Abstract
There is a need for fast-acting, non-oral medication options for migraine because some attacks develop rapidly and some are accompanied by nausea, vomiting, and gastroparesis, which can hinder oral medication uptake and absorption. The most commonly prescribed migraine medications are oral triptans, with sumatriptan as the most common. However, oral triptans are associated with adverse events (AEs) of atypical sensations that may be problematic for patients. Subcutaneous (SC) injectable sumatriptan and conventional liquid triptan nasal spray formulations are also available, but the frequency of atypical sensations is the highest with SC sumatriptan, and the intense bitter taste of conventional liquid triptan nasal spray discourages use. AVP-825 (ONZETRA® Xsail®) is an intranasal medication delivery system containing 22 mg sumatriptan nasal powder that is now available in the USA for the acute treatment of migraine with or without aura in adults. The objective of this review is to summarize the development of AVP-825, which utilizes unique features of nasal anatomy to achieve efficient absorption and reduced systemic exposure. Literature searches for “sumatriptan nasal powder”, “AVP-825”, and “sumatriptan intranasal” were conducted. Review articles and pharmacokinetic, Phase II and Phase III studies were evaluated. AVP-825 demonstrates an earlier onset of efficacy and lower rate of atypical sensations than the oral standard of care, which can be attributed to its fast absorption and low systemic exposure. AEs of abnormal taste are predominantly mild. These results confirm the initial design concept for AVP-825, which aligned pharmacokinetics, anatomy, and drug presentation in a novel device to achieve optimal outcomes for the acute treatment of migraine.
Introduction
Migraine: a headache disorder
Migraine is a primary headache disorder characterized by attacks of moderate-to-severe headaches, typically on one side of the head, which have a pulsating quality, are worsened by physical activity, and are associated with nausea and/or vomiting, photophobia, and phonophobia.Citation1,Citation2 In up to a third of patients, migraines are preceded or accompanied by aura, which most commonly consists of visual symptoms.Citation1,Citation3 In the most recent Global Burden of Disease Study, migraine was the sixth most prevalent disorder and the second leading cause of years lived with disability worldwide.Citation4 In a recent US survey, 20.0% of women and 9.7% of men aged ≥18 years had a severe headache or migraine within the past 3 months.Citation5 Beyond the reduction in disability that would accompany successful treatment of individual migraine attacks, improved treatment may also reduce the risk of chronic migraine development.Citation6
Migraine medication: the importance of formulation and delivery route
The current mainstays of migraine treatment are orally administered over-the-counter pain relievers (acetaminophen, nonsteroidal anti-inflammatory drugs) and prescription triptans (serotonin [5-HT]1B/D receptor agonists).Citation7,Citation8 Oral medications are easy to use but can have drawbacks, particularly for migraine patients. For example, those who experience nausea and/or vomiting with their migraines may have difficulty taking or retaining oral medication.Citation9,Citation10 In addition, gastroparesis (delayed emptying of the stomach) may occur in migraine patients during or between migraines and appears to reduce absorption, consistency, and effectiveness of oral medications.Citation9,Citation11,Citation12 Oral medications are also subject to gastrointestinal (GI) and hepatic first-pass metabolism, which can change their pharmacokinetics and clinical effects. For example, orally administered sumatriptan tablet, the most commonly prescribed triptan, is largely converted to an inactive metabolite through first-pass metabolism and, combined with incomplete absorption, has a bioavailability of about 15% in humans.Citation13,Citation14 Notably, one-third of patients in clinical trials fail to obtain headache relief with sumatriptan oral.Citation15
While there are a limited number of ways of bypassing the GI tract, non-oral formulations of some triptans are available and primarily include subcutaneous (SC) injection of sumatriptan (needle and needle-free) and liquid intranasal triptans (sumatriptan and zolmitriptan). SC sumatriptan injection is fast-acting but has a substantial rate of injection-site reactions and triptan-associated atypical sensations (eg, tingling, numbness, hot sensation, flushing, and chest/jaw/neck tightness), and fewer than 10% of eligible migraine patients use SC sumatriptan for treatment.Citation16–Citation21 Intranasal triptans are also fast-acting and effective with different nasal delivery methods available. This review highlights the nose as a medication delivery route and currently available intranasal delivery methods for migraine, including the Breath Powered® sumatriptan dry nasal powder, AVP-825, medication delivery system approved by the US Food and Drug Administration as ONZETRA® Xsail® (sumatriptan nasal powder; Avanir Pharmaceuticals, Inc. Aliso Viejo, CA) for the acute treatment of migraine with or without aura in adults.Citation22
