 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Treatment of growth hormone disorders typically involves daily injections of human growth hormone (GH) over many years, incurring substantial costs. We assessed the extent of undesired GH loss due to leakage in the course of pen preparation prior to injection, and differences between the prescribed dose, based on patient weight, and the actual delivered dose based on pen dosing increments in five GH administration devices.
Methods
Norditropin® prefilled FlexPro®, NordiFlex®, NordiLet®, and durable NordiPen®/SimpleXx® 5 mg pens (Novo Nordisk A/S, Bagsværd, Denmark) and durable Omnitrope® Pen-5 devices (Sandoz, Holzkirchen, Germany) were tested (n = 40 for each device type). Product wastage was measured in accordance with validated protocols in an ISO (International Organization for Standardization) 11608-1 and Good Manufacturing Practice compliant laboratory. The average mass of wasted GH from each device type was measured in simulations of dripping with the needle attached prior to injection and while setting a dose. Statistical significance (P < 0.05) was confirmed by Student’s t-test, and a model was constructed to estimate mean annual GH wastage per patient in cohorts of pediatric patients with GH disorders.
Results
Mean GH mass wasted with the needle on prior to injection was 0.0 μg with Norditropin pens, relative to 98 μg with Omnitrope Pen-5. During dose dialing, 0.0–2.3 μg of GH was lost with Norditropin pens versus 0.8 μg with Omnitrope Pen-5. All Norditropin and Omnitrope device comparisons were statistically significant. Modeling GH wastage in a US cohort showed 5.5 mg of annual GH wastage per patient with FlexPro versus 43.6 mg with Omnitrope, corresponding to 7–8 additional pens per patient annually.
Conclusion
Overall, Norditropin pens resulted in significantly less wastage than the Omnitrope Pen-5. The study suggests that GH devices of the same nominal volume exhibit differences that may affect the frequency of GH prescription refills required to remain adherent to therapy.
Introduction
Human growth hormone (GH), secreted from the somatotroph cells of the anterior pituitary gland, is involved in the regulation of lipid and glucose homeostasis and plays a pivotal role in the promotion of linear growth during childhood.Citation1,Citation2 In patients with GH deficiency, the secretion of GH is either deficient or absent and is phenotypically manifest in the form of growth failure, low lean body mass, abnormal lipid profiles, impaired cardiac function, and retardation of bone maturation.Citation3–Citation7 Treatment of GH deficiency, along with short stature arising from conditions such as Turner syndrome, Noonan syndrome, children born small for gestational age, and adult GH deficiency involves daily subcutaneous injections of GH. To date, a number of commercially available GH formulations have been produced, varying in terms of the cell cultures used for expression and the buffering and preservative agents used in the final liquid GH formulation.Citation8 The products are typically referred to as biosimilar, are interchangeable, and physicians consider the available brands of GH to exhibit similar clinical effects.Citation9
Given this assumption of clinical equivalence, key features leading to differences between GH products include the characteristics of GH delivery devices, the GH formulation, and patient support services offered by the GH manufacturer.Citation8,Citation10,Citation11 In terms of characteristics of GH administration devices, there are numerous factors that affect patient preferences for the different GH brands, including the intuitiveness of handling the device, pain and stinging associated with injections, the need to mix/reconstitute the medication, and the requirement to store the GH solution at appropriate temperatures.Citation11–Citation15 However, there are also a number of factors that may influence the choice of administration devices from an economic perspective, namely those affecting the quantity of GH that is available for dosing. Such factors include pen dosing increments and GH wastage that may occur during dose setting or injection. Any wastage arising from these delivery system characteristics may have a material effect on the frequency with which patients need to refill their GH prescriptions and, given the per milligram cost of GH, may affect the cost of treatment from the health care payer perspective.Citation10 The aim of the present study was therefore to assess the extent of GH wastage (including undesired GH loss due to leakage in the course of pen preparation prior to injection and differences between the prescribed dose, based on patient weight, and the actual delivered dose based on pen dosing increments) in five current GH delivery systems. Because the rationale for performing the study stemmed from an economic perspective, the analysis focused on the pediatric population with GH deficiency, which comprises at least one child per 3,480 in the US, compared with one adult per 10,000 with adult-onset GH deficiency.Citation16,Citation17 Based on these prevalence data and treatment patterns (including lower typical doses in adult patients than pediatric patients), any differences in wastage between devices would be likely to have the largest impact in the pediatric population.
