.
The procedure is performed under conscious sedation using flexible bronchoscope which is introduced and wedged into the segmental or subsegmental airway leading to emphysematous part of the lung. To collapse distal airways, suction is applied through the bronchoscope. First, the enzymatic solution (eg, porcine trypsin) is instilled into the airway to detach epithelial cells from bronchial wall and to prepare mucosa for fibroblast adherence. After 2 minutes, that solution had to be washed out and a dual lumen catheter with thrombin and fibrinogen placed through the bronchoscope. The contents are instilled and pushed distally, mixing in the distal airway. The liquid component must fill the alveoli and block collateral ventilation. Each subsegmental application takes ~10 minutes and four to eight subsegments are treated during a single procedure.Citation28
This method was partially successful in the study of patients affected by heterogeneous, predominant upper lobe emphysema, showing mild-to-moderate improvement (improvement in pulmonary function, better dyspnea scores and quality of life), which lasted up to 6 months.Citation26 Similar results were reported in a study of 25 patients with bilateral homogeneous emphysema in whom high- or low-dose hydrogel was administered to eight subsegments.Citation13
Another observational study compared the effect of single lobe versus scattered double lobe Bio-LVR for predominant upper lobe emphysema.Citation28 Single lobe treatment led to a greater improvement in forced expiratory volume in 1 second (FEV1) at 12 weeks after the procedure compared to scattered double lobe approach. It can be explained by reducing collateral ventilation to other lobes by performing complete treatment of only one lobe.Citation27
There is also an alternative method that uses a synthetic polymeric foam called emphysematous lung sealant (ELS) with similar application technique. Herth et alCitation29 performed initial tests using ELS (AeriSeal® System; Pulmonx Corporation, Redwood City, CA, USA) in 25 patients with heterogeneous emphysema in 2011. There was an improvement in some lung function parameters (increased FEV1, FVC, 6-minute walk test and decreased residual volume [RV]/total lung capacity [TLC]), but only the improvement in FVC was statistically significant. Results were better in 14 patients in Global Initiative on Obstructive Lung Disease (GOLD) stage III compared with 11 subjects in GOLD stage IV for whom the benefit was less relevant.Citation29,Citation30 Over 90% of patients treated with Bio-LVR experienced flu-like symptoms, such as fever, dyspnea, pleurisy, nausea, headache and leukocytosis, within 24 hours of the procedure. These symptoms resolved in 24–48 hours.Citation27,Citation29
Magnussen et alCitation31 investigated the effect of interlobar fissure integrity on responses to treatment with an endoscopic tissue sealant (AeriSeal ELS) that collapses hyperinflated lung. They demonstrated that fissure integrity did not contribute to posttreatment changes in FEV1, RV/TLC ratio or lobar volume measured by CT analysis in patients with severe upper lobe predominant emphysema.
Safety and efficacy of ELS were assessed in 57 patients who were randomly assigned to two groups: the first group had ELS (two subsegments in each upper lobe) and received medical therapy and the second group had medical therapy alone.Citation31 Significant improvements were noted in lung function, dyspnea and quality of life in the ELS and medical therapy groups, and benefits persisted for 6 months. However, serious adverse events requiring hospitalization occurred in 44% of patients. Treatment responders tended to be those experiencing respiratory adverse events.Citation32
Recent study showed promising results in maintaining the improvement of volumetric HRCT and FEV1/FVC ratio.Citation33 It was performed in 15 patients in which autologous blood and fibrin glue were used to achieve BLVR and showed promising results in maintaining HRCT volumetry improvement and FEV1/FVC ratio over 12-week assessment period. Within both groups, there was statistically significant improvement in dyspnea, quality of life and exercise tolerance at 12 weeks postprocedure compared with baseline value.Citation33
Kramer et al, assessed the safety and efficacy of bilateral AeriSeal Emphysematous Lung Sealant System (ELS) in the treatment of patients with advanced emphysema. The study demonstrated short procedure time and length of hospital stay (average 1.1 day), successful primary end point of a reduction at 3 months in upper lobe lung volume analyzed by quantitative CT scan, with improvements in spirometry, gas trapping, diffusing capacity of lung for carbon monoxide, symptom scores (modified Medical Research Council dyspnea score) and health-related quality of life (St. George Respiratory Questionnaire) measured at 6 and 12 months. In the further studies, Kramer et alCitation35 summarized the safety and efficacy data of patients from the initial ELS study after 2 years and suggested that the beneficial effects of lung sealant therapy, previously reported at 1 year after treatment, persist to 2 years and beyond with a favorable long-term safety profile.