The nose as a route for drug delivery
The nose is an attractive delivery route for medication, as it has a large mucosal surface area and is highly vascular, making it well suited for rapid absorption of drugs into the systemic circulation.Citation23,Citation24 As the primary purpose of the nasal airway is to protect the lungs from hazardous exposure, some aspects of nasal anatomy and physiology present obstacles to efficient nasal drug delivery.Citation23 The richly vascular mucosa most conducive to drug absorption lies in the upper posterior nose, beyond the narrow nasal valve.Citation23,Citation24 The small dimensions of the nasal valve and its triangular shape that narrows further during nasal inhalation are impediments to drug delivery beyond it.Citation23
Conventional liquid nasal sprays
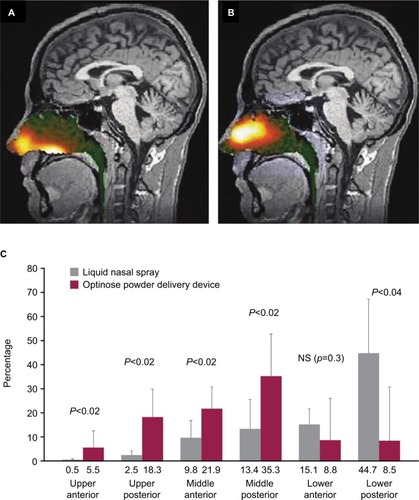
Intranasal drug delivery commonly has been accomplished using liquid nasal spray delivered through a metered-dose mechanical pump,Citation23 which is the case for the conventional liquid intranasal sumatriptan formulation. Activating the spray pump propels liquid medication outward in a hollow spray cone, and even if the spray pump is inserted deep into the nostril, there is a mismatch between the geometry of the circular spray cone and the narrow triangular nasal valve opening.Citation23 Imaging studies using a liquid solution of radiolabeled diethylene triamine pentaacetic acid (DTPA) show that deposition from a conventional liquid intranasal spray pump largely occurs anterior to the nasal valve and on the interior floor of the nose, with only a limited portion of the liquid penetrating beyond the nasal valve to the highly absorptive upper posterior nasal cavity ().Citation23–Citation25 Pharmacokinetic studies of conventional sumatriptan liquid nasal spray indicate that a substantial portion of the dose administered through conventional liquid spray pump is subsequently swallowed.Citation24–Citation28 Once swallowed, the medication becomes, in effect, an orally administered medication that is subject to the disadvantages noted previously. In addition, bitter taste is often reported with these nasal sprays because bitter-sensing taste buds at the base of the tongue are exposed to the liquid medication.Citation19,Citation24
Figure 1 Imaging of intranasal delivery of (A) conventional liquid spray (radiolabeled diethylene triamine pentaacetic acid) vs (B) powder (radiolabeled lactose) with the breath-powered device and (C) quantification of deposition patterns.
Breath-powered exhalation delivery of sumatriptan dry nasal powder: AVP-825
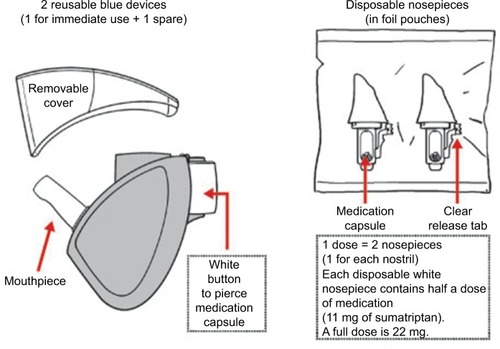
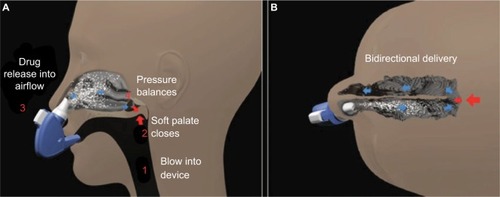
AVP-825 was designed to improve intranasal medication delivery by taking greater advantage of nasal anatomy to achieve more effective drug delivery to the upper posterior absorptive surfaces of the nasal cavity.Citation22,Citation24 AVP-825 consists of a breath-powered exhalation device and a low-dose dry powder formulation of sumatriptan (22 mg), supplied as a reusable mouthpiece and two disposable nosepieces each containing a capsule filled with 11 mg sumatriptan nasal powder (). A dry powder formulation has several potential advantages over liquid for intranasal delivery, including greater stability, reduced need for preservatives, and greater adherence to the upper posterior nasal mucosa.Citation23 To use AVP-825, the user inserts a nosepiece into the device, presses a button to pierce the medication capsule, and then slides the nosepiece into one nostril until it forms a seal with the nostril opening. This action expands the nasal valve. The user then rotates the device to place the mouthpiece into the mouth and blows into it, which leads to closing of the soft palate and further expansion of the nasal passage (). The closing of the soft palate isolates the nasal cavity from the throat, which prevents medication deposition into the throat, lungs, or GI tract. In addition, the positive pressure created by blowing into the device allows airflow to enter one nostril, diffuse within the upper nasal cavity, and exit through the other nostril, resulting in Bi-Directional® delivery (). The user then repeats the process with the second nosepiece in the opposite nostril. Each nosepiece delivers an average dose of 7.5–8.1 mg sumatriptan, providing a total dose of 15–16.2 mg sumatriptan per treatment episode from two nosepieces.Citation22
Figure 3 Illustration of breath-powered intranasal delivery.
