Abstract
Background
Government regulations require postmarket surveillance for cleared/approved medical devices. Trend analysis of newly marketed devices may help to confirm device-related safety or uncover other device or procedure-related problems.
Methods
Complaints related to the use of 3D-printed triangular titanium implants for sacroiliac joint (SIJ) fusion were compared with those of the prior machined version of the device manufactured with a titanium plasma spray (TPS) coating. Event rates were calculated either by dividing event counts by numbers of surgeries or, for late events, using Kaplan–Meier survival analysis.
Results
Three types of complaints with nontrial frequencies were identified. Issues in instruments occurred at a low and constant rate (1.3%). Using Kaplan–Meier analysis, pain-related complaints occurred at a low and similar rate in both groups (<0.5%). The 1-year cumulative probability of surgical revision was low in both the 3D and machined versions of the device (1.5% for machined and 1% for 3D printed, P=0.0408 for difference). No implant breakages or migrations were identified in either group, and overall rates were similar to a previously published report.
Conclusion
The 3D-printed version of triangular titanium implant was associated with complaint and adverse event rates similar to those for the prior machined version of the device.
Introduction
Following regulatory approvals, both medical device and pharmaceutical manufacturers must perform postmarket surveillance to ensure that use of their products in the commercial setting continues to be safe and effective. As per USCitation1 and European regulations,Citation2 medical device manufacturers must track and evaluate all complaints. US regulations define a complaint as “any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance of a device after it is released for distribution”. A complaint can be solely a device-related issue without patient impact or an issue that causes harm to a patient. These records are commonly inspected by regulatory authorities. Such tracking aids manufacturers in confirming the safety use of commercialized devices and helps to detect potential trends in safety problems (eg, for hip implants).Citation3 In the US, complaints resulting in patient harm are submitted to United States Food and Drug Administration and available for public review.Citation4
The sacroiliac joint (SIJ) connects the lower lumbar spine with the pelvis. The SIJ is thought to be involved in 15–30% of all chronic lower back pain.Citation5–Citation9 Nonsurgical treatments for SIJ pain are commonly used, but evidence supporting long-term effectiveness is lacking and chronic persistent pain is relatively common. With the commercial availability of purpose-built devices, interest in surgical fusion of the SIJ is growing. Prospective trials of SIJ fusion using titanium plasma spray (TPS)-coated triangular titanium implants (iFuse; SI-BONE, Inc., Santa Clara, CA, USA) have shown the procedure to be safe and effective, providing long-term pain and disability relief for most patients with chronic SIJ dysfunction.Citation10–Citation13
Previously, we reported postmarket surveillance findings for both procedure-related safety eventsCitation14 and surgical revisions after SIJ fusion using TPS-coated triangular titanium implants.Citation15 The former study reported a relatively low rate of complaints resulting in patient harm. The reported rate was similar to the device and procedure-related adverse events subsequently observed in three prospective trials of the same device.Citation10–Citation12 The latter study reported an important but less common adverse event, namely pain requiring surgical revision.Citation15 Surgical revision rates were low and decreased over time, possibly related to improved surgical techniques and/ or improved training.
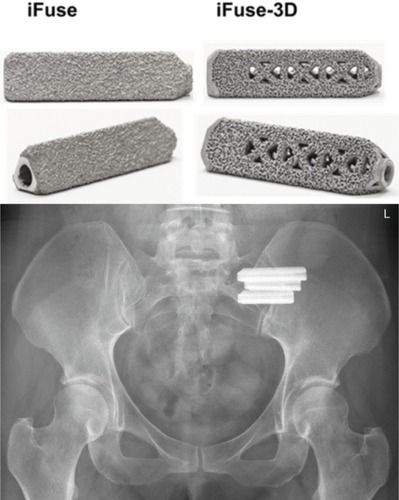
Recently, a 3D-printed version of the iFuse implant (iFuse-3D) gained market clearance in the US and EU (). The implant, made from the same underlying material (titanium, Ti-6Al-4V ELI, ASTM F136) but produced via additive manufacturing (3D printing) has an optimized porous surface as well as fenestrations designed to allow bone to grow onto and through the center of implant. Using methods derived from the prior published safety studies, we report a comparison of perioperative complaints as well as surgical revision rates of the TPS-coated and 3D-printed implants.
Figure 1 Top: triangular titanium implants for SIJ fusion. Left: machined iFuse implant (commercially available since 2009). Right: 3D-printed implant (iFuse-3D, commercially available since early 2017). Bottom: X-ray image of implants after placement across the left SIJ.
