Abstract
Carotid artery stenting technologies are rapidly evolving. Options for endovascular surgeons and interventionists who treat occlusive carotid disease continue to expand. We here present an update and overview of carotid stenting devices. Evidence supporting carotid stenting includes randomized controlled trials that compare endovascular stenting to open surgical endarterectomy. Carotid technologies addressed include the carotid stents themselves as well as adjunct neuroprotective devices. Aspects of stent technology include bare-metal versus covered stents, stent tapering, and free-cell area. Drug-eluting and cutting balloon indications are described. Embolization protection options and new direct carotid access strategies are reviewed. Adjunct technologies, such as intravascular ultrasound imaging and risk stratification algorithms, are discussed. Bare-metal and covered stents provide unique advantages and disadvantages. Stent tapering may allow for a more fitted contour to the caliber decrement between the common carotid and internal carotid arteries but also introduces new technical challenges. Studies regarding free-cell area are conflicting with respect to benefits and associated risk; clinical relevance of associated adverse effects associated with either type is unclear. Embolization protection strategies include distal filter protection and flow reversal. Though flow reversal was initially met with some skepticism, it has gained wider acceptance and may provide the advantage of not crossing the carotid lesion before protection is established. New direct carotid access techniques address difficult anatomy and incorporate sophisticated flow-reversal embolization protection techniques. Carotid stenting is a new and exciting field with rapidly advancing technologies. Embolization protection, low-risk deployment, and lesion assessment and stratification are active areas of research. Ample room remains for further innovations and developments.
Introduction
Ramsay-Hunt reported in 1913 the clinicopathological findings of what he called “thrombotic hemiplegia,” where stroke patients were found to have a weakened carotid pulse, and postmortem pathology revealed carotid thrombus ipsilateral to gross ischemic changes in the brain.Citation1 Cervical carotid artery stenosis was recognized by C Miller Fisher in the 1950s to be a major cause of stroke.Citation2 Carotid endarterectomy has been implemented in the at-risk patient pool with significant success in reducing the risk of subsequent stroke compared to medical therapy alone.Citation3–Citation5 The innovative options offered by endovascular techniques have created a rapidly evolving field of medical devices whose judicious use allows for safe, often less invasive, alternatives to carotid endarterectomy for carotid revascularization. Carotid stenting is now a widely accepted alternative to endarterectomy in specific situations.Citation6,Citation7 We here systematically describe the current state of carotid revascularization devices and the evidence to support them. Although endovascular treatment of occlusive carotid disease is indispensable during acute stroke intervention, we will focus predominantly on elective and semielective revascularization.
The carotid artery is unique because of its end organ. Whereas the primary concern during revascularization in peripheral vessels is restoration of flow and hemodynamic balance, even minor distal embolization in the carotid artery can be associated with devastating neurological injury. Conversely, although distal embolization remains a concern when instrumenting and manipulating intracranial vasculature, these vessels lack significant muscularity and have a lower resistance bed. The unique goal of carotid device technology is to achieve adequate endoluminal recanalization against a centripetal muscular force, while minimizing distal embolic events.
Conceptually, we categorize carotid technologies into three groups: stent, balloon angioplasty, and embolization prevention devices. Other miscellaneous technologies are also presented.