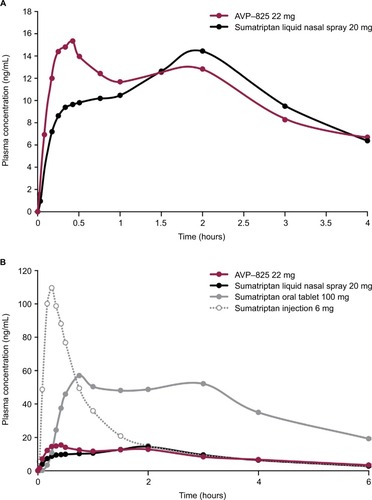
Delivery of powder (radiolabeled lactose) through the breath-powered exhalation technology yields significantly greater deposition in the upper posterior, highly absorptive regions of the nasal cavity beyond the nasal valve () compared to conventional liquid spray (radiolabeled DTPA) (), as shown by imaging studies.Citation23–Citation25 Pharmacokinetic data comparing AVP-825 and conventional sumatriptan liquid nasal spray (20 mg) support these imaging studies, as AVP-825 shows faster absorption, with an earlier and significantly higher peak plasma concentration and a 61% higher systemic sumatriptan exposure in the first 30 minutes (), despite a 20% lower delivered dose.Citation29 AVP-825 also produces faster sumatriptan absorption compared to 100 mg sumatriptan oral tablet () and a lower peak and total exposure than the 100 mg oral tablet or 6 mg SC sumatriptan injection,Citation29,Citation30 indicating the possibility of improved tolerability with AVP-825.
Figure 4 Sumatriptan plasma concentration–time profiles over (A) the first 4 hours after AVP-825 (22 mg) and conventional sumatriptan liquid nasal spray (20 mg) and (B) the first 6 hours of the entire 14-hour sampling period for AVP-825, conventional sumatriptan liquid nasal spray (20 mg), sumatriptan oral tablet (100 mg), and subcutaneous sumatriptan injection (6 mg).
Evaluating AVP-825 efficacy and safety in clinical trials
AVP-825 was evaluated as an acute treatment of migraine in one Phase II and two Phase III randomized, double-blind, controlled trials in adults.Citation31–Citation33 Two of these trials compared AVP-825 to placebo in a single migraine attack (the Phase II studyCitation32 and the Phase III TARGET study [NCT01462812]Citation31), and one trial compared AVP-825 to 100 mg sumatriptan oral tablet (well-established standard of care) over the course of multiple migraine attacks, a randomized, controlled comparative efficacy trial (The COMPASS study, NCT01667679).Citation33 In these trials, AVP-825 demonstrated superior efficacy compared to placebo and an earlier onset of efficacy compared to 100 mg sumatriptan oral tablets. Advantages on other measures of efficacy (eg, rates of nausea, photophobia, and phonophobia, assessment of disability, and patient-assessed meaningful relief) were also observed. AVP-825 treatment was well tolerated in all three trials, with no serious adverse events (AEs) and few discontinuations due to AEs. The most common AEs in all trials related to the site of administration were predominantly mild in severity, and AEs related to abnormal taste did not lead to any discontinuations.Citation34 There was only one case of a mild atypical sensation (paresthesia) in the single-attack Phase II and III placebo-controlled trials, and in the multiattack Phase III COMPASS trial, atypical sensations occurred at a significantly lower rate with AVP-825 vs 100 mg sumatriptan oral tablet.