Materials and methods
The present study was performed in two stages, the first of which comprised a series of tests of five GH delivery systems, including four systems used to administer Norditropin®, namely the FlexPro®, NordiFlex®, NordiLet®, and NordiPen®/SimpleXx® 5 mg pens (Novo Nordisk A/S, Bagsværd, Denmark), and one used to administer Omnitrope®, the durable Omnitrope Pen-5 (Sandoz International GmbH, Holzkirchen, Germany). Characteristics of each of these devices are detailed in . The second stage of the study involved the construction of a simulation model of GH wastage designed to estimate the annual GH wastage using data from the laboratory tests and the device package inserts.
Table 1 Characteristics of devices and growth hormone formulations included in the analysis
Laboratory analysis
A series of analyses were performed in accordance with validated protocols in a Good Manufacturing Practice and ISO (International Organization for Standardization) 11608-1 compliant laboratory to evaluate the following characteristics of the five GH delivery systems: dripping with the needle attached prior to injection and dripping while setting/dialing a dose.Citation18 For each analysis, Norditropin prefilled FlexPro, NordiFlex, and NordiLet, and durable NordiPen/SimpleXx 5 mg pens and durable Omnitrope Pen-5 devices were tested (n = 40 for each device type). All tests were performed with needles as recommended by the device manufacturer; FlexPro, NordiFlex, NordiPen/SimpleXx, and NordiLet pen tests were performed with NovoFine G 31 × 6 mm needles, while the Omnitrope Pen-5 durable devices were tested with Micro-Fine™ BD 31 G × 5 mm needles (Becton-Dickinson, Franklin Lakes, NJ, USA). All tests were performed at 18°C–28°C and 25%–75% room humidity and pens were stored in these conditions for 30 minutes prior to performing each test.
For each test, the pen was connected to an XP205DR analytical balance (Mettler Toledo AG, Greifensee, Switzerland) using 500 mm of G 22 tubing connected with a G 31 × 6 mm needle for Norditropin pens or a BD 31 G × 5 mm needle for Omnitrope pens. The mass of wasted GH solution from each pen was then measured using validated METDose software (Cirkom A/S, Roskilde, Denmark) and averaged over all devices. An example test procedure (for measuring wastage differences between NordiPen/SimpleXx and Omnitrope Pen-5) is outlined in detail in . In line with the laboratory’s compliance with ISO 11608-1, a measurement uncertainty budget was performed in which the uncertainty associated with test personnel, machine/equipment, environment, measurement, and materials was evaluated. Concerns arising from the uncertainty budget were addressed by trained test operators in line with standard operating procedures in the laboratory. Statistical significance was ascertained in a series of pairwise comparisons of the Omnitrope Pen-5 versus each Norditropin device using two-tailed, unpaired Student’s t-tests, with a significance threshold of P < 0.05.
