Abstract
Background
Metabolic syndrome is diagnosed using clinical and laboratory data. Electro interstitial scan (EIS) is a rapid and noninvasive screening. It measures and calculates the parameters to reflect hypertension, sympathetic activity, stiffness of the arteries, body fat composition, leptin and insulin resistance. Metabolic syndrome will be diagnosed if calculated score ≥10 CU.
Purpose
To evaluate the accuracy, validity and appropriate cut-off score to diagnose metabolic syndrome.
Materials and Methods
A cross-sectional study was conducted using the population-based approach. Metabolic syndrome was diagnosed according to the modified National Cholesterol Education Program (NCEP ATP III) and International Diabetes Federation (IDF) criteria. The appropriate cut-off score to diagnose metabolic syndrome by instrument was determined.
Results
A total of 253 participants were enrolled with mean age of 40.06±6.33 years, and 64.43% (163/253) were female. Metabolic syndrome was diagnosed among 123 (48.62%) and 104 (41.11%) patients according to the NCEP ATP III and IDF criteria, respectively. The diagnostic indices of metabolic syndrome score ≥10 CU had a sensitivity of 23.6% and 27.9%, a specificity of 100% and area under ROC of 0.62 and 0.64, according to the NCEP ATP III and IDF criteria, respectively. The best cut-off level of metabolic syndrome score was ≥9 CU with a sensitivity of 79.67% (95% CI, 71.5–86.4%) and 88.46% (95% CI, 80.7–93.9%), a specificity of 96.92% (95% CI, 92.3–99.2%) and 93.29% (95% CI, 88.0–96.7%) and area under ROC 0.89 (95% CI, 0.85–0.93) and 0.92 (95% CI, 0.88–0.95), respectively, according to the NCEP ATP III and IDF criteria.
Conclusion
Screening of metabolic syndrome using ES TECK in a Thai population demonstrated inadequate accuracy when using metabolic syndrome score ≥10 CU. We recommend using a metabolic syndrome score ≥9 CU to provide the best accuracy. This instrument is safe, fast and easy to use for metabolic syndrome screening.
Keywords:
Introduction
Metabolic syndrome is a cluster of conditions characterized by abdominal obesity, dyslipidemia, hypertension and insulin resistance. It increases the risk of various chronic diseases, especially type 2 diabetes, cardiovascular disease and stroke.Citation1–Citation4 Metabolic syndrome was diagnosed according to clinical criteria and laboratory investigations. However, a rapid and noninvasive tool is needed for mass screening. Currently, an alternative screening tool is available using electro interstitial scan named ES TECK. Nevertheless, no clinical study has been published validating metabolic syndrome diagnosed using the electro interstitial scan. This device has been approved for clinical use by the US Food and Drug Administration and certified by ISO 9001. It also passed European regulatory approval CE 0459.
ES TECK (LD Technology, Miami, FL, USA) is a computer-aided medical screening device conducted using two measuring methods; an oximeter and electro interstitial scan, which is an impedance method, as one single measurement. The screening procedure is noninvasive, and takes around five minutes to complete the examination. Algorithms of electro interstitial technology provide measuring data related to the sympathetic system activity. Some data are calculated and based on electrochemical principles.
The parameters measured by this device are of three types. The first constitutes body composition analysis involving evaluating the proportion of different types of tissues in the body such as fat mass, percentage of body fat and body mass index as the main parameters. Second, mathematical cross analysis evaluates the overall operation of various organ systems using a mathematical algorithm to determine a risk score using values 1 to 5, from highest to the lowest. Third, body system analysis involves a metabolic syndrome indicator by measuring and calculating sympathetic activity, the stiffness of the carotid artery, elasticity of target artery, estimated renin secretion, body fat composition, leptin resistance and estimated insulin resistance. Each parameter indicates the cut-off score’s value in normal and abnormal range. Metabolic syndrome can be diagnosed when metabolic syndrome score is ≥10 conventional units (CU).
