Abstract
The measurement of urinary flow is a vital medical indicator for critically ill patients in intensive care units. However, there is a clinical need to automate the real-time measurement of diuresis using Internet of Medical Things devices, allowing continuous monitoring of urine flow. A systematic review of scientific literature, patents, and available commercial products was conducted, leading to the conclusion that there is no suitable device to fulfill this need. We identified six characteristics that such a device should possess: minimizing contact with urine, detecting changes in flow patterns, the ability to record minute-by-minute data, capable of sending early alerts, not relying on exclusive disposable components, and being user-friendly for clinical professionals. Additionally, cost-effectiveness is crucial, encompassing the device, infrastructure, maintenance, and usage.
Plain Language Summary
Continuous monitoring of urinary output in intensive care patients provides vital insights, potentially revealing low cardiac output, renal hypoperfusion, or deteriorating renal function. Currently, these measurements are done manually, prompting a need for automated, real-time monitoring using Internet of Medical Things (IoMT) devices. Current automatic urinometers are not widely used due to high costs, maintenance requirements, and logistical complications. However, we propose developing an affordable, updated urinometer that meets clinician needs and can be widely used in hospitals.
This innovation would enable early intervention strategies to reverse or improve renal perfusion and prevent potential toxic treatments. The shift from manual to automated monitoring is likely, facilitating real-time data integration such as blood pressure, heart rate, and temperature. This can lead to personalized medical approaches and predictive algorithms for renal function deterioration.
As technology, IoMT, big data, and artificial intelligence (AI) evolve, these advancements will be indispensable for hospital medicine, supporting precision medicine and efficient clinical operation. While we cannot predict the definitive method for continuous diuresis measurement, we anticipate the adoption of these technologies in the near future, improving diagnostics and clinical practice. If successful, the application could extend beyond critical care into postoperative or home care, not only for diuresis but for any fluid flow, enabling early pathology identification and potentially revealing diagnostic patterns through AI.
Introduction
The care of critically ill patients in the Intensive Care Unit (ICU) requires real-time monitoring of vital signs. An automated early warning system would allow healthcare professionals to respond to significant or potentially dangerous variations in diuresis.Citation1,Citation2 Urinary flow measurement provides insights into renal function, serving as an indicator to assess the degree of vascular perfusion of organs in general.Citation3 In terms of quality, the most commonly used indicator is urine flow, measured as the rate of flow over time, although additional parameters could be measured.Citation4 Ultimately, monitoring urine production could serve as a continuous early warning system for acute renal failure, akin to a caged canary in a coal mine signaling an impending multiorgan failure.Citation5
The current system for measuring urinary flow involves a collection container with volume measurements, allowing manual measurements by nursing staff every hour. This system is prone to measurement/transcription errors and may lead to a delay of one or more hours in detecting anuria (<100mL/24h or <15mL/h) or oliguria (<400mL/24h or <25 mL/h),Citation6 in addition to the time required for measurements. We estimate that, in a 15-bed ICU, hourly measurements and 2 minutes per measurement per patient amount to 12 hours per day.
Therefore, it would be convenient to have an automatic urinometer that can identify oliguria (reduced urine production) or anuria (complete interruption) through a pattern detection system in critically ill patients.Citation7–13
This article will systematically review the available scientific evidence in the literature, presented patents, and commercial products. Subsequently, the requirements for an automatic urinometer will be proposed to address current needs and provide a clinically valid solution that is practical, scalable, and cost-effective.
The proposed system should be integrated with the current technology based on data harvesting, specifically in the clinical context, thus positioning it within the realm Internet of Medical Things (IoMT).
The question we want to answer is whether there is a smart urinometer in the market or in academia that could solve the needs of automatic measurement of diuresis in critically ill ICU probed patients.
Methodology
The systematic review of scientific evidence consists of three parts: scientific articles, patent publications, and commercial devices.
The first author conducted the extraction of the studies on three occasions, with the initial selection results aligning each time. Moreover, the second author undertook the search following the same methodology but did so independently, yielding the same initial results. The selection of articles was done by the first author.
For the patents, their authenticity and validity were verified by checking the official identity number from the official patent office. This ensures that the findings derived from these sources are reliable and legally supported.
In the context of commercial products, a meticulous review of the manufacturers’ official websites was conducted. Additionally, the information directly provided by the manufacturers through direct communication was considered. This approach guarantees the accuracy of the data related to commercial products and its applicability in the clinical practice setting.