Table 2 Example procedure for measuring growth hormone wastage with NordiPen®/SimpleXx® 5 mg or Omnitrope® Pen-5
In order to convert the mass of GH solution (as measured in the laboratory wastage tests) to the mass of active GH substance, the density of each GH solution (Norditropin and Omnitrope) was ascertained using an oscillating transducer (DMA 4500; Anton Paar GmbH, Graz, Austria). Density measurements were performed on both 5 mg and 10 mg formulations of each GH solution in accordance with the European Pharmacopoeia.Citation19
Budget impact model
Based on the laboratory analyses and data from device package inserts, a computer-based cohort simulation model was constructed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) to estimate the mean annual GH wastage per patient in hypothetical US, Japanese, and European cohorts of pediatric patients with GH disorders. The model captured wastage prior to injection with the needle attached, wastage during dose setting, and wastage arising from different pen dosing increments. The analysis was conducted in terms of mass of active GH substance (as opposed to mass of GH solution). Since the laboratory analyses reported GH solution mass, all measured values were converted to mass of active GH substance (solute) using the density and concentration of the respective GH solutions:
where m denotes mass in mg, ρ denotes density in mg/mL, and square brackets denote concentration in mg/mL.
The concentration of both GH solutions was taken to be 3.3 mg/mL based on 5 mg of GH being available in 1.5 mL of solution and 6.6 mg/mL for the concentration of both 10 mg solutions. In the sensitivity analyses involving the 10 mg devices, it was assumed that the same mass of GH solution would be wasted with the 10 mg device as with the corresponding 5 mg device, but the mass of wasted GH solute (as used in the modeling analyses) was derived from the GH solution mass using the 10 mg solution density as established in the oscillating transducer assay.
Wastage analyses were conducted in typical US, Japanese, and European cohorts of patients with growth disorders using country-specific bodyweight assumptions and median GH dosing data. Dosing data were taken from three post-marketing GH observational studies: NordiPAD (for the Japanese setting), the NordiNet® International Outcome Study and the NovoNet® ANSWER Program® (for the European and US settings, respectively).Citation20,Citation21 In the US, mean bodyweight was taken to be 35 ± 5 kg with an average daily dose of 0.048 mg/kg/day. In the European setting, mean bodyweight was assumed to be 30 ± 4 kg with an average daily dose of 0.035 mg/kg/day, and in the Japanese setting, mean bodyweight was taken to be 25 ± 3 kg with an average daily dose of 0.026 mg/kg/day. Based on these parameters and assuming a Gaussian distribution of bodyweight, the model calculated the proportion of the cohort in each 1 kg bin across the whole distribution.
To calculate any excess dose arising from pen dosing increments, the target daily GH dose was first calculated by multiplying the midpoint of each 1 kg bodyweight bin by the recommended daily dose per kg, multiplying the resulting values by the proportion of patients in each bin, and summing them to give the mean daily dose over the whole cohort. To calculate the actual administered dose (which differs from the target dose as a result of the pen dosing increments), the model assumed that all patients would avoid administering doses lower than the target dose and instead dose to the nearest dose increment above the recommended dose.Citation10 The dose was therefore calculated by finding the nearest integer number of dose increments that would exceed the recommended dose and multiplying it by the size of the dose increment.
Wastage of GH product occurring during dose setting and prior to injection with the needle attached was calculated by multiplying the values from the laboratory analyses by the number of injections performed per year (assuming 365 injections per year). These wastage parameters were assumed to be independent of the administered dose and the volume of remaining GH in the device at the time of injection.
Results
Laboratory analysis
Laboratory measurements showed the density of Norditropin and Omnitrope 5 mg solutions to be 1014.2 mg/mL and 1013.4 mg/mL, respectively, while measurement of the 10 mg solutions yielded density values of 1015.1 mg/mL and 1009.3 mg/mL for Norditropin and Omnitrope, respectively.
All wastage comparisons with Omnitrope Pen-5 were statistically significant at P < 0.05 (). Wastage with the needle attached prior to injection was significantly higher with Omnitrope Pen-5 than with Norditropin devices. The measured volume of GH wasted with the needle attached prior to injection was slightly negative (>−0.01 mg) with all Norditropin devices due to capillary phenomena occurring when changing devices. Because the analysis was purely comparative (and the effect is consistent across devices within the measurement error of the analytical balance), the capillary effects were left uncompensated and, for the purposes of the modeling analysis, all negative values were rounded up to zero. These values contrast with an average of 98 μg of solution wastage with Omnitrope Pen-5. During dose setting, 0.0, 0.2, 1.9, and 2.3 μg of GH was wasted with NordiLet, NordiFlex, NordiPen/SimpleXx, and FlexPro respectively, compared with 0.9 μg with Omnitrope Pen-5 (each comparison with Omnitrope Pen-5 was significant at P < 0.05).