Methods
Complaint database
Since 2009, key data regarding product complaints reported to SI-BONE, Inc., have been actively maintained. Data captured include procedure date, surgeon name, implant catalog number/lot number, and complaint details. All complaints are investigated as to root cause, and appropriate actions are undertaken as required (eg, device redesign, fixing manufacturing issues, and surgeon retraining) in response to such root causes. When a complaint involves a potential adverse event, a staff surgeon (WCR) reviews the event and may contact the involved surgeon to gather further details. All complaints are evaluated for reportability as required by the US FDA’s Medical Device Reporting regulationCitation1 as well as EuropeanCitation2 and other relevant regulations. Institutional review board approval was not required for this study as it was based on an analysis of internal company data routinely collected during postmarket surveillance.
In this report, a surgical revision was defined as an additional surgical procedure on an SIJ treated with the company’s device (iFuse or iFuse-3D). Index cases representing unapproved uses or uses to address the failure of another device were excluded. In addition, analysis focused solely on complaints occurring in the USA and Canada, as these are likely reported with the greatest fidelity. The current analysis included complaints reported to the manufacturer between January 1, 2015, and June 30, 2018.
Statistical analysis
For acute intraoperative events (eg, instrument breakage) or immediate postoperative events (bleeding), rates are reported as the number of events per quarter divided by the number of surgical cases per quarter. Where relevant, acute events were compared across device types with a Fisher’s test.
For events that are more likely to be delayed (eg, surgical revision and pain), Kaplan–Meier survival analysis was additionally used. For patients with revision surgery, “days to event” was defined as days from index surgery date to revision surgery; for patients with pain complaints, days to event was defined as index surgery date to complaint date. Patients without such complaints were censored according to days from index surgery to a fixed date (July 1, 2018). Kaplan–Meier analyses were restricted to surgeries (and their associated complaints) taking place from January 1, 2015, to present. Given the relatively young mean age of patients undergoing this procedure (55.8Citation15), expected overall survival is high and lack of vital status information, which could cause early censoring, is not expected to meaningfully affect calculated rates. Survival rates were compared using the log-rank test.
An unknown but small (estimated 10%) proportion of patients undergo staged bilateral surgery, meaning index surgery on one side, followed weeks to months later by index surgery on the other side. The reported number of sides therefore slightly overestimates the number of treated patients.
Results
From January 1, 2015, to June 30, 2018, 14,210 SIJ fusions were performed using either iFuse implants (11,070 cases) or iFuse-3D implants (3,140 cases). iFuse cases occurred at a steady rate until the second quarter of 2017, when iFuse-3D was introduced. By the second quarter of 2018, iFuse-3D was used in >80% of all index surgeries.
During this period, 837 complaints (USA) were reported to SI-BONE, Inc. (). Most events occurred at a very low rate and were therefore not compared across implant types due to the lack of statistical power. Analysis below focuses on events occurring with more than very low frequencies, namely instrument problems, pain complaints, and revision surgeries. shows days elapsed from index surgery date to complaint report date and includes events that typically occur soon after surgery (eg, cardiovascular events and infection) and events that can occur at later times (eg, pain recurrence and revision surgery). We note that the two cases of metal sensitivity were not confirmed via MELISACitation16,Citation17 (metal-linked immunosorbent assay, see http://www.melisa. org) or LTT (lymphocyte transformation test,Citation18,Citation19 https://www.orthopedicanalysis.com/).
Table 1 Complaints (USA) reported to manufacturer between January 1, 2015, and June 30, 2018
Table 2 Days from index surgery to complaint by complaint type and device type. In some cases, surgery or complaint dates were not available.
Instrument problem
For iFuse implants, the rate of damaged instruments occurred at a mean rate of 1.3% with no obvious changes over time (). As the same instruments are used to place iFuse and iFuse-3D, no analysis across implant type was performed.
Table 3 Instrument issue rate (USA) by quarter between January 1, 2015, and June 30, 2018
Pain complaints
A total of 173 pain complaints (170 with iFuse and three with iFuse-3D) were reported to the manufacturer between January 2015 and June 2018. For surgeries performed after January 1, 2015, the probability of a pain complaint event, evaluated by Kaplan–Meier methodology, was very low (1-year rate <0.5%) and showed no difference across device type (log rank P=0.138). These complaints represented a variety of issues, including transient pain after surgery, wound infection, persistent pain, and pain recurrence. None of these complaints resulted in a surgical revision of the treated side.
Revision surgery
The company was notified of a total of 435 revision surgeries between January 2015 and June 2018: 409 in iFuse cases and 26 in iFuse-3D cases. For surgeries performed after January 1, 2015, the 1-year product-limit estimate of the cumulative rate of revision surgery was 1.5% for iFuse and 1.0% for iFuse-3D (P=0.0408, ). For these revisions, implant malposition resulting in symptomatic nerve impingement was the most common reason (n=151, 54% of all revisions, ), occurring at a median of 29 days after index surgery. There were no differences in time to revision for symptomatic implant malposition across device types (29 days for iFuse vs 41 days for iFuse-3D, P=0.9). Revisions for other causes occurred at a median of 367–478 days after surgery. These late revisions include procedures performed to treat symptomatic pseudoarthrosis, lack of improvement in malpositioned implants (not placed fully across the SIJ), and a small number procedures where implants were removed because the patients obtained no pain relief from the index procedure, possibly due to misdiagnosis.