In addition to the quality assessment of the aforementioned, an analysis of the utility of the information obtained from the perspective of research team members with a clinical profile has been conducted. Criteria such as the relevance of the technology, its applicability in daily practice, and its suitability for intensive care professionals in their daily work have been taken into account.
Systematic Review of Scientific Articles
The following terms were entered into PubMed and Google Scholar: “urinometer”, “electronic AND urinometer”, “automatic urinometer”, published from January 1st, 2000, to March 14th, 2023 (date of the search), published in Spanish or English. The inclusion criteria were as follows: articles that described a sensor or electronic device for measuring urine flow and/or an automatic system (real-time automatic urinometer) for managing this variable. The exclusion criteria were as follows: articles related to a specific pathology (eg, diabetes) or articles that compared a manual urinometer with other automatic ones without technical details. All references of the selected articles were reviewed to find any significant article that may have been missed during the systematic review.
Systematic Review of Patents
The terms “urinometer” and “urine AND flow monitor” were entered into Google Patents, searching for publications from January 1st, 2000 (inclusive) to March 25th, 2023 (date of the search), published in Spanish or English in all regions of the world. We also reviewed all possible patents identified in the references of the selected patents, similar articles, and citations of these patents, as well as patents mentioned in the search results of commercial products (explained below). The inclusion criteria were the same as described for the review of scientific articles. The exclusion criteria were as follows: patents that did not describe an electronic device (only a manual one) or those that were purely theoretical (not oriented towards commercial production) due to anticipated difficulties in their development or manufacturing.
Systematic Review of Commercial Products
The following terms were entered into the Google search engine: “urinometer” “electronic urinometer”, “automatic urine flow”, “automatic urinometer”, and “automatic urine flow” published on any date and in any language. The search was conducted on March 28th, 2023.
Additionally, with the same keywords, an image search was performed, and the links to the device pages were reviewed. When the language was not Spanish or English, Google Translate was used for automatic translation.
Some commercial brands were directly searched using the model/brand reference found during the previous review of scientific literature (eg, Sippi, Urinfo2000, Urexact, etc.).
Results and Discussion
Scientific Articles
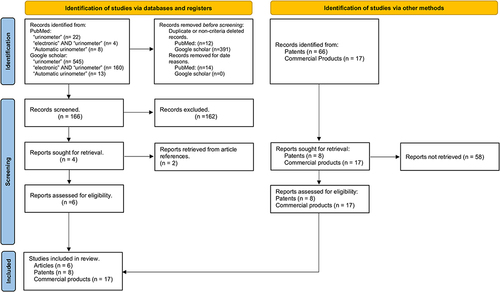
The initial review yielded 166 abstracts. After applying the inclusion and exclusion criteria, we were left with four abstracts, from which we obtained the full articles. Additionally, all references of the selected articles were reviewed, and two additional articles were found. provides a summary of the six selected articles. The six selected articles were published in journals indexed in the Journal Citation Reports (JCR), which suggests an enhanced level of quality given that they have undergone peer review.
Table 1 Abstract of Scientific Articles Selected in the Systematic Review of Automatic Urinometers (2023)
The research conducted by the Otero group focuses on accurately measuring fluids. They have tested different methods, among which the most precise and feasible method is the weighing method.Citation14–17 Although the measurement methods are highly accurate, these devices are not intended for commercialization.
The work carried out by VillarrubiaCitation18 focuses on network connectivity using the weighing method. A study on network connectivity between different devices via Wi-Fi is initiated, outlining the key points to be developed when creating network connections.
The Raam teamCitation19 studied a device that utilizes weight. The measured values are sent to an Android application, which forwards the received data to a web-based cloud storage application for remote monitoring.
Patents
Sixty-six patents were obtained from the initial search. After applying the inclusion and exclusion criteria, we ended up with a total of eight patents (see ).
Table 2 Summary of Patents Selected in the Systematic Review (2023)
The proposal by FernándezCitation20 describes a urinometer based on a capacitive sensor, although there is no remote connection. Additionally, the digitization of the measurement is performed locally. This patent discusses the history of patents with different measurement methods and their disadvantages. Albuminuria and hemoglobinuria cause a progressive accumulation of biofilms on the capacitance sensors of urinometers, which decreases the functionality of the devices. According to Slettengren,Citation21 it measures accurately, but we believe it is not practical for manufacturing and does not provide real-time monitoring.