The study of Kawada and OtsukaCitation5 showed that secondary derivative photoplethysmography (SDPTG) is an indicator of arterial stiffness and clarified indices of the SDPTG in combination with components of the metabolic syndrome. They revealed age, d/a, serum uric acid, serum C-reactive protein, and regular exercise as independent determinants of the risk of metabolic syndrome. The study of Otsuka et alCitation6 showed that SDPTG is useful to estimate the risk of coronary heart disease in the general population. They also showed the prevalence of hypertension and hyperlipidemia was approximately 30%. The study of Rand et alCitation7 showed that electrical stimulation induced an increase in sweat response between the two electrodes. Control and subjects with diabetes showed a 20.2% and 18.2% increase in sweat rate, respectively. Electrical stimulation does act as a predictive tool for early signs of autonomic nervous system dysfunction among people with diabetes by measuring their sweat response and constitutes a modality that can be used in any physical therapy practice providing an inexpensive method to assess endothelial impairment. In addition, the study of Adami et alCitation8 showed that the homeostasis model assessment of insulin resistance (HOMA2-IR), HbA1c and plasma glucose level highly correlated to the algorithms derived from bio-impedance and spectrophotometric devices. However, no studies have reported the association between electro interstitial scan measured by this device and metabolic syndrome. Therefore, our study aimed to evaluate the accuracy, validity and appropriate cut-off score to diagnose metabolic syndrome.
Materials and Methods
Participants and Setting
A cross-sectional study using a population-based approach was conducted. The Inclusion criteria comprised healthy participants, aged more than 30 years old. We excluded participants with cardiovascular diseases, dermatological lesions at the examined area, having metal pins, prostheses, cardiac pacemaker, implanted electronic devices, defibrillators, pregnancy, presence of fever, infection or acute diarrhea. Furthermore, participants receiving diuretics, beta-blocker, antihistamine, pseudoephedrine, energy drinks, alcohol, caffeine, excessive perspiration (hyperhidrosis), stream or sauna treatment within 12 hours before test were excluded.Citation9 In all, 295 participants were screened at the center of anti-aging medicine (Apex Medical Center, Bangkok, Thailand) and primary care unit (Mith Mitree Medical Clinic, Bangkok Thailand) from September 2019 to February 2020. Informed consent was provided by all participants before entering the study.
Sample Size Estimation and Statistical Analysis
The sample size was calculated based on a study related to the EIS system.Citation6,Citation8,Citation10 Stata Software, Version 12.0 was used to calculate sample size, and one-sample comparison of proportions to hypothesized values in which the sensitivity of EIS was determined to be 90%. Therefore, based on the two-sided test, 5% α error and 90% power with 30% estimated prevalence of disease,Citation6,Citation11,Citation12 the number of patients needed for the study was calculated to be 117. We considered a p value of less than 0.05 to establish the level of significance. All statistical analyses and graphics were performed using Stata Software, Version 15.0 (StataCorp). Data are presented as number, percentage and mean ± standard deviation. Diagnostic results of the tests (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy) were presented as percentages. The study was approved by the human research ethics committee of Thammasat University (Faculty of Medicine) with protocol number of MTU-EC-ES-0-122/62, and adhered to the ethical principles of the Declaration of Helsinki. Each participant signed an informed consent form, and confidentiality was maintained for all participants.
Procedures of Anthropometric and Laboratory Measurements
All participants underwent height, weight, blood pressure and waist circumference measurement. Blood pressure, measured using an appropriately sized cuff, was recorded in duplicate and values were reported as means. Waist circumference was measured at the midpoint between the iliac crest and lowest rib when a participant was in the standing position with feet 15 cm apart.Citation13 Blood samples after 10 to 12 hours fast were obtained and then electro interstitial scan was performed. The biochemical measurements contained tests of fasting plasma glucose, HDL cholesterol and plasma triglycerides.