Stents
Background
A stent is a scaffolding device that is often tubular and is used to hold tissue in a specific stretched or taut position. Modern intravascular stents are available in balloon-expandable and self-expanding varieties. In the setting of carotid bifurcation disease where prominent chronic compressive forces and a substantial drop in caliber between the common and internal carotid arteries (ICAs) as well as their mobile nature, self-expanding stents (eg, Wallstent; Boston Scientific, Natick, MA, USA) have become standard of care.Citation8–Citation11 Though early carotid interventionists reserved balloon-expandable stents for especially tortuous, ostial, and very calcified lesions,Citation8 advances in stent technology have reduced the role of this type of stent even in those lesions. The two most common alloys for carotid stents are cobalt-chromium and nickel-titanium (known as nitinol). Self-expanding stents are usually manufactured in a ready-to-use compressed form within a catheter-based sheath that functions as a delivery system. After insertion into the target artery, expansion is achieved by “unsheathing” the stent, at which time the mechanically predetermined centrifugal spring-like force is no longer neutralized by the sheath structure and the stent expands.Citation12
Carotid stenting trials
The initial impetus to develop endovascular alternatives to endarterectomy was to provide a noninferior treatment modality for patients who were suboptimal surgical candidates. Medical morbidities that increase surgical risk include severe cardiac, pulmonary, and renal disease. Poor anatomical factors include a very high carotid bifurcation, in which case carotid access/exposure poses an inevitable risk of mandibular dislocation. Historically, adjunct procedures to improve access to the carotid artery have included mandibulonasal wiring, mandibular subluxation, and mandibular condyle resection.Citation13–Citation15 Other anatomic considerations include previous extensive neck dissection and/or irradiation. Patients with previous injury to nervous structures with partial laryngeal palsy are at high risk for developing debilitating and life-altering pharyngolaryngeal dysfunction with surgery contralateral to the compromised side. Prohibitive spinal immobility and other anatomic hindrances to intubation are also considerations.Citation16,Citation17
Early endovascular alternatives to carotid endarterectomy were predominantly balloon-based. A randomized study comparing carotid endarterectomy and angioplasty was terminated after enrolling only ten endarterectomy and seven angioplasty patients due to a high rate of mortality in the latter group.Citation18 The Carotid and Vertebral Artery Transluminal Angioplasty Study investigators found no significant difference in rates of stroke or death between carotid balloon angioplasty with or without stenting and endarterectomy in symptomatic carotid stenosis cases.Citation19 However, the study was underpowered to make a claim of equal efficacy.
Trials conducted in North America (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy [SAPPHIRE]) and Europe (Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis [EVA-3S] and Stent-Protected Angioplasty versus Carotid Endarterectomy [SPACE]) attempted to demonstrate noninferiority for stenting compared to endarterectomy.Citation20–Citation22 The SAPPHIRE trial enrolled high-risk (eg, significant cardiac disease, recurrent stenosis, and age >80 years) symptomatic and asymptomatic patients.Citation22 The composite end point (death, stroke, or myocardial infarction [MI] within 30 days of intervention or death or ipsilateral stroke within 1 year) occurred less frequently in the stenting with embolic protection group compared with the endarterectomy group in a noninferiority analysis (12.2% versus 20.1%, P=0.004). The EVA-3S trial, on the other hand, was terminated prematurely because of safety and futility concerns. A significant increase in 30-day risk of stroke or death was found in the stenting group compared to the endarterectomy group (9.6% versus 3.9%, P=0.01).Citation20 Follow-up results demonstrated a higher cumulative probability of periprocedural stroke or death and nonprocedural ipsilateral stroke after up to 4 years in the stenting group (11.1% in the stenting group versus 6.2% in the endarterectomy group, P=0.03).Citation23 In the SPACE trial, no significant difference was found in the 30-day and 2-year risk of stroke or death between stenting and endarterectomy.Citation24 Nevertheless, the study failed to prove noninferiority, likely due to insufficient power. Additionally, the rate of recurrent stenosis was higher in the stenting group. Though neither European trial proved noninferiority of stenting, data suggested that the greatest risk was concentrated in the periprocedural period and that the risk of mid-term to long-term recurrent stroke was low. Both were criticized for lacking standardization of stent type and neurointerventionist experience.
More recently, the International Carotid Stenting Study (ICSS) investigators randomized 1,713 patients with >50% symptomatic carotid stenosis in 50 mostly European centers.Citation25 Within the 120-day follow-up period, the incidence of stroke, death, or periprocedural MI was significantly higher in the stenting group. Endarterectomy was significantly more likely to be associated with cranial neuropathies and wound complications. In a subsequent subset analysis, significantly more ischemic brain lesions were found on magnetic resonance (MR) diffusion-weighted imaging after stenting compared to endarterectomy.Citation26
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) is the largest randomized controlled trial comparing stenting with embolic protection “whenever feasible” and endarterectomy in both symptomatic and asymptomatic carotid stenosis.Citation7 Patients from 117 centers in North America were enrolled in the trial. Up to 4 years of follow-up data were collected. No statistical difference was noted in the primary end point of a composite of 30-day stroke, MI, or death. Isolation of the end points revealed a higher risk of periprocedural stroke or death in the stenting group (4.4% versus 2.3%, P=0.005), whereas MI was more common in the endarterectomy group (1.1% versus 2.3%, P=0.03). The change in measured outcomes in this trial compared to previous trials has been attributed to improving technology and advanced interventionist skills. More comprehensive screening protocols have also been invoked; for example, the overall rate of MI was much higher in CREST than in ICSS (1.7% versus 0.5%), likely due to cardiac biomarkers being screened in all patients in CREST and only in patients complaining of cardiac symptoms in ICSS.