Fruchter et alCitation36 demonstrated that previous BLVR treatment was not associated with different outcomes following lung transplantation, but with evident increased bacterial colonization rates. However, when investigating correlation among airway bacterial colonization (ABC), serum C-reactive protein (CRP) level and the risk of COPD exacerbation within 1 month following BLVR, Fruchter et alCitation37 demonstrated that ABC is common in severe COPD patients undergoing BLVR, and along with elevated CRP level they are associated with high risk of immediate postprocedural COPD exacerbation.
Common inclusion and exclusion criteria for BLVR are summarized in . Patients with heterogenous “scattered” distribution of emphysema are not candidates for BLVR. Patients with heterogenous emphysema and COPD, GOLD stage III, had better treatment results.
Table 1 Common inclusion and exclusion criteria for Bio-BLVRCitation2,Citation11,Citation25,Citation28,Citation9
The safety of Bio-BLVR was analyzed in clinical studies. There were no treatment-related deaths, pulmonary emboli, episodes of heart failure, cardiac ischemia or myocardial infarction or severe cardiac arrhythmia. In addition, there were no serious procedural or immediate postprocedural complications, pneumothorax, bleeding episodes, respiratory failure requiring ventilator support, empyemas and lung abscesses. A few patients experienced spillage of material from the administration site into the central airways, but the material was cleared safely by suctioning through the bronchoscope in all patients.
However, BLVR treatment was associated with significant side effects. All patients who underwent BLVR experienced a transient inflammatory reaction, ie, “flu-like” reaction characterized by leukocytosis, elevated sedimentation rate and/or CRP, fever and malaise within 8–24 hours of treatment. In most cases, this reaction was self-limited and resolved within 24–96 hours with supportive care (nonsteroidal anti-inflammatory medications, acetaminophen, corticosteroids, bronchodilators and antibiotics as indicated). Some of these patients experienced COPD exacerbation, and some of them were related to the treatment procedure.
Although the study data showed that BLVR is a potential alternative to LVRS, questions about the techniques arise, such as how to prevent and reduce the treatment-associated inflammatory response, to predict the clinical outcome and to maintain the long-term efficacy with promising safety data.
Conclusion and future perspectives
The currently available data on the efficacy of numerous Bio-BLVR methods are not consistently conclusive.
Lung sealant technology is a novel and an effective approach for BLVR in patients with advanced emphysema. The results of the current studies about this treatment method are promising because they demonstrated bronchoscopic administration of sealants to be an effective method of improving respiratory function, exercise tolerance and quality of life in patients with advanced emphysema. It seems that subjective benefits in dyspnea scores and quality of life are more marked than improvements in lung function tests.
It is difficult to make the definite conclusion about the effectiveness of bronchoscopic administration of lung sealants LVRS due to small patient populations analyzed in current studies. More randomized large controlled studies are required in establishing the role of Bio-LVR in the treatment of emphysema, especially in the light of current achievements in emphysema phenotyping.
Disclosure
The authors report no conflicts of interest in this work.
References
- HoggJCSeniorRMChronic obstructive pulmonary disease – part 2: pathology and biochemistry of emphysemaThorax200257983083412200530
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- GagnonPGuenetteJALangerDPathogenesis of hyperinflation in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2014918720124600216
- MascalchiMCamiciottoliGDiciottiSLung densitometry: why, how and whenJ Thorac Dis2017993319334529221318
- ArmstrongHFDussaultNEThirapatarapongWLemieuxRSThomashowBMBartelsMNVentilatory efficiency before and after lung volume reduction surgeryRespir Care2015601637125371397
- O’BrienGMCrinerGJSurgery for severe COPD and lung volume reduction for emphysemaChest200412623824815249467
- FishmanAMartinezFNaunheimKA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- National Emphysema Treatment Trial Research GroupA randomized trial comparing lung-volume reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- ValipourAHerthFJFBurghuberOCTarget lobe volume reduction and COPD outcome measures after endobronchial valve therapyEur Respir J201443238739623845721