Table 3 Laboratory analysis findings for 5 mg devices including dripping with the needle attached and when setting the GH dose, reported in mg of active GH (converted from mass of GH solution)
Budget impact model
When projected over the course of a year, the modeling analysis showed substantial differences in wasted GH between the Omnitrope and Norditropin devices in the US, Europe, and Japan (). In the US setting, modeling total annual GH waste showed wastage of 5.5 mg, 4.7 mg, 8.3 mg, and 12.0 mg of GH with 5 mg FlexPro, NordiFlex, NordiPen/SimpleXx, and NordiLet, respectively, equating to between approximately 1 and 2.5 pens (or cartridges in the case of the durable NordiPen/SimpleXx device) of lost GH product per patient per annum. The corresponding wastage with Omnitrope was projected to be 43.6 mg, equivalent to over 8 cartridges of lost GH product per patient per annum (). Similar results were observed in the European and Japanese settings, but with slight differences driven by the cohort bodyweight and dosing assumptions, which directly affect the quantity of GH that is administered beyond the prescribed dose as a result of the device dosing increments ().
Figure 1 Modeled growth hormone wastage per patient per year for 5 mg administration devices in US, European, and Japanese settings.
Abbreviation: GH, growth hormone.
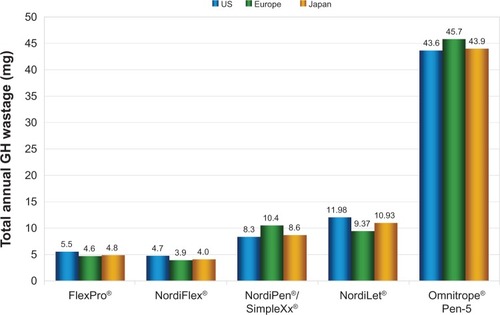
Table 4 Modeled annual GH wastage per patient with 5 mg and 10 mg devices in simulated US, European, and Japanese cohorts of pediatric patients on GH treatment
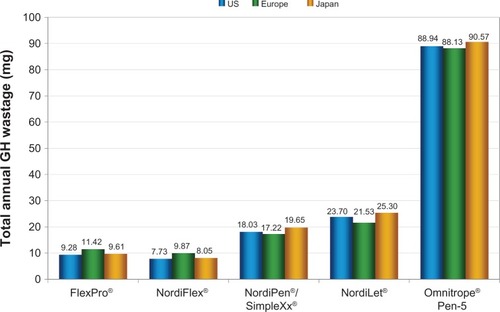
With the 10 mg sensitivity analyses in the US setting, FlexPro, NordiFlex, NordiPen/SimpleXx, and NordiLet showed wastage of 9.3 mg, 7.7 mg, 18.0 mg, and 23.7 mg of GH per annum, equivalent to between roughly 0.75 and 2.5 pens of lost GH product per patient per annum. As in the 5 mg analyses, wastage with the Omnitrope Pen-5 was substantially higher, at 88.9 mg per annum, equivalent to nearly nine cartridges of wasted GH product per patient per annum. These findings were mirrored in the European and Japanese settings, with slight changes arising from differences in bodyweight and dosing assumptions ().
Figure 2 Growth hormone wastage per patient per year for 10 mg administration devices in US, European, and Japanese settings.
Abbreviation: GH, growth hormone.
Discussion
Laboratory analyses of prefilled and durable Norditropin and Omnitrope Pen-5 devices showed substantial differences in the amount of wasted GH. By far the largest driver of differences was the wastage that occurred as a result of dripping with the needle attached. In these analyses, all Norditropin administration devices exhibited significantly less GH wastage with the needle on compared with the Omnitrope Pen-5 devices. Differences during dose setting were substantially smaller but more varied, with Omnitrope Pen-5 exhibiting significantly less wastage than FlexPro and NordiPen/SimpleXx but significantly more than NordiFlex and NordiLet.