Metabolic Syndrome Diagnosis
Metabolic syndrome was defined using The National Institute of Hyperlipidemia (NCEP ATP III, 2001, the modified NCEP ATP III, 2004) and The World Diabetes Federation (IDF, 2005) criteria. The IDF criteria required central obesity assessed by waist circumference ≥90 cm among Asian men or ≥80 cm among Asian women. Also, the criteria included the presence of any two or more of the following: 1) HDL cholesterol <40 mg/dl (1.0 mmol/L) among men, <50 mg/dl (1.3 mmol/L) among women, 2) plasma triglycerides ≥150 mg/dl (1.7 mmol/L) or drug treatment for elevated triglycerides, 3) fasting plasma glucose ≥100 mg/dl (5.6 mmol/L) or drug treatment for elevated plasma glucose and 4) blood pressure >130/85 mmHg or drug treatment for hypertension. In contrast to the IDF criteria, the NCEP ATP III criteria requires any three of five criteria.Citation14–Citation16
Electro Interstitial Scan
The electro interstitial scan instrument consists of three parts of Electro-Sensor (ES) software which manages and integrates data as described below. First, the Electro sensor Oxi (ESO) device was used. The oximeter module requires a spectrophotometric pulse oximeter. The ESO device displays the arterial waveform and heart rate. From the arterial waveform, photo-electrical plethysmography analysis estimates the arterial stiffness index. The device also assesses heart rate variability. The mathematical calculation from SDPTG evaluates vascular aging in hypertension. SDPTG is obtained from double differentiation of the finger photoplethysmogram (PTG). It provides functional and structural properties of both central and peripheral arteries.Citation17 Additionally, SDPTG correlated with age and vascular aging, and other risk factors of atherosclerosisCitation18–Citation20 Second, the Electro Interstitial Scan – Galvanic Skin (EIS–GS) Module was performed. The module used the electrodermal response device technique by measuring the electrical response of the body through the skin.Citation9,Citation21 The measurements are performed by electrical stimulation of the post sympathetic cholinergic fiber with weak DC current and voltage (1.28V) applied in the bipolar mode.Citation22 Third, ES-BC was conducted. The bio-impedance module is used to estimate body composition and accurately assess body composition compared with standardized instruments.
EIS can be used as a tool for metabolic syndrome screening. This constitutes a galvanic skin response device based on bio-impedance and is performed by electrical stimulation of the post-sympathetic cholinergic fiber. It uses bio-impedance and electrical conductivity of eleven pathways of the body through six tactile electrodes placed symmetrically on the palms of the hands, soles of the feet and on the forehead. A weak DC current is sent alternatively between two electrodes using a sequence. It records the electrical conductance of eleven various pathways of the body, recorded and sent for signal processing analysis.Citation22
Metabolic syndrome parameter is one type of body system analysis, an indirect parameter obtained by combining measurement of data regarding eight direct parameters. This system measures eight parameters. First, (-d/a): is the SDPTG parameter which decreases with age, and represents the index of hypertension. Second, low frequency (LF) of the fast Fourier transform and low-frequency baroreceptor reflex activity reflects sympathetic activity.Citation23 Its value increase with hypertension. Third, the Augmentation Index (AI) indicates the systemic arterial stiffness and risk factor of cardiovascular disease. Fourth, the Estimated Renin secretion regulates blood pressure. Fifth, the Ejection Elastic Index (EEI) indicates the elasticity of target arteries, left ventricular ejection and compliance/elasticity of large arteries. A low EEI denotes vasoconstriction, arteriosclerosis or left ventricular ejection insufficiency, while a high EEI indicates vasodilation of large arteries, anemia, increased ejection power, hyperthyroidism or congested heart failure.Citation24 Sixth, fat mass represents the whole body fat composition. Seventh, the Leptin Resistance Index and eighth is the Estimated Insulin Resistance Index. The software algorithm combines the eight direct parameters of data reported as CU using the Cole equation to estimate metabolic syndrome parameters from the EIS device.Citation25 The cut-off score is estimated and validated using the manufacturer’s standardization.Citation26
Results
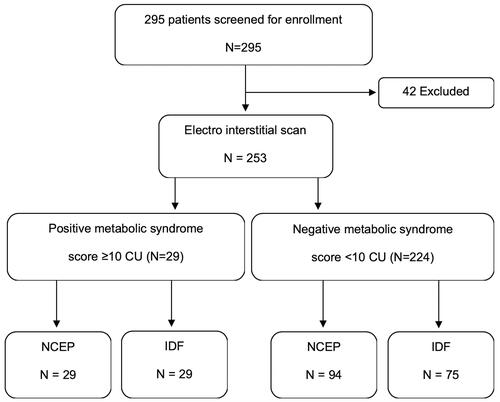