The CREST-2 trial currently under way utilizes a four-armed parallel study design to compare composite 30-day stroke and death outcome differences between medical therapy alone versus endarterectomy and stenting, respectively, in conjunction with best medical therapy, in an asymptomatic patient pool.Citation27 Once the two parallel studies are completed, the difference in gain, if any, from adding one of the two revascularization procedures to medical therapy will be compared.
Evolution of stent technology
The Wallstent (Boston Scientific), initially approved for biliary use in Europe, has subsequently been approved for use in various internal organs and the iliac artery.Citation10,Citation28,Citation29 Use in the carotid artery has been off-label. Given its limited expansion force, the diameter of the Wallstent must usually be oversized by 2–3 mm relative to the lumen of the ICA. Additionally, it is difficult to predict where and to what degree the stent will be foreshortened, challenging the interventionist’s capacity to ensure that the lesion is entirely crossed by the stent. Further, its sharp wire ends create the potential for intimal injury. In cases of vessel tortuosity, this problem is compounded by unevenly distributed circumferential pressure, predisposing to kinking at bends and flaring at the ends. The Wallstent is an alloy of cobalt, chromium, nickel, molybdenum, iron, manganese, and stainless steel woven into a cylindrical superstructure. Tantalum has been added to its composition to enhance radiopacity. The Symphony (Boston Scientific) is a welded nitinol stent that is more rigid than the Wallstent and has a tendency to kink in tortuous vessels. It is thus primarily used for common carotid and brachiocephalic arteries with fairly straight trajectories. It has been reported to foreshorten less than 8%.Citation30 The SMART Stent (Cordis Corporation, Bridgewater, NJ, USA) is laser cut from a single nitinol tube and has a deployment mechanism and wall coverage that are similar to the Wallstent. The Acculink (Abbott Vascular, Abbott Park, IL, USA) was specifically designed for use in the extracranial carotid artery. Numerous other unique sophisticated stent designs are available at present, some of which are listed in .
Table 1 Characteristics of commonly used carotid stents
Open-cell versus closed-cell design
The open-cell versus closed-cell distinction identifies the free-cell area or the amount of space between the latticework of a stent. In closed-cell stents, each free space (cell) between the tines of a stent is completely separated from all other spaces by tines, whereas in open-cell designs the cell is connected to other cells through incomplete tines. An open-cell stent allows for larger uncovered gaps and is more malleable, making it better suited for tortuous areas. The reduced need for manipulation associated with this stent design is likely to reduce embolic potential, as well as kinking. Conversely, the greater free-cell area allows for more exposed plaque, possibly increasing embolic potential.Citation31 Closed-cell stents may provide greater plaque coverage but are more rigid and require more vigorous manipulation to implant. Closed-cell stents are associated with possibly decreased levels of platelet aggregation.Citation32–Citation34 In addition, elevated carotid artery velocities have been demonstrated in closed-cell stents.Citation35 However, the implications for clinical correlates and restenosis rate are unclear.
A prospective randomized trial found no significant difference in diffusion restricting lesions on MR imaging when comparing closed- and open-cell designs.Citation36 A retrospective comparison found that the open-cell design was associated with significantly fewer diffusion-weighted detectable embolic events on MR imaging when carotid atherosclerosis was treated without distal protection devices.Citation37 However, the difference in rate of clinical events did not reach statistical significance. It should be noted that these retrospective studies do not address the possibility that the embolic imaging findings may be related to the necessary process of crossing the lesions with the protection device itself before distal protection is established.
Some studies suggest that closed-cell stents afford more protective lesion coverage and a possible decrease in transient ischemic attacks.Citation38,Citation39 Yet, another study found no statistically significant differences in neurologic complications, stroke, and mortality risk between carotid stent cell designs.Citation40
It should be noted that dichotomizing free-cell area into “open” and “closed” cell designs is slightly misleading. Not only is there variability in quantitative free-cell area among so-called “open-cell” stents, but also the final free-cell area will inevitably be significantly affected by the size of stent chosen for a particular carotid artery caliber and lesion – ie, whether the interventionist chooses to “oversize” or “undersize.”Citation41 Additionally, newer hybrid stent designs have attempted to combine the benefits of open- and closed-cell designs by introducing a variable free-cell area in different regions of the stent ().