The proposal by AkinfievCitation20 modifies some aspects of Fernández’s development. However, it does not provide a real-time electronic system, although it envisions wireless connectivity, and focuses on improving weight measurements. Weight measurement is highly accurate but does not result in a viable system for manufacturing and commercialization because it shows a mechanical balance and weight system without considering the size or possible placement of the device (on the patient’s bed) or whether the patient is catheterized.
The proposal by BustinCitation28 presents a urinometer that also relies on weight and provides Wi-Fi connectivity but does not provide detailed information on how the data is sent. It foresees the use of unmodified urine bags. Regarding the connection, the patent itself does not specify which route it would use, which means several options are possible. It only raises some questions regarding the wireless network.
The proposal by ZhangCitation22 is associated with patent CN-302528015-S (expired). It describes a urinometer that monitors the bladder filling level and alerts medical personnel when it reaches a certain point. It offers very little information. In particular, it does not provide information about the connections.
The proposal by NishtalaCitation24 belongs to the brand CR Bard and measures creatinine and urine flow. Creatinine is measured by an optical sensor that is in contact with the urine. It does not provide specific details about the flow measurement method. Wireless or cloud connections are also not mentioned. This patent transforms into one of the studied commercial devices.
The proposal by BurnettCitation27 belongs to Potrero Medical and describes a device for analyzing bodily fluids. In addition to urine, it also measures other parameters. This patent transforms into one of the studied commercial devices.
Finally, the proposal by BustinCitation28 belongs to Renalsense. It measures through weighing. This patent transforms into one of the studied commercial devices.
Commercial Products
A total of 17 commercial products were found (see ).
Table 3 Summary of the Systematic Review of Commercial Urinometers (2023)
In general, there is very little information available regarding their technical details and commercial availability. We know that at least three of them have been discontinued, some due to not functioning correctly (mainly in terms of producing measurement errors), and others due to requiring expensive disposable parts (lack of sustainability). Commercially, the devices utilize weight measurement solutions or photoelectric systems. Many include network connections (wired), and some are wireless, but none of them describe their use in the cloud. It is worth mentioning that many of them are only certified for marketing in certain regions.
Conclusions
In a comprehensive review, we analyzed 6 articles, 8 patents, and 17 commercial devices concerning urine flow measurement. Notably, none of these devices are currently feasible for widespread clinical implementation due to technical challenges, construction complexities, or prohibitive costs.
While scientific literature affirms the accuracy of urine flow measurements using weight systems or photoelectric detection, the primary focus has not been on device development. These articles, predating the Internet of Medical Things (IoMT) era, warrant modernization. Although patents offer innovative concepts, only half have transitioned into commercial products. Some of these commercialized patents were later abandoned due to economic non-viability or remain too costly for general adoption, a situation exacerbated by advancements in electronic technology and cloud computing.
Commercially available products typically entail expensive equipment reliant on high-cost, proprietary disposables. Such disposables, often necessitating replacement every 4–7 days, represent a significant financial burden for healthcare centers. Additionally, feedback from healthcare professionals highlights the absence of such devices in standard hospital operations globally. The primary barriers include prohibitive costs, operational intricacies, and limited or complicated remote connectivity features.
Our research underscores the pressing need for an IoMT-based automatic urinometer that is compact, user-friendly, and economically scalable for intensive care units (ICUs). The ideal device should encompass six primary attributes: minimized urine contact, capability to identify flow pattern alterations, independence from exclusive disposables, minute-by-minute data recording, ease of use for healthcare staff, and cost-effectiveness. Ideally, it should streamline data collection processes for medical professionals. Advanced features, such as measuring additional parameters like colorimetry and integrating cloud connectivity with intelligent alert systems, can further enhance the device’s value. Using IoMT and machine learning, the proposed device can potentially automate decision-making support, offering predictive models and insights into various pathologies for ICU physicians.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The group IASalud is supported by the Chair BHD-IASalud from the Universidad Europea de Madrid, Spain.