Totally, 253 participants (90 males and 163 females) were enrolled and met the inclusion criteria (). According to NCEP and IDF definitions, metabolic syndrome was diagnosed among 123 (48.6%) and 104 patients (41.1%), respectively (), and 29 (23.6%) and 29 (27.9%) of those tested positive using the cut-off metabolic syndrome score ≥10 CU, respectively. Diagnostic indices of metabolic syndrome scores ≥10 CU using the EIS instrument showed a sensitivity of 23.6 (95% CI, 16.4–32.1) and 27.9 (95% CI, 19.5–37.5) and a specificity of 100% (95% CI, 97.2–100) and 100% (97.6–100), respectively. The ROC area indicated 0.62 (95% CI, 0.58–0.66) and 0.64 (95% CI, 0.60–0.68), respectively, according to NCEP and IDF criteria ().
Table 1 Demographic Characteristics of the 253 Patients with Metabolic Syndrome Diagnosed by NCEP and IDF Criteria
Table 2 Diagnostic Indices of Metabolic Syndrome Score ≥10 CU Derived from Electro Interstitial Scan Instrument with the Reference Standards
Figure 1 Study flow diagram of diagnostic accuracy of electro interstitial scan when using metabolic score ≥10 CU as a cut-off level to diagnose metabolic syndrome compared with NCEP and IDF as reference standards.
Metabolic syndrome score ≥9 CU exhibited the best discriminative and predictive indices. According to NCEP and IDF definitions, this cut-off level revealed a sensitivity of 79.7 (95% CI, 71.5–86.4) and 88.5 (95% CI, 80.7–93.7), a specificity of 96.9% (95% CI, 92.3–99.2) and 93.9% (88.0–96.7) and ROC area of 0.88 (95% CI, 0.0.85–0.93) and 0.91 (95% CI, 0.88–0.95), respectively ().
Table 3 Diagnostic Indices of Metabolic Syndrome Score ≥9 CU Derived from Electro Interstitial Scan Instrument with the Reference Standards
Discussion
Presently, metabolic syndrome, a new noncommunicable disease, has become a major health hazard of the modern worldCitation12 Because early diagnosis and treatment of metabolic syndrome is important, screening for metabolic syndrome using EIS, and having a specific module to diagnose metabolic syndrome may provide an alternative. Currently, few companies could manufacture electro interstitial scan devices such as Medeia Inc, USA, LD technology, etc. Our study, enrolling participants aged 30 to 65 years, according to NCEP and IDF criteria, diagnosed 123 and 104 metabolic syndrome cases, representing a prevalence of 48.6% and 41.1%, respectively. We found that the prevalence of metabolic syndrome using the NCEP criteria was 7.5% greater than that using the IDF criteria as reported in other studiesCitation11,Citation27-Citation29 The metabolic syndrome score measured by this instrument is derived from eight subparameters, namely, 1) -d/a, 2) LF, baroreceptor reflex activity, indicator of sympathetic activity increased with hypertension, 3) AI, indicator of the stiffness of the carotid artery and hypercholesterolemia, 4) estimated renin secretion: indicator of the blood pressure regulation 5) EEI, indicator of elasticity of target artery 6) fat mass (%), 7) leptin resistance (%) and 8) estimated insulin resistance (CU), calculated using the software’s algorithm. The results of diagnostic accuracy showed that the analysis of the metabolic syndrome score using the cut-off score of ≥10 CU demonstrated inadequate accuracy; the discriminative and predictive indices are reported in . Results of cut-off score analysis showed the best cut-off score was a metabolic syndrome score of ≥9 CU using both the NCEP and IDF criteria. Therefore, based on the new cut-off score result in a Thai population and age group from 30 to 65 years, in terms of clinical applications from diagnostic accuracy, we suggest that the metabolic syndrome score ≥9 CU should be used in clinical practice. The new cut-off score, lower than default, may be due to the Asian race having a smaller physical appearance. Possibly, the amount of accumulated fat mass may be related to waist circumference, waist-height ratio and body mass index. However, we suggest further study employing any body system analysis. We observed that the cut-off score at metabolic syndrome score ≥8 CU according to the NCEP and IDF criteria, showed a sensitivity of 100%, which may be useful but the specificity was only 0.67 and 0.77, respectively, providing inadequate accuracy in terms of clinical application. The metabolic syndrome score from the instrument could only be reported for patients aged 30 years and older. The instrument issue may be beneficial in selecting the age groups intended to be at risk, related to the study, for which the risk of metabolic syndrome was associated with increasing age.Citation30 The strength of this study was in recruiting participants who intended to receive a diagnosis of metabolic syndrome, and to compare using two criteria, as is widely used in Thailand as a reference standard.