Bare-metal versus covered stents
Some investigators suggest that bare-metal stents with insufficient luminal wall coverage predispose to periprocedural embolic events and increase long-term embolic potential due to the exposed atherosclerotic surface.Citation42 Studies have been mixed in demonstrating a clinical difference between bare-metal and covered stents. Materials used to cover stents include porous expanded polytetrafluoroethylene polymer and semipermeable porous silicone-polyurethane.Citation43–Citation46 An extensive review of covered stents from a materials engineering perspective has been published.Citation47
One disadvantage of covered stents is their propensity for blocking branch vessels. Because carotid artery disease usually involves the carotid bifurcation and the cervical carotid artery, this is unlikely to be a major concern except in the setting where the stent is anticipated to cross the external carotid artery (ECA). Although occlusion of the extracranial carotid circulation may be of limited consequence in the setting of robust ICA flow, patients with long-standing cerebrovascul-opathy and chronic ICA low-flow states are often dependent on extensive networks of angiographically visible and hidden extracranial-to-intracranial anastomoses.Citation48
A covered stent with an opening on the side has been devised but has not gained wide acceptance because of the difficulty in deploying it with the opening in the exact orientation of the ECA. A recent stent design places slits on a covered stent in the direction of the long axis of the stent. This design is intended to allow blood flow into the ECA while covering the plaque and preventing embolization of atherosclerotic debris.Citation7 This device is awaiting clinical testing.
Stent tapering
The caliber of the common carotid artery (CCA) is usually significantly and predictably larger than that of the ICA. Endovascular attempts to address this issue have included the use of tapered stent designs. In a nontapered stent, the distal and proximal diameters of the stent are engineered to be the same. Manufacturers have argued that self-expanding stents are naturally “self-tapering” and will contour to the inner lumen of the carotid artery. Tapered stents provide a decrement in diameter in the proximal and distal edges. Two subcategories are available. One type, represented by the Acculink and Xact stents (Abbott Vascular, Abbott Park, IL, USA), incorporates a gradual diminution of diameter, referred to as a conical taper. A “shouldered” variety, exemplified by the Protégé stent (Covidien, Irvine, CA, USA), has a punctuated decrease in stent caliber. The transitional point is referred to as the shoulder and should be positioned at the level of the ECA takeoff. Data regarding possible benefits of tapered stents is still inconclusive.Citation49
Unfavorable anatomy and direct carotid access
Direct carotid artery puncture provides enhanced access in tortuous vessels.Citation50,Citation51 Initially reported in intracranial angioplasty and stenting cases,Citation52 it has now been extended for use in carotid bifurcation lesions. Although age >70 or 80 years is often presented as an intrinsic independent risk factor for embolic events with endovascular stenting, close reevaluation has found that unfavorable arch anatomy is most important in predisposing for adverse embolic events, rather than age alone.Citation53–Citation55 Vessel tortuosity and atherosclerotic aortic arch disease were found to be the predominant factors contributing to the increased rate of stroke associated with carotid stenting in patients >70 years compared with carotid stenting in younger patients and endarterectomy among all ages; this suggests that the increased risk of embolic events is likely related to difficult access.Citation54,Citation55 When patients with an acute angle between the aortic arch and the stented CCA were compared to patients with more favorable arch anatomy in a high-volume single-center retrospective study, a significant difference (P=0.0073) in adverse embolic events was observed.Citation53 When controlling for arch anatomy, no statistically significant difference between patients aged ≥80 years and <80 years could be found.