References
- Davoudi A, Malhotra KR, Shickel B, et al. Intelligent ICU for autonomous patient monitoring using pervasive sensing and deep learning. Sci Rep. 2019;9(1):8020. doi:10.1038/s41598-019-44004-w
- Yoon JH, Jeanselme V, Dubrawski A, Hravnak M, Pinsky MR, Clermont G. Prediction of hypotension events with physiologic vital sign signatures in the intensive care unit. Crit Care. 2020;24(1):661. doi:10.1186/s13054-020-03379-3
- Hariri G, Joffre J, Leblanc G, et al. Narrative review: clinical assessment of peripheral tissue perfusion in septic shock. Ann Intensive Care. 2019;9(1):37. doi:10.1186/s13613-019-0511-1
- Strasinger SK, Lorenzo D. Análisis de orina y de los líquidos corporales [Urinalysis and body fluid tests]. 5a ed. Editorial Panamericana; 2008.
- Mehta RL. Acute kidney injury: urine output in AKI--The canary in the coal mine? Nat Rev Nephrol. 2013;9(10):568–570. doi:10.1038/nrneph.2013.178
- Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338(10):671–675. doi:10.1056/NEJM199803053381007
- Eklund A, Van der Linden J. A new automatic urinometer shows lower bias, no loss of precision due to temporal deviation and higher user evaluation when compared with a manual standard urinometer in an ICU setting. Crit Care. 2014;18(Suppl 1):P163. doi:10.1186/cc13353
- Minor J, Smith A, Deutsch F, Kellum JA. Automated versus manual urine output monitoring in the intensive care unit. Sci Rep. 2021;11(1):17429. doi:10.1038/s41598-021-97026-8
- Hersch M, Kanter L. A new electronic urine meter (UREXACT) is more accurate in measuring urine output than the standard Urinometer: a comparative study. Crit Care. 2005;9(Suppl 1):P409. doi:10.1186/cc3472
- Shalman A, Klein Y, Toledano R, et al. The clinical significance of fluctuations in the minute-to-minute urine flow rate and in its minute-to-minute variability during septic events in critically ill patients. Rom J Anaesth Intensive Care. 2020;27(2):1–5. doi:10.2478/rjaic-2020-0013
- Klein Y, Grinstein M, Cohn SM, et al. Minute-to-minute urine flow rate variability: a new renal physiology variable. Anesth Analg. 2012;115(4):843–847. doi:10.1213/ANE.0b013e3182625813
- Hersch M, Einav S, Izbicki G. Accuracy and ease of use of a novel electronic urine output monitoring device compared with standard manual urinometer in the intensive care unit. J Crit Care. 2009;24(4):629.e13–629.e17. doi:10.1016/j.jcrc.2008.12.008
- Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26(2):509–515. doi:10.1093/ndt/gfq332
- Otero A, Palacios F, Akinfiev T, Fernández R. A device for automatically measuring and supervising the critical care patient’s urine output. Sensors. 2010;10(1):934–951.
- Otero A, Palacios F, Akinfiev T, Apalkov A. A low cost device for monitoring the urine output of critical care patients. Sensors. 2010;10(12):10714–10732. doi:10.3390/s101210714
- Otero A, Fernandez R, Apalkov A, Armada M. An automatic critical care urine meter. Sensors. 2012;12(10):13109–13125. doi:10.3390/s121013109
- Otero A, Apalkov A, Fernández R, Armada M. A new device to automate the monitoring of critical patients. Urine Output BioMed Res Int. 2014;2014:1–8. doi:10.1155/2014/587593
- Villarrubia G, Hernández D, De Paz JF, Bajo J. Combination of multi-agent systems and embedded hardware for the monitoring and analysis of diuresis. Int J Distrib Sens Netw. 2017;13(7):155014771772215. doi:10.1177/1550147717722154
- Raam KG, Jeelani A, P SP, Nagaiyan S, Joseph J, Sivaprakasam M. Design, development and clinical validation of a novel urine output monitor. 2017 IEEE International Symposium on Medical Measurements and Applications (Memea) [Internet]; Rochester, MN: IEEE; 2017:188–92.
- Akinfiev T, Fernández R, Otero A, Palacios F. Dispositivo para la medición de la cantidad de líquido que fluye y el procedimiento para su medición[Device for measuring the amount of liquid flowing and the procedure for measuring it]. ES-2354794-B1, 2012:15. ES-2354794-B1: Available from: https://patentimages.storage.googleapis.com/b7/79/14/b1ee7758bb00a0/ES2354794B1.pdf. Accessed November 28, 2023.