This instrument encountered limitation of measurement in certain conditions such as age less than 30 years old, presence of fever, infection, diarrhea, excessive perspiration (hyperhidrosis) and stream or sauna treatment within 12 hours before the test. Furthermore, participants with dermatological lesions at the examined area, having metal pins, prostheses, cardiac pacemaker, implanted electronic devices, defibrillators, being pregnant, receiving diuretics, beta-blocker, antihistamine, pseudoephedrine, energy drinks, alcohol and caffeine could not be accurately tested.Citation22 Results from our study are considered applicable to Thais aged from 30 to 65 years. Because data were collected from participants with 10 to 12 hours fast, it could not be applied to fasting participants. A research study to define the cut-off score for metabolic syndrome among non-fasting participant should be conducted.
Conclusion
Metabolic syndrome screening among Thais using the electro interstitial scan named ES TECK, demonstrated inadequate accuracy when using the default metabolic syndrome score ≥10 CU. We recommend using a metabolic syndrome score ≥9 CU to provide the best accuracy. This instrument is safe, fast and easy to use for metabolic syndrome screening.
Acknowledgments
We wish to express our appreciation to all research participants, the staff of Apex Medical Center and Mith Mitree Medical Clinic for their continuous support and contribution to the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet (London, England). 2014;383(9921):999–1008. doi:10.1016/S0140-6736(13)61752-3
- Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program – Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8–13. doi:10.2337/dc06-1414
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
- Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012;10:138. doi:10.1186/1741-7015-10-138
- Kawada T, Otsuka T. Factor structure of indices of the second derivative of the finger photoplethysmogram with metabolic components and other cardiovascular risk indicators. Diabetes Metab J. 2013;37(1):40–45. doi:10.4093/dmj.2013.37.1.40
- Otsuka T, Kawada T, Katsumata M, Ibuki C. Utility of second derivative of the finger photoplethysmogram for the estimation of the risk of coronary heart disease in the general population. Circ J. 2006;70(3):304–310. doi:10.1253/circj.70.304
- Rand S, Petrofsky JS, Zimmerman G. Diabetes: sweat response and heart rate variability during electrical stimulation in controls and people with diabetes. J Appl Reliab. 2008;8(1):48.