Balloon angioplasty for in-stent restenosis
Cutting balloon
In-stent restenosis has been reported in 8% of carotid stenting cases.Citation56 The CREST investigators reported a 6% restenosis rate in both stenting and surgical endarterectomy cases.Citation7 Consensus and rigorous evidence are lacking for the management of in-stent restenosis. Successful use of a cutting balloon, previously well known to interventional cardiologists for partial atherectomy in cases of primary or delayed lesion recurrence after coronary stenting, has been reported in small carotid stenting case series.Citation57–Citation59 The same technology has been successfully reported for severely calcified primary carotid lesions as an adjunct to stenting to minimize hemodynamic instability from carotid bulb compression.Citation60
Drug-eluting balloon
Drug-eluting balloon technology has a long track-record in the coronary arteries and has recently been applied to the carotid artery. The dominant opinion is that these devices should not be used for direct mechanical dilatation but should rather be used as a drug delivery system once predilation has been successfully achieved.Citation61,Citation62
Embolization prevention
Statistically significant microembolic events have been recorded using transcranial Doppler ultrasonography during carotid stenting.Citation63 Noninferiority of stenting with distal protection compared to historical results with endarterectomy has been demonstratedCitation64 and was confirmed in the prospective randomized controlled SAPPHIRE trial.Citation22 Periprocedural neuroprotection is thought to provide clinical benefit by preventing distal migration of macroscopic and microscopic emboli that may cause clinically significant ischemic events.
Three categories of embolic prevention systems are currently available. Distal nonocclusive systems ideally prevent distal embolization while preserving distal flow. Distal occlusive systems prevent flow of both blood and embolic material distally. Proximal devices occlude CCA and ECA flow, thereby producing flow reversal from the ICA, removing embolic material from the carotid bifurcation region into the CCA, and, eventually, returning blood via a filter into the venous system (GORE Flow Reversal System; WL Gore and Associates, Flagstaff, AZ, USA) or direct aspiration (Mo.Ma; Medtronic, Minneapolis, MN, USA).
Distal protection
Stenting with distal filtration technologies (distal nonocclusive systems) demonstrated noninferiority to endarterectomy, and outcomes following multicenter cohort studies as well as the SAPPHIRE trial were promising.Citation22,Citation65,Citation66 The Angioguard (Cordis, Bridgewater, NJ, USA) used in SAPPHIRE is a low profile guidewire-based filtering device that allows for easy simultaneous delivery of stents and balloons. The wire is then pulled out at the end of the case, along with the filter basket. The NeuroShield Cerebral Protection System (MedNova, Galway, UK; subsequently acquired by Abbott Vascular and marketed as the Emboshield) is a temporary implantable design composed of a porous nonocclusive polyurethane membrane deployed over a preformed nitinol expansion wire construct. A retrieval system is included with a flexible distal portion to the outer catheter that expands as the inner catheter is passed. Commonly used distal protection devices are listed in .
Table 2 Distal embolization protection devices
That distal protection devices perform their intended function of catching emboli is clear,Citation67–Citation69 yet the incidence of periprocedural ischemic events has not been found to be affected by the load of embolic material found in distal filters.Citation67–Citation71 Despite initially promising multicenter results, retrospective studies in which distal protection devices were used have not shown significantly improved outcomes, though a trend toward decreased risk of embolic ischemic events may be present.Citation72 However, it should be noted that in the United States, carotid stenting is not reimbursed by Medicare unless it is performed with distal embolic protection.
Several disadvantages of distal protection devices have been described. These devices must be advanced across the stenotic lesion before embolic protection can be established; and during this process, distal embolization may occur. Their size is typically in the range of 3-French, and therefore they are generally stiffer and bulkier than proximal occlusion devices, increasing navigation difficulty and the likelihood of aggressive vessel/intimal manipulation across tortuous vessels or critically stenotic carotid arteries.Citation73 They are also frequently associated with focal vasospasm and, infrequently, vessel injury. Further, retrieval and removal of a deployed protection device may provide yet another opportunity for periprocedural distal embolization.Citation30,Citation74,Citation75 Acute periprocedural distal stent occlusion has been reported as well.Citation76 One explanation for this potentially devastating complication may be a phenomenon known as “slow flow,” in which focal flow diminution immediately proximal to the distal filter device may predispose to a >9% 30-day risk of stroke or death, and was found to occur in approximately 10% of carotid stenting cases in a single-center registry.Citation77 Previously symptomatic and older patients were more likely to be affected. Indeed, an unacceptably high rate of complications has been reported in older patients undergoing carotid artery stenting in several studies utilizing distal protection devices.Citation78–Citation80 Nevertheless, rapid advances in endovascular stenting and associated protection techniques have been associated with improved outcomes in the recent past; the anticipation is that this trend will continue and new technologies are likely to further mature with time.Citation81
Concentric versus eccentric filter
Concentric and eccentric distal protection devices differ based on the position of the wire that tethers the filter: centered versus to the side. Although one study identified no difference between filter type,Citation82 another study reported a statistically significant decrease in embolic events with eccentric filters when analyzing all clinical findings, including transient ischemic attacks.Citation39 However, this difference was not significant when transient clinical findings were excluded and only stroke and death were analyzed. Eccentric filters may engage the vessel wall more stably, allowing for better embolization protection.