The prospect of a device that could efficiently deliver low-dose sumatriptan led to the initial design of AVP-825. The results were quicker onset of efficacy of sumatriptan compared to the oral formulation with lower systemic exposure and fewer side effects, confirming the design concepts and the pharmacokinetic promise.
Phase II study (N=117)
Two doses of sumatriptan dry nasal powder (11 and 22 mg) delivered through the breath-powered exhalation system were compared to an identical placebo delivery system in the treatment of a single migraine attack of moderate-to-severe intensity.Citation32 After treatment, a significantly greater proportion of patients in each sumatriptan nasal dry powder dose group (11 and 22 mg) vs placebo were pain free at 120 minutes (54% and 57% vs 25%, P<0.05, primary end point), had pain relief as early as 60 minutes (73% and 74% vs 38%; P<0.01), and had pain relief at 120 minutes (84% and 80% vs 44%, P<0.001 and P<0.01). Efficacy was sustained as both sumatriptan nasal dry powder dose groups showed significantly greater rates of sustained pain freedom from 120 minutes up to 48 hours compared to placebo (47% and 49% vs 22%, P<0.05). AEs occurred in 18% and 23% of patients in the 11 and 22 mg sumatriptan nasal powder groups, respectively, vs 5% for placebo, and the most common AE was bitter or metallic taste (10% and 13% vs 0%). No events of chest discomfort or pain, paresthesia, or asthenia were reported for the active treatment groups, and there were no serious AEs or withdrawals due to AEs.
Phase III TARGET study (N=230)
AVP-825 (22 mg) was compared to an identical placebo delivery system (containing lactose powder) in the treatment of a single migraine attack of moderate-to-severe intensity.Citation31 A significantly greater proportion of patients treated with AVP-825 vs placebo were pain free at 120 minutes (34.3% vs 17.3%, P=0.008), had pain relief as early as 30 minutes (41.7% vs 26.9%; P=0.03), and had pain relief at 120 minutes (67.6% vs 45.2%, P=0.002, primary end point). Efficacy was sustained as AVP-825 showed a significantly greater rate of sustained pain freedom from 120 minutes up to 48 hours compared to placebo (20.4% vs 8.7%, P=0.02). The most common AEs (≥2% in any treatment group) were abnormal product taste (22% AVP-825 vs 4% placebo), nasal discomfort (13% vs 2%), rhinorrhea (5% vs 3%), and rhinitis (3% vs 0%). One patient reported mild paresthesia with AVP-825, but there were no other reports of atypical sensations. There were no serious AEs, and only one patient withdrew because of AEs (placebo treatment).
Phase III COMPASS study (N=275)
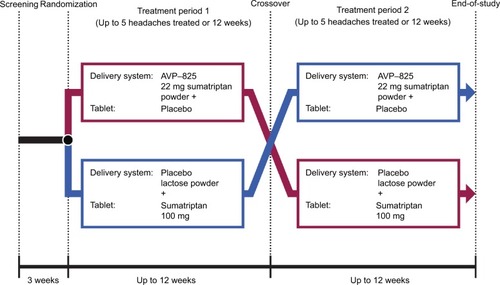
AVP-825 (22 mg) was compared to 100 mg sumatriptan oral tablet using a double-dummy, crossover trial design in the treatment of multiple migraine attacks ().Citation33 Treatment began within 1 hour of the onset of the migraine attacks, even if pain was mild. Patients treated up to five migraines per treatment period (up to 12 weeks) with AVP-825 plus oral placebo tablet or an identical placebo device (containing lactose powder) plus 100 mg sumatriptan oral tablet for the first double-blind treatment period and then switched treatment for the next period. Overall, >1,500 migraine attacks were evaluated.
Figure 5 COMPASS study design.