- Adami CE, Gobato RC, Gestic MA, Cazzo E, Pimentel MU, de Carvalho Ramos M. Correlations of HOMA2-IR and HbA1c with algorithms derived from bioimpedance and spectrophotometric devices. Obes Surg. 2012;22(12):1803–1809. doi:10.1007/s11695-012-0683-3
- Lewis JE, Tannenbaum SL, Gao J, et al. Comparing the accuracy of ES-BC, EIS-GS, and ES Oxi on body composition, autonomic nervous system activity, and cardiac output to standardized assessments. Med Devices (Auckl). 2011;4:169–177. doi:10.2147/MDER.S24291
- Caudal F. New marker using bioimpedance technology in screening for attention deficit/hyperactivity disorder (ADHD) in children as an adjunct to conventional diagnostic methods. Psychol Res Behav Manag. 2011;4:113–117. doi:10.2147/PRBM.S22924
- Aekplakorn W, Chongsuvivatwong V, Tatsanavivat P, Suriyawongpaisal P. Prevalence of metabolic syndrome defined by the International Diabetes Federation and National Cholesterol Education Program criteria among Thai adults. Asia Pac J Public Health. 2011;23(5):792–800. doi:10.1177/1010539511424482
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
- Tsai -S-S, Chu -Y-Y, Chen S-T, Chu P-H. A comparison of different definitions of metabolic syndrome for the risks of atherosclerosis and diabetes. Diabetol Metab Syndr. 2018;10:56. doi:10.1186/s13098-018-0358-x
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
- Rezaianzadeh A, Namayandeh S-M, Sadr S-M. National Cholesterol Education Program Adult Treatment Panel III Versus International Diabetic Federation definition of metabolic syndrome, which one is associated with diabetes mellitus and coronary artery disease? Int J Prev Med. 2012;3(8):552–558.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
- Takazawa K, Tanaka N, Fujita M, et al. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension. 1998;32(2):365–370. doi:10.1161/01.HYP.32.2.365
- Bortolotto LA, Blacher J, Kondo T, Takazawa K, Safar ME. Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photoplethysmogram versus pulse wave velocity. Am J Hypertens. 2000;13(2):165–171. doi:10.1016/S0895-7061(99)00192-2
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–39. doi:10.1088/0967-3334/28/3/R01
- Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18(1 Pt 2):3s–10s. doi:10.1016/j.amjhyper.2004.10.009
- Vitale GI, Quatrale RP, Giles PJ, Birnbaum JE. Electrical field stimulation of isolated primate sweat glands. Br J Dermatol. 1986;115(1):39–47. doi:10.1111/j.1365-2133.1986.tb06218.x
- Maarek A. Electro interstitial scan system: assessment of 10 years of research and development. Med Devices (Auckl). 2012;5:23–30.
- Electrophysiology, Task Force of the European Society. Heart rate variability. Circulation. 1996;93(5):1043–1065. doi:10.1161/01.CIR.93.5.1043
- von Wowern E, Östling G, Nilsson PM, Olofsson P. Digital photoplethysmography for assessment of arterial stiffness: repeatability and comparison with applanation tonometry. PLoS One. 2015;10(8):e0135659–e0135659. doi:10.1371/journal.pone.0135659
- Ayllon D, Seoane F, Gil-Pita R. Cole equation and parameter estimation from electrical bioimpedance spectroscopy measurements – a comparative study. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3779–3782. doi:10.1109/IEMBS.2009.5334494
- Creaform Hlo. The ES TECK screening system techniques scientific basis with references. Available from: http://www.halsokunskap.se/wp-content/uploads/2015/12/Es-Teck-scientific-2013.pdf. Published 2013. Accessed August 18, 2020.
- Chackrewarthy S, Gunasekera D, Pathmeswaren A, et al. A comparison between revised NCEP ATP III and IDF definitions in diagnosing metabolic syndrome in an urban Sri Lankan population: the Ragama Health Study. ISRN Endocrinol. 2013;2013:7. doi:10.1155/2013/320176
- Paula HA, Ribeiro Rde C, Rosado LE, Pereira RS, Franceschini Sdo C. Comparison of the different definition criteria for the diagnosis of the metabolic syndrome in elderly women. Arq Bras Cardiol. 2010;95(3):346–353. doi:10.1590/S0066-782X2010005000100
- Moy FM, Bulgiba A. The modified NCEP ATP III criteria maybe better than the IDF criteria in diagnosing metabolic syndrome among Malays in Kuala Lumpur. BMC Public Health. 2010;10:678. doi:10.1186/1471-2458-10-678
- Moreira GC, Cipullo JP, Ciorlia LAS, Cesarino CB, Vilela-Martin JF. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One. 2014;9(9):e105056–e105056. doi:10.1371/journal.pone.0105056