Distal occlusion
Distal occlusion, as opposed to distal filtration, involves isolation of the distal cerebrovascular distribution from the atherosclerotic carotid segment being manipulated. This can be most simply done with temporary balloon occlusion, which was the first distal embolization protection mechanism reported.Citation83 A more sophisticated approach is required to ensure that the embolic material is cleared before the balloon is removed. This is accomplished by applying suction to the sheath proximally or attempting to flush the embolic material proximally in hope of driving the debris into the external carotid system. The GuardWire Protection system (PercuSurge, Sunnyvale, CA, USA), for example, is designed for distal occlusion that is supplemented with an aspiration catheter called the Export (Medtronic). Despite the difficulties associated with debris removal with distal occlusion devices rather than distal protection devices, there is likely a decreased risk of device-induced vasospasm with balloon devices compared to filter devices. A significantly greater likelihood of the need for unprotected predilation was found with distal filter devices compared to distal balloon occlusion devices.Citation39,Citation84 Some authors recommend the use of filter devices in preference to balloon occlusion because of a somewhat lower risk of distal embolization, unless the vessels are tortuous and critically narrowed.Citation85 Though concern has been expressed regarding increased radiation exposure with the added use of distal embolic protection devices, this has not been borne out by quantitative dosimetric studies.Citation86
Flow reversal
Proximal occlusion aims not to isolate the diseased area undergoing intervention from the distal area but rather to create flow reversal from contralateral carotid and vertebrobasilar collaterals intracranially, thereby flushing emboli proximally into the CCA. Although endarterectomy has the advantage of allowing the placement of a shunt to direct flow from proximal to the CCA clamp to distal to the ICA clamp, yet another maneuver utilized in endarterectomy is replicated by flow reversal technology. Unclamping is performed in a specific order – first the ICA, then the ECA, and finally the CCA – to allow for temporary flow reversal and flushing of potentially embolic material into the extracranial carotid circulation. An advantage of proximal occlusion devices over distal protection devices is that the lesion is not crossed in an unprotected state.
The initial introduction of flow reversal technology for proximal embolic protection was met with skepticism given the significantly lower resistance bed that comprises the intracranial carotid system compared to the extracranial carotid system; even small extracranial collateral communications from the external carotid or vertebrobasilar system may compromise the ability to achieve meaningful flow reversal. Early authors argued that to achieve flow reversal, one must first induce flow stasis by placing the occlusive balloon distal to the ECA takeoff; and if this is done, clearance of embolic materials becomes problematic.Citation30
A creative solution to this dilemma was the development of a proximal protection device in which a triple-lumen catheter with a double-balloon was utilized to occlude the CCA and ECA; the reversed flow was then directed through the catheter to a filtered external femoral venous system.Citation87 The popularity of proximal protection increased with the introduction of sophisticated technologies, such as the GORE Flow Reversal and Mo.Ma systems (). Although the GORE system induces true flow reversal, as described above, Mo.Ma is simply a focal flow arrest without any venous return. Trials and meta-analyses confirm a 30-day stroke risk of <2% associated with carotid stenting using flow reversal with both systems.Citation88–Citation92 It should be noted that there is a reported intolerance to flow arrest or flow reversal in a small subset of patients (<2%) with isolated intracranial circulation.
Table 3 Proximal embolization protection devices
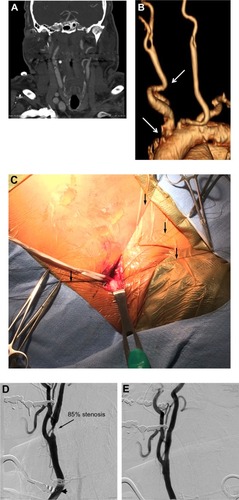
An innovative approach aimed at obviating the need for accessing an unfavorable aortic arch is being evaluated in the Reverse Flow Used During Carotid Artery Stenting Procedure safety and efficacy study. The MICHI Neuroprotection System (Silk Road Medical Inc., Sunnyvale, CA, USA) has demonstrated initial safety and noninferiority to historical endarterectomy dataCitation93 using direct carotid puncture access to establish flow reversal and diversion into the femoral venous system with the intent to maximize the interventionist’s control of flow parameters. A case in which this transcervical access neuroprotection system was used for carotid stenting is illustrated in .