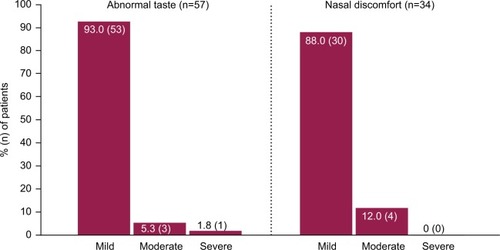
A significantly greater reduction in migraine pain intensity occurred with AVP-825 treatment vs sumatriptan oral in the 30 minutes postdose, as assessed by summed pain intensity differences (SPID-30, 10.80 vs 7.41, P<0.001, primary end point), and this was true regardless of whether attacks were treated when pain was mild or moderate/severe.Citation34 As early as 15 minutes postdose, rates of pain freedom (7.2% vs 3.7%, P=0.008) and pain relief (27.9% vs 20.9%, P=0.007) were significantly greater with AVP-825 vs sumatriptan oral, and significant differences in favor of AVP-825 continued through every time point up to and including 90 minutes postdose. Sustained efficacy was similar between treatments, as measured by rates of sustained pain relief and sustained pain freedom from 120 minutes up to 48 hours. AEs occurred in 53.9% and 32% of patients for AVP-825 and 100 mg sumatriptan oral tablet, respectively, with local administration site AEs such as abnormal taste (26% vs 4%) and nasal discomfort (16% vs 1%) driving the difference in overall AE rate.Citation34 However, these AEs were predominantly mild in severity () and led to few discontinuations (one patient each with nasal discomfort, throat irritation, and throat tightness).Citation33,Citation34 The rate of atypical sensations was significantly lower with AVP-825 compared to 100 mg sumatriptan oral tablet (2% vs 5%, P=0.02), and throughout the trial, no serious AEs occurred, and <2% of patients receiving either treatment withdrew because of AEs.
Figure 6 Severity of the most common treatment-emergent adverse events associated with AVP-825 in COMPASS.
Expert opinion
Development of AVP-825: from bench to medication delivery
Early efficacy and tolerability are important aspects of patient satisfaction with migraine therapy.Citation35–Citation37 A key advantage of AVP-825 is that it allows for quick drug absorption and thereby an earlier onset of action relative to typical oral migraine therapies. In addition, AVP-825 delivers a lower sumatriptan dose relative to sumatriptan oral, which results in lower systemic exposure and a correspondingly lower incidence of atypical sensations. Based on clinical experience with migraine patients who have experienced triptan-associated atypical sensations and the resulting negative impact on compliance and tolerability, we argue that this difference is clinically important and should be considered in the treatment decision-making process.
For migraine, which can be accompanied by nausea, vomiting, and gastric stasis, a non-oral medication option such as AVP-825 can be optimal. Furthermore, while nausea is often a symptom of migraine, it can also be a result of treatment.Citation38,Citation39 Recent longitudinal modeling analyses of data from the COMPASS trial explored the impact of treatment on nausea and showed that AVP-825 led to more rapid early reductions in nausea rates during the first hour after treatment, reduced odds of nausea from 30 minutes to 2 hours following treatment, and a reduced risk of treatment-emergent nausea compared to sumatriptan oral.Citation40
AVP-825 uptake in the clinic may be hampered by an assumption that it is “just another nasal spray”, which may lead to concerns that AVP-825 is associated with many of the same disadvantages noted with traditional nasal sprays. While some medication is swallowed with any intranasal delivery method, AVP-825 was designed to reduce medication swallowing through the breath-powered closing of the soft palate. Pharmacokinetic data for AVP-825 as well as severity data for taste-related AEs in the AVP-825 COMPASS trial are consistent with this advantage. In COMPASS, 93% of the cases of abnormal taste were classified as mild.
AVP-825 should be considered for patients who need rapid migraine relief and those who fail to tolerate triptans in oral, injectable, or conventional liquid nasal spray formulations. It may be especially useful in those with migraine with concurrent nausea or vomiting, patients who have difficulty in swallowing, and/or patients with gastroparesis or other GI problems. In addition, patients who have struggled with atypical sensations with higher doses of other triptan formulations may be ideal candidates for AVP-825. It should also be noted that migraine treatment is likely not going to be limited to AVP-825. Rather, AVP-825 may be an option along with other sumatriptan formulations for patients who use an attack-based care model to treat their migraines,Citation19 in which the characteristics of an individual migraine attack dictate the use of a particular treatment.
Breath-powered technology has the potential to be adapted for other nasal and central nervous system-acting agents. A recently approved fluticasone propionate product for the treatment of nasal polyps employs this technology,Citation23,Citation41 and additional applications for this technology are under investigation.Citation42
Key takeaways
The need for non-oral medication is clear in migraine, due to speed of some attacks and the presence of nausea, vomiting, and gastroparesis.