- Slettengren M, Linnros M, van der Linden J. Silicone oil decreases biofilm formation in a capacitance-based automatic urine measurement system. Sens Basel. 2021;21(2):445. doi:10.3390/s21020445
- Zhang R, Zhang S. Portable urine output monitor (with screen). China; CN302552968S; 2013.
- Fernández Saavedra RE. Dispositivo Para La Medición Automática De La Cantidad De Líquido Que Fluye Y El Procedimiento Para Su Medición[Device for automatic measurement of the amount of liquid flowing and the procedure for its measurements]. CEU SAN PABLO DE MADRID, University of Consejo Superior de Investigaciones Cientificas CSIC Universidad Politecnica de Madrid; ES2395501B1; 2013:20. ES2395501B1: Available from: https://patentimages.storage.googleapis.com/49/96/1c/3bfc785007536e/ES2395501B1.pdf. Accessed November 28, 2023.
- Nishtala V. Renal monitor. EEUU; US8715254B2; 2014.
- Yehoshua J, Grinstein M. Apparatus, system, and methods for urinalysis[Internet]. world; US20170367636A1. Available from: https://patents.google.com/patent/US20170367636A1/en. Accessed November 28, 2023.
- Lark T, Hadas N. Apparatus And Method Of Monitoring Intra-Abdominal Pressure And Urine Output [Internet]. Israel; WO2019106675; 2019. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019106675. Accessed November 28, 2023.
- Burnett D, Keenan R. Systems, devices and methods for draining and analyzing bodily fluids. US10391275B2; 2019. Available from: https://patents.google.com/patent/US10391275B2/en. Accessed November 28, 2023.
- Bustin G, Coats T, Holt J, Sims M. Urine weighing apparatus [Internet]. AU-2017225337-B2; 2017:26. Available from: https://patentimages.storage.googleapis.com/d6/4b/fd/71b6dd7beae0d0/AU2017225337B2.pdf. Accessed November 28, 2023.
- Sippi [Internet]. Sippi – innovative medtech for a critical unmet need; 2022. Available from: https://observemedical.com/sippi/. Accessed November 28, 2023.
- Clarity RMS [Internet]. Clarity RMS; 2022. Available from: https://www.renalsense.com/clarity-rms-critical-care-monitoring-system/. Accessed November 28, 2023.
- Acuuryn [Internet]. critical care. Smarter; 2022. Available from: https://potreromed.com/product/. Accessed November 28, 2023.
- URINFO 2000 is the first medical device developed for accurate measurement and monitoring of urine output in the hospital. Israel High-Tech & Investment Report; 2013. Available from: http://www.ishitech.co.il/0413ar8.htm. Accessed November 28, 2023.
- Go with the flow to monitor kidney injuries [Internet]; 2012 [cited August 8, 2022]. Available from: https://www.israel21c.org/go-with-The-flow-to-monitor-kidney-injuries/. Accessed November 28, 2023.
- Criticore® monitor [Internet]. 2005 [cited June 8, 2022]. Available from: https://pdf.medicalexpo.com/pdf/bard-medical/criticore-monitor/78646-133265.html. Accessed November 28, 2023.
- RenalGuard [Internet]. RenalGuard. [cited June 8, 2022]. Available from: https://renalguard.com/. Accessed November 28, 2023.
- Clinical urinary system dynamic monitoring instrument: development status and analysis of key technology at home and abroad: [internet]. Daily Headlines. 2018 [cited Jun 8, 2022]. [cited Jun 8, 2022]: Available from: https://kknews.cc/zh-sg/health/nrkbraq.html. Accessed November 28, 2023.
- Sentinel [Internet]. [cited June 8, 2022]. Available from: https://www.serenno-med.com/. Accessed November 28, 2023.
- Akas - urine output monitoring [Internet]. [cited 2022 June 8, 2022]. Available from: https://www.akasinfusions.com/urometer. Accessed November 28, 2023.
- Sensica orine output monitor [Internet]. 2022 [cited June 8, 2022]. Available from: https://www.bd.com/en-us/products-and-solutions/products/product-page.SCCS1002. Accessed November 28, 2023.
- Uros- output monitor [Internet]. [cited June 8, 2022]. Available from: https://es.trademed.com/products/4313/Urine-Monitoring-System.html. Accessed Nov 28, 2023.