Figure 1 Case illustration of the Reverse Flow Used During Carotid Artery Stenting Procedure (ROADSTER).
Comparison of embolization prevention devices
Variable results with embolic protection devices have been reported. Recent MR diffusion imaging and ultrasound transcranial Doppler data have shown embolic events to be less common with flow reversal than distal filter protection.Citation94 The opposite conclusion was reached by another group studying postprocedure follow-up MR imaging diffusion restriction.Citation95 The results of a study comparing carotid stenting with flow reversal, carotid stenting with distal filter protection, and endarterectomy suggest that the increased risk of perioperative stroke in carotid stenting compared to endarterectomy may be applicable to stenting with distal filter devices but not with flow reversal.Citation96 However, no significant differences among these techniques were found when long-term outcomes, such as death and neurological disability, were assessed. Another study found no difference between distal and proximal protection outcomes but noted that distal filters were used in more symptomatic and complex lesions as determined by plaque ulceration, calcification, and length.Citation97 The variable results may be due to the novelty of the various embolic-protection devices and their fairly recent introduction into broad use. Flow-reversal is associated with approximately five more steps than distal protection, perhaps making the use of flow-reversal devices more learning-curve and operator-experience dependent.Citation98
Modeling and risk stratification
As stated above in reference to the Wallstent, one problem that is commonly encountered during carotid stenting is the inability to predict the exact final position of the stent. The use of finite-element analysis to study the effect of carotid stenting on each individual vessel wall is currently a costly and lengthy process that requires several days and significant technological resource expenditure. With improvements in modeling efficiency and computational technology, such analyses may in the future be a helpful addition to clinical practice.Citation99
Carotid tortuosity can be categorized and quantified for risk-stratification on the basis of an intraprocedural angiogram or a preprocedural computed tomographic or MR luminogram.Citation55 A group of clinicians in Poland have championed a “tailored” carotid artery stenting algorithm for the elderly; they posit that an intimate knowledge of the specific benefits and characteristics of each device and tailoring the specific combination of devices to the particular vessel anatomy, pathological process, and radiographic qualities of the patient may be especially important in older patients in whom the risks of carotid stenting are especially prominent.Citation100 Though at this time insufficiently generalizable or reproducible, a systematic, reportable algorithm may ensue with sufficient experience and effort.
Intravascular ultrasound
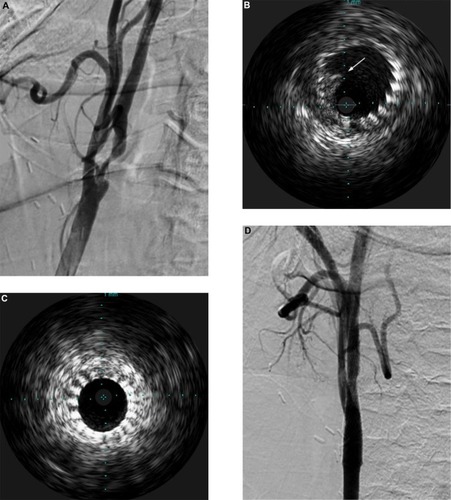
Ultrasound-based technologies and techniques can be used to identify and crudely quantify the echolucency of carotid plaque, which may be a marker for embolic potential.Citation101 When choosing devices, relative embolic risk of the devices under consideration and characteristics of the patient’s plaque can be taken into account. Intravascular ultrasound (IVUS) is not currently equipped to provide sophisticated quantitative data. Nevertheless, it provides real-time plaque architecture data that has been shown to have good predictive value for embolic potential.Citation102–Citation104 An ex vivo study has demonstrated good concordance between plaque morphology on IVUS and its histological correlates.Citation105 The utility of IVUS in the setting of intraluminal thrombus encountered during carotid stenting is illustrated in . It is also important to note that IVUS provides superior intraluminal visualization as compared to angiography, particularly poststenting and angioplasty, allowing visualization of plaque prolapse through the tines of the stent, which can be addressed prior to removal of protection devices. This is particularly germane in proximal protection cases where IVUS can be performed while the carotid artery is still under flow reversal/arrest so no anterograde injection of contrast material, which can carry embolic debris, is required before confirming plaque constraint and optimal stent placement.