The AEs associated with both SC and sumatriptan oral, the triptan atypical sensations, also suggest the need for an improved, more tolerable sumatriptan delivery system.
The intense bitter taste of conventional liquid nasal sumatriptan discourages use.
The development of a breath-powered dry nasal powder sumatriptan delivery system, AVP-825, was predicated on delivery of a lower dose of sumatriptan to the upper posterior of the nose for optimal absorption and a closure of the soft palate to reduce oral exposure and the bad taste.
The comparative efficacy COMPASS trial, directly comparing the breath-powered dry nasal powder AVP-825 system repeatedly to oral 100 mg sumatriptan in a double dummy design, in the same patients and across multiple patients and attacks, demonstrated earlier onset of efficacy with a lower dose and fewer AEs for AVP-825 over the oral standard of care, confirming the initial engineering and aligning the pharmacokinetics, anatomy, and drug presentation of the novel device with optimal outcomes for the acute treatment of migraine.
Acknowledgments
The authors thank Jennifer Hepker, PhD, from Prescott Medical Communications Group (Chicago, IL, USA) for editorial assistance.
Disclosure
Stewart J Tepper, MD, has consulted and/or participated in advisory boards for Acorda, Alder, Allergan, Amgen, ATI, Avanir Pharmaceuticals, Inc., Cefaly, Charleston Laboratories, DeepBench, Dr. Reddy’s, electroCore, eNeura, Eli Lilly, GLG, Guidepoint Global, Impax, Pfizer, Scion Neurostim, Slingshot Insights, Supernus, Teva, and Zosano, received research support (no personal compensation, not tied to salary) from Alder, Allergan, Amgen, ATI, Avanir, Dr. Reddy’s, electro-Core, eNeura, Scion Neurostim, Teva, and Zosano, holds stock options from ATI, receives salary from Dartmouth-Hitchcock Medical Center and the American Headache Society, and receives royalties from Springer. The authors report no other conflicts of interest in this work.
References
- Headache Classification Committee of the International Headache Society (IHS)The International Classification of Headache Disorders, 3rd editionCephalalgia2018381121129368949
- MarmuraMJSilbersteinSDSchwedtTJThe acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapiesHeadache201555132025600718
- LoderETriptan therapy in migraineN Engl J Med20103631637020592298
- GBD 2016 Disease and Injury Incidence and Prevalence CollaboratorsGlobal, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016Lancet2017390101001211125928919117
- QuickStats: percentage of adults aged ≥18 years who reported having a severe headache or migraine in the past 3 months, by sex and age group – National Health Interview Survey, United States, 2015MMWR2017662465428640794
- LiptonRBFanningKMSerranoDReedMLCadyRBuseDCIneffective acute treatment of episodic migraine is associated with new-onset chronic migraineNeurology201584768869525609757
- BeckerWJAcute migraine treatment in adultsHeadache201555677879325877672
- DiamondSBigalMESilbersteinSLoderEReedMLiptonRBPatterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention studyHeadache200747335536317371352
- JohnstonMMRapoportAMTriptans for the management of migraineDrugs201070121505151820687618
- SilbersteinSDPractice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology200055675476210993991
- Camara-LemarroyCRRodriguez-GutierrezRMonreal-RoblesRMarfil-RiveraAGastrointestinal disorders associated with migraine: a comprehensive reviewWorld J Gastroenterol201622368149816027688656
- TepperSJRapoportAMThe triptans: a summaryCNS Drugs1999125403417
- IMITREX (sumatriptan succinate) tablets, for oral use [Prescribing information]GlaxoSmithKline Research Triangle ParkNC, USA2013
- DixonCMSaynorDAAndrewPDOxfordJBradburyATarbitMHDisposition of sumatriptan in laboratory animals and humansDrug Metab Dispos19932157617697902233
- DodickDWTriptan nonresponder studies: implications for clinical practiceHeadache200545215616215705122
- GallagherRMKunkelRMigraine medication attributes important for patient compliance: concerns about side effects may delay treatmentHeadache2003431364312864756
- CadyRKMunjalSCadyRJManleyHRBrand-SchieberERandomized, double-blind, crossover study comparing DFN-11 injection (3 mg subcutaneous sumatriptan) with 6 mg subcutaneous sumatriptan for the treatment of rapidly-escalating attacks of episodic migraineJ Headache Pain20171811728176235