Modeling these differences in typical populations of pediatric patients with GH disorders in the US, European, and Japanese settings showed substantial differences in the annual quantity of usable GH in 5 mg and 10 mg pens, thereby confirming that, even in GH devices of the same nominal volume, differences exist in the usable GH volume that may affect the frequency with which patients must refill their GH prescriptions in order to remain adherent to therapy.
A limitation of the present study is in the modeling approach, which necessarily incorporates some assumptions that may or may not reflect the real-world use of GH delivery devices. Notably, the model assumes that patients follow dosing instructions accurately and that all dispensed GH product is used. For instance, the model assumes that, in the case where a patient is unable to inject a full dose using the GH remaining in a pen, the remainder of the pen content would be injected and the full dose made up from a new device. As the model accounts only for the usable amount of GH over one year, changes in the weight-based prescribed dose due to pediatric patient growth are not taken into account because averaged values across an entire patient population are used. Nevertheless, we consider the model to be a reasonable representation of GH use and any incremental differences in the estimates of wastage arising as a result of the assumptions would be negligible.
When interpreting the findings of the present study, it is important to consider the results in the broader context of product wastage and how it relates to the amount of GH available to the patient. Specifically, there are a number of “real-world” factors that may contribute to wastage, such as device breakages, devices being left unrefrigerated, dosing errors, and devices going unused beyond their expiry date. However, there is currently a paucity of data on how these factors may differ between devices and, while the omission of these factors could be considered a limitation of the study from a real-world use perspective, the goal of the study was to establish how different device attributes, rather than factors that are under the patient’s control, can contribute to product wastage.
One final potential concern is the possibility that the GH wastage observed in the present study may be offset by the total content of the device being greater than advertised. However, regulatory guidelines in the European setting mandate that device content must fall within a tightly defined range around the advertised or nominal content.Citation22,Citation23 As such, any effect that device underfill or overfill would have on cost outcomes is likely to be negligible.
Given that the currently available GH products contain the same active ingredient and are clinically equivalent, health care decision-makers must look to other factors when deciding which GH products to prescribe. As noted in previous studies, product differentiation generally focuses on the delivery devices, formulation, the price per milligram of active substance and patient support services offered by each manufacturer. As illustrated in the present study, in addition to patient-centric considerations, such as injection site pain and device storage and handling, there are differences between the GH delivery systems that may affect the volume of GH that is available for injection relative to the nominal content. Given the annual per patient costs associated with GH treatment, such differences may lead to material cost savings that would not be apparent from the per milligram list prices of the various GH products.
Acknowledgments
This study was funded by Novo Nordisk A/S, Bagsværd, Denmark.
Disclosure
A-MK is a full-time employee of, and owns stock options in Novo Nordisk A/S. TW is a full-time employee of Novo Nordisk Inc (USA). LS and YQ are full-time employees of Novo Nordisk A/S. RP is a full-time employee of Ossian Health Economics and Communications GmbH, which received consultancy fees from Novo Nordisk A/S to perform the modeling analysis and prepare the manuscript. The authors report no other conflicts of interest in this work.