- IngenitoEPBergerRLHendersonACReillyJJTsaiLHoffmanABronchoscopic lung volume reduction using tissue engineering principlesAm J Respir Crit Care Med2003167577177812406835
- RefaelyYDransfieldMKramerMRBiologic lung volume reduction therapy for advanced homogeneous emphysemaEur Respir J2010361202719926742
- ShahPLGompelmannDValipourAThermal vapour ablation to reduce segmental volume in patients with severe emphysema: STEP-UP 12 month resultsLancet Respir Med201649e44e4527451345
- SciurbaFCCrinerGJStrangeCRENEW Study Research GroupEffect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW Randomized Clinical TrialJAMA2016315202178218927179849
- CardosoPFGSnellGIHopkinsPClinical application of airway bypass with paclitaxel-eluting stents: early resultsJ Thorac Cardiovasc Surg2007134497498117903516
- Falkenstern-GeRFIngerlHKohlhäuflMSevere emphysema treated by endoscopic bronchial volume reduction with lung sealant (AeriSeal)Case Rep Pulmonol2013201336139123710404
- BelboulADernevikLAljassimOSkrbicBRadbergGRobertsDThe effect of autologous fibrin sealant (Vivostat) on morbidity after pulmonary lobectomy: a prospective randomised, blinded studyEur J Cardiothorac Surg20042661187119115541982
- D’AndrilliAAndreettiCIbrahimMA prospective randomized study to assess the efficacy of a surgical sealant to treat air leaks in lung surgeryEur J Cardiothorac Surg200935581782019269837
- BertolacciniLLyberisPMannoELung sealant and morbidity after pleural decortication: a prospective randomized, blinded studyJ Cardiothorac Surg201054520509919
- AnnabiNYueKTamayolAKhademhosseiniAElastic sealants for surgical applicationsEur J Pharm Biopharm201595Pt A273926079524
- MontanaroLArciolaCRCenniECytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical useBiomaterials2001221596611085384
- AssmannAVeghAGhasemi-RadMA highly adhesive and naturally derived sealantBiomaterials201714011512728646685
- ItanoHThe optimal technique for combined application of fibrin sealant and bioabsorbable felt against alveolar air leakageEur J Cardiothorac Surg200833345746018243004
- HillerdalGGustafssonGWegeniusGEnglessonSHedenströmHHedenstiernaGLarge emphysematous bullae. Successful treatment with thoracoscopic technique using fibrin glue in poor-risk patientsChest19951075145014537750347
- ReillyJWashkoGPinto-PlataVBiological lung volume reduction: a new bronchoscopic therapy for advanced emphysemaChest200713141108111317426216
- CrinerGJPinto-PlataVStrangeCBiologic lung volume reduction in advanced upper lobe emphysema: phase 2 resultsAm J Respir Crit Care Med2009179979179819179484
- ErnstAAnanthamDEndoscopic management of emphysemaClin Chest Med201031111712620172437
- BergerRLDecampMMCrinerGJCelliBRLung volume reduction therapies for advanced emphysema: an updateChest2010138240741720682529
- StrangeCCrinerGLeedsWImproved efficacy of biological lung volume reduction (BLVR) therapy with lobar targeting [abstract]Am J Respir Crit Care Med2009179A4391
- HerthFJGompelmannDStanzelFTreatment of advanced emphysema with emphysematous lung sealant (AeriSeal®)Respiration2011821364521228545
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [homepage on the Internet]Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2017 Available from: www.goldcopd.orgAccessed April 15, 2017
- MagnussenHKramerMRKirstenAMEffect of fissure integrity on lung volume reduction using a polymer sealant in advanced emphysemaThorax201267430230822374920
- ComeCEKramerMRDransfieldMTA randomised trial of lung sealant versus medical therapy for advanced emphysemaEur Respir J201546365166225837041
- BakeerMAbdelgawadTTEl-MetwalyREl-MorsiAEl-BadrawyMKEl-SharawySLow cost biological lung volume reduction therapy for advanced emphysemaInt J Chron Obstruct Pulmon Dis2016111793180027536091
- KramerMRRefaelyYMaimonNRosengartenDFruchterOBilateral endoscopic sealant lung volume reduction therapy for advanced emphysemaChest201214251111111722722233
- KramerMRRefaelyYMaimonNRosengartenDFruchterOTwo-year follow-up in patients treated with emphysematous lung sealant for advanced emphysemaChest201314451677168024189860
- FruchterOFridelLKramerMRThe pathological features of bronchoscopic lung volume reduction using sealant treatment assessed in lung explants of patients who underwent lung transplantationRespiration201386214314423797158
- FruchterORosengartenDGoldbergEBen-ZviHTorRKramerMRAirway bacterial colonization and serum C-reactive protein are associated with chronic obstructive pulmonary disease exacerbation following bronchoscopic lung volume reductionClin Respir J201610223924525196428