- DerryCJDerrySMooreRASumatriptan (all routes of administration) for acute migraine attacks in adults – overview of Cochrane reviewsCochrane Database Syst Rev20145CD009108
- SilbersteinSDMarcusDASumatriptan: treatment across the full spectrum of migraineExpert Opin Pharmacother201314121659166723786565
- IMITREX (sumatriptan succinate) injection, for subcutaneous use [Prescribing information]GlaxoSmithKline Research Triangle ParkNC, USA2015
- Sumavel DosePro (sumatriptan injection), for subcutaneous use [Pre-scribing information]Endo Pharmaceuticals IncMalvern, PA, USA2016
- ONZETRA® Xsail® (sumatriptan nasal powder) [Prescribing information]Avanir Pharmaceuticals, IncAliso Viejo, CA, USA2016
- DjupeslandPGNasal drug delivery devices: characteristics and performance in a clinical perspective – a reviewDrug Deliv Transl Res201331426223316447
- DjupeslandPGMessinaJCMahmoudRABreath powered nasal delivery: a new route to rapid headache reliefHeadache201353Suppl 2728424024605
- DjupeslandPGSkrettingANasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray pumpJ Aerosol Med Pulm Drug Deliv201225528028922251061
- FuseauEPetricoulOMooreKHBarrowAIbbotsonTClinical pharmacokinetics of intranasal sumatriptanClin Pharmacokinet2002411180181112190330
- DuquesnoyCMametJPSumnerDFuseauEComparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administrationEur J Pharm Sci199862991049795022
- DerryCJDerrySMooreRASumatriptan (intranasal route of administration) for acute migraine attacks in adultsCochrane Database Syst Rev20122CD009663
- ObaidiMOffmanEMessinaJCarothersJDjupeslandPGMahmoudRAImproved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powderHeadache20135381323133323992438
- LuthringerRDjupeslandPGSheldrakeCDRapid absorption of sumatriptan powder and effects on glyceryl trinitrate model of head-ache following intranasal delivery using a novel bi-directional deviceJ Pharm Pharmacol20096191219122819703372
- CadyRKMcAllisterPJSpieringsELA randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (The TARGET Study)Headache20145518810025355310
- DjupeslandPGDocekalPCzech Migraine Investigators GroupIntranasal sumatriptan powder delivered by a novel breath-actuated bi-directional device for the acute treatment of migraine: a randomised, placebo-controlled studyCephalalgia201030893394220656704
- TepperSJCadyRKSilbersteinSAVP-825 breath-powered intra-nasal delivery system containing 22 mg sumatriptan powder vs 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS Study): a comparative randomized clinical trial across multiple attacksHeadache201555562163525941016
- SilbersteinSWinnerPKMcAllisterPJEarly onset of efficacy and consistency of response across multiple migraine attacks from the Randomized COMPASS Study: AVP-825 Breath Powered® Exhalation Delivery System (Sumatriptan Nasal Powder) vs Oral SumatriptanHeadache201757686287628497569
- DaviesGMSantanelloNLiptonRDeterminants of patient satisfaction with migraine therapyCephalalgia200020655456011075838
- LiptonRBHamelskySWDaynoJMWhat do patients with migraine want from acute migraine treatment?Headache200242Suppl 13911966858
- SmeltAFLouterMAKiesDAWhat do patients consider to be the most important outcomes for effectiveness studies on migraine treatment? Results of a Delphi studyPLoS One201496e9893324932784
- FerrariMDGoadsbyPJRoonKILiptonRBTriptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trialsCephalalgia200222863365812383060
- PierceMOral triptans and nausea: treatment considerations in migraineHeadache201353Suppl 1172023721286
- LiptonRBMcGinleyJSShulmanKJSilbersteinSDWirthRJBuseDCAVP-825 (sumatriptan nasal powder) reduces nausea compared to sumatriptan tablets: results of the COMPASS Randomized Clinical TrialHeadache201858222924229034453
- XHANCE™ (fluticasone propionate) nasal spray, for intranasal use [Prescribing Information] Available from: https://www.xhance.com/wp-content/themes/xhance/assets/pdf/XHANCE_Full_Prescribing_Information.pdf2017Accessed February 27, 2018
- OptiNose US, IncResearch & Development Available from: https://www.optinose.com/research-and-development/overview-chart2017Accessed February 27, 2018