Figure 2 Cumulative probability of implant revision surgery after iFuse or iFuse-3D.
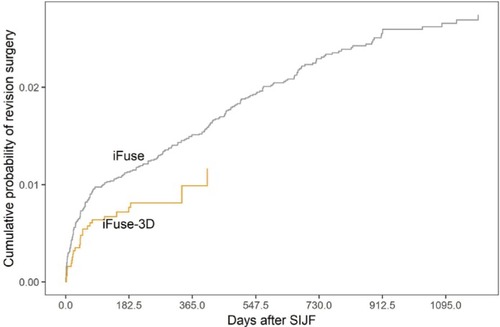
Table 4 Suspected cause for surgical revisions (USA only) by device type for surgeries performed between January 1, 2015, and June 30, 2018
Discussion
The major findings of our postmarket surveillance analysis are as follows: 1) complaints related to use of triangular titanium implants for SIJ fusion occur at a low rate and 2) the complaint rate for iFuse-3D (newer version of device) appears to be qualitatively similar to the older, machined version of the device. Notably, we did not observe any instances of device breakage or migration. No new types of complaints related to iFuse-3D were identified.
The complaint rate for instrument issues (primarily damaged instruments) has been consistent at ~1.3% of all US cases. There were no reported adverse events related to damaged instruments. Instrument damage due to user error is the most common occurrence (eg, bending or cutting of guide pins due to noncollinearity during drilling, damaged chisel edges when the removal guide is not correctly lined up with the iFuse Implant and guide pin clamps that are not used correctly). Other instrument damages such as breaking of soft tissue protector heads, through which other instruments used for device placement are passed, may have been due to heat-related damage during re-sterilization.
The rate of pain complaints to the manufacturer was very low, and reported incidents were highly variable. None required revision surgery.
Surgical revisions after any surgical procedure are an important clinical outcome. Previously, we reported that surgical revision rates with triangular titanium implants observed in the commercial and clinical trial settings were both low and of similar magnitude.Citation15 One-year surgical revision rates after SIJ fusion surgery (~1.5%) appear to be substantially lower than those reported for lumbar stenosis surgery (4.1% at 1 year)Citation20 and lumbar arthrodesis (4–5%).Citation21
Surgical revisions after SIJ fusion with iFuse implants fall into two general categories: early and late revisions. Early revisions are typically for symptomatic implant malposition, which can result in the impingement of the L5 or S1 nerve roots that causes immediate postoperative radiating leg pain. Pain typically responds favorably to repositioning of the offending implant. Late revisions are performed to treat symptomatic pseudoarthrosis (recurrent pain, sometimes associated with implant loosening) or to remove implants in cases where a patient never had pain relief (most likely as a result of misdiagnosis). Failure of pain to improve after SIJ fusion can be due to poor implant position with insufficient stabilization or initial misdiagnosis. Our data provide insight into the relative occurrence rates of these events. Based on reported complaints, approximately half of surgical revisions, typically performed soon after the index procedure, are due to implant malposition resulting in nerve impingement (). Of revisions performed after the perioperative period, causes are primarily insufficient fixation (~40%), radiolucency (23%), malposition (14%), and failure to relieve pain (13%). The number of late revisions with iFuse-3D was too small to make any rate comparisons across device type. Our data do not allow any conclusions regarding the rate of misdiagnosis (ie, patient had a condition other than SIJ pain).
Previously, we reported a reduction in the rate of surgical revisions from initial product launch (2009) to 2015, which we attributed to increased physician experience and perhaps improved training.Citation15 The 2-year revision rate for 2012–2014 reported in the prior study (2.2%) was the same as that observed in the current study from 2015 to present (2.3%).
Our study has several strengths. The number of index surgeries performed is carefully recorded since implants, in almost all cases, are provided directly through company representatives. A company representative is almost always in attendance for both index procedures and surgical revisions. Revisions typically require special instrumentation manufactured by SI-BONE, Inc., and the removed implant is often replaced with another SI-BONE implant. Finally, company staff are instructed to report all complaints, especially revisions, to complaint-handling staff.