References
- BlethenSLBaptistaJKuntzeJFoleyTLaFranchiSJohansonAAdult height in growth hormone (GH)-deficient children treated with biosynthetic GH. The Genentech Growth Study GroupJ Clin Endocrinol Metab19978224184209024229
- RossJCzernichowPBillerBMGrowth hormone: health considerations beyond height gainPediatrics20101254e906e91820308212
- RadovickSDiVallSApproach to the growth hormone-deficient child during transition to adulthoodJ Clin Endocrinol Metab20079241195120017409338
- LongobardiSCuocoloAMerolaBLeft ventricular function in young adults with childhood and adulthood onset growth hormone deficiencyClin Endocrinol (Oxf)19984821371439579223
- BootAMEngelsMABoermaGJKrenningEPDe Muinck Keizer-SchramaSMChanges in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiencyJ Clin Endocrinol Metab1997828242324289253311
- CapaldoBPattiLOlivieroUIncreased arterial intima-media thickness in childhood-onset growth hormone deficiencyJ Clin Endocrinol Metab1997825137813819141519
- MerolaBCittadiniAColaoACardiac structural and functional abnormalities in adult patients with growth hormone deficiencyJ Clin Endocrinol Metab1993776165816618263155
- KappelgaardAMBojesenASkydsgaardKSjögrenILaursenTLiquid growth hormone: preservatives and buffersHorm Res200462 Suppl 39810315539807
- RoehrBThe many faces of human growth hormoneBETA2003154121612691033
- BazaloGRJoshiAVGermakJComparison of human growth hormone products’ cost in pediatric and adult patients. A budgetary impact modelManag Care2007169455117969748
- LaursenTHansenBFiskerSPain perception after subcutaneous injections of media containing different buffersBasic Clin Pharmacol Toxicol200698221822116445598
- FuchsGSMikkelsenSKnudsenTKKappelgaardAMEase of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: an open-label, uncontrolled usability testClin Ther200931122906291420110030
- PfütznerAHartmannKWinterFFuchsGSKappelgaardAMRohrerTRIntuitiveness, ease of use, and preference of a prefilled growth hormone injection pen: a noninterventional, randomized, open-label, crossover, comparative usability study of three delivery devices in growth hormone-treated pediatric patientsClin Ther201032111918193421095487
- KappelgaardAMMikkelsenSKnudsenTKFuchsGSPatient preference for a new growth hormone injection device: results of an open-label study in Japanese pediatric patientsJ Pediatr Endocrinol Metab2011247–848949621932587
- KappelgaardAMMikkelsenSBaggerCFuchsGSChildren and adolescent acceptability of a new device system to administer human growth hormone- a pilot studyJ Pediatr Endocrinol Metab2012253–428529422768658
- LindsayRFeldkampMHarrisDRobertsonJRallisonMUtah Growth Study: growth standards and the prevalence of growth hormone deficiencyJ Pediatr1994125129358021781
- National Institute for Clinical ExcellenceTechnology Appraisal 64: Human growth hormone (somatropin) in adults with growth hormone deficiency Available from: http://www.nice.org.uk/nicemedia/live/11504/32665/32665.pdfAccessed June 4, 2013
- International Organization for StandardizationNeedle-based injection systems for medical use. Requirements and test methods. Part 1: Needle-based injection systems Available from: http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?csnumber=52525Accessed June 26, 2013
- European Directorate for the Quality of MedicinesEuropean Pharmacopoeia Section 2.2.5: Relative density Available from: http://www.edqm.eu/site/european-pharmacopoeia-publications-1401.htmlAccessed June 26, 2013
- Novo Nordisk Pharma LtdClinical trial NCT01604161Non-interventional study of patients using Norditropin®for growth hormone deficiency or Turner Syndrome (NordiPAD) Available from: http://clinicaltrials.gov/ct2/show/NCT01604161Accessed June 6, 2013
- HöybyeCSävendahlLChristesenHTThe NordiNet® International Outcome Study and NovoNet® ANSWER Program®: rationale, design, and methodology of two international pharmacoepidemiological registry-based studies monitoring long-term clinical and safety outcomes of growth hormone therapy (Norditropin®)Clin Epidemiol2013511912723658497
- European Medicines AgencyGuideline 3 AQ11 A: specifications and control tests on the finished product1992 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003368.pdfAccessed June 26, 2013
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human UseICH Harmonised Tripartite Guideline: pharmaceutical development Q8(R2)2009 Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdfAccessed June 26, 2013