Limitations to the study are as follows. First, despite rigorous training, some complaints may not be reported and some physicians may choose not to report a complaint (eg, if a revision was done with another product) to the manufacturer; the extent of underreporting cannot be determined. Nonetheless, the revision rate calculated in the current study is similar to that reported in fully monitored prospective trials, suggesting that under-reporting may not be marked.Citation22 Second, because iFuse-3D is newer, the follow-up period for patients undergoing treatment with the 3D-printed implant is shorter than with the machined implant. The relatively shorter follow-up period likely explains the higher proportion of revision cases in the 3D implant that are for acute causes (implant malposition resulting in nerve impingement). However, through the use of Kaplan–Meier methods to adjust for differences in follow-up times, our data provide relatively strong evidence that late revisions with the 3D products are not increased compared to the prior machined version of the device. iFuse-3D’s surface is designed to increase bone on-growth and allow through-growth, but whether these features result in lower revision rates will require further follow-up. Third, our analysis disregards the chance that patients could be censored due to death from other causes, which could result in the underestimation of revision rates.
Despite these limitations, our study provides evidence to support a postmarket surveillance safety profile for iFuse-3D that is similar to the original device.
Conclusion
Complaints and adverse events with the use of a 3D-printed triangular titanium implant for SIJ fusion occur at low rates similar to those of the prior machined version of the device.
Disclosure
All authors are employees of SI-BONE, Inc., which manufactures the implants described herein. The authors report no other conflicts of interest in this work.
References
- FDA [webpage on the Internet]Medical Device Reporting. Code of Federal Regulations, Title 21, Part 803 Available from: http://www.access-data.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=803Accessed August 18, 2018
- Guidelines on a medical devices vigilance systemMEDDEV 2.12.1 rev 82013 Available from: http://ec.europa.eu/DocsRoom/documents/15506Accessed September 10, 2018
- UrbanRMJacobsJJTomlinsonMJGavrilovicJBlackJPeoc’hMDissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacementJ Bone Joint Surg Am200082445747710761937
- FDA [webpage on the Internet]MAUDE – Manufacturer and User Facility Device Experience Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed July 11, 2018
- BernardTNKirkaldy-WillisWHRecognizing specific characteristics of nonspecific low back painClin Orthop Relat Res1987217266280
- SchwarzerACAprillCNBogdukNThe sacroiliac joint in chronic low back painSpine199520131377709277
- MaigneJYAivaliklisAPfeferFResults of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back painSpine19962116188918928875721
- IrwinRWWatsonTMinickRPAmbrosiusWTAge, body mass index, and gender differences in sacroiliac joint pathologyAm J Phys Med Rehabil2007861374417304687
- SembranoJNPollyDWHow often is low back pain not coming from the back?Spine2009341E27E3219127145
- PollyDWSwoffordJWhangPGTwo-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint DysfunctionInt J Spine Surg201610 Article 28
- DenglerJDKoolsDPflugmacherR1-Year Results of a Randomized Controlled Trial of Conservative Management vs. Minimally Invasive Surgical Treatment for Sacroiliac Joint PainPain Physician201720653755028934785
- DuhonBSBitanFLockstadtHKovalskyDCherDHillenTSIFI Study GroupTriangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter TrialInt J Spine Surg201610 Article 13
- VanaclochaVHerreraJMSáiz-SapenaNRivera-PazMVerdú-LópezFMinimally Invasive Sacroiliac Joint Fusion, Radiofrequency Denervation, and Conservative Management for Sacroiliac Joint Pain: 6-Year Comparative Case SeriesNeurosurgery2018821485528431026
- MillerLERecklingWCBlockJEAnalysis of postmarket complaints database for the iFuse SI Joint Fusion System®: a minimally invasive treatment for degenerative sacroiliitis and sacroiliac joint disruptionMed Devices201367784
- CherDRecklingWCCapobiancoRImplant survivorship analysis after minimally invasive sacroiliac joint fusion using the iFuse Implant System®Med Devices (Auckl)2015848549226648762
- StejskalVDCederbrantKLindvallAForsbeckMMELISA-an in vitro tool for the study of metal allergyToxicol In Vitro199485991100020693060
- Valentine-ThonESchiwaraHWValidity of MELISA for metal sensitivity testingNeuro Endocrinol Lett2003241-2576412743534
- HallabNJLymphocyte transformation testing for quantifying metal-implant-related hypersensitivity responsesDermatitis2004152829015473335
- HallabNJMikeczKVermesCSkiporAJacobsJJDifferential lymphocyte reactivity to serum-derived metal-protein complexes produced from cobalt-based and titanium-based implant alloy degradationJ Biomed Mater Res200156342743611372061
- DeyoRAMartinBIKreuterWJarvikJGAngierHMirzaSKRevision surgery following operations for lumbar stenosisJ Bone Joint Surg Am201193211979198622048092
- MartinBIMirzaSKComstockBAGrayDTKreuterWDeyoRAReoperation rates following lumbar spine surgery and the influence of spinal fusion proceduresSpine200732338238717268274
- DenglerJDuhonBWhangPPredictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint: A Pooled AnalysisSpine201742211664167328350586