Figure 2 Case illustration of the utility of intravascular ultrasound (IVUS).
Abbreviation: CCA, common carotid artery.
Future directions
A novel carotid stent (Gore Scaffold; WL Gore and Associates) is being evaluated in a trial that enrolled its first patient in late 2013 (Gore Carotid Stent Clinical Study for the treatment of carotid Artery stenosis in patients at increased risk For adverse events From CarOtid enDarterectomy [SCAFFOLD]). The Scaffold stent has an open-cell design that is left uncovered on the outside with a polymer film coat on the inside. The aim is to improve plaque stabilization and decrease distal embolic events with the aid of the internal coating which also has a layer of bound heparin. The design of the Casper stent (MicroVention, Tustin, CA, USA) also embraces this similar new-generation technology.
Intravascular ultrasonography allows us to address concerns associated with the use of proximal protection devices. The adjunctive use of this technology during carotid stenting requires further systematic study from device integration and practice protocol perspectives.
Conclusion
Advances in endovascular approaches to carotid artery revascularization include stent and neuroprotection technologies and techniques, as well as therapies for subsequent restenosis. Intimate knowledge of device technology, along with the flaws and advantages of each technology, is essential to tailoring care to the particular risk profile of a patient. Better designed prospective trials and registries are needed to further delineate the unique indications for new sophisticated technologies.
Author contribution
All authors are responsible for concepts and design, and all authors contributed intellectually. All authors acquired, ana-lyzed, and interpreted the data. The manuscript was prepared by Morr. All authors reviewed and made critical revision of the manuscript.
Acknowledgments
The authors thank Paul H Dressel, BFA for assistance with preparation of the illustrations and Debra J Zimmer for editorial assistance.
Disclosure
Siddiqui’s research grants (not related to present study) include National Institutes of Health (coinvestigator: NINDS 1R01NS064592-01A1 and NIBIB5RO1 EB002873-07), University at Buffalo (Research Development Award). Financial interests include Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, Lazarus Effect; associations as consultant include Codman and Shurtleff, Inc., Concentric Medical, Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra, Stryker Neurovascular, Pulsar Vascular; speakers’ bureaus include Codman and Shurtleff, Genentech; national steering committees member for Penumbra 3D Separator Trial, Covidien SWIFT PRIME Trial; advisory board involvement with Codman and Shurtleff, Covidien Vascular Therapies; honoraria incurred from Abbott Vascular and Codman and Shurtleff, Inc. for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. Lin and Morr report no conflicts of interest in this work.
References
- HuntJRHunt: the role of the carotid arteries: the role of the carotid arteries, in the causation of vascular lesions of the brain, with remarks on certain special features of the symptomatology. 1914Am J Med Sci2013346650450924263078
- FisherMOcclusion of the internal carotid arteryAMA Arch Neurol Psychiatry1951653346377
- North American Symptomatic Carotid Endarterectomy Trial CollaboratorsBeneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosisN Engl J Med199132574454531852179
- MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70%–99%) or with mild (0%–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative GroupLancet19913378752123512431674060
- MaybergMRWilsonSEYatsuFCarotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist GroupJAMA199126623328932941960828
- BrottTGHalperinJLAbbaraS2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular SurgeryJ Am Coll Cardiol20115781002104421288680
- BrottTGHobsonRWHowardGCREST InvestigatorsStenting versus endarterectomy for treatment of carotid-artery stenosisN Engl J Med20103631112320505173
- IschingerTACarotid stenting: which stent for which lesion?J Interv Cardiol200114661762312053383
- MathurADorrosGIyerSSVitekJJYadavSSRoubinGSPalmaz stent compression in patients following carotid artery stentingCathet Cardiovasc Diagn19974121371409184284
- SerruysPWStraussBHde FeyterPUrbanPThe Wallstent: a self-expanding stentJ Invasive Cardiol19913312713410149122
- von BirgelenCGilRRuygrokPOptimized expansion of the Wallstent compared with the Palmaz-Schatz stent: on-line observations with two- and three-dimensional intracoronary ultrasound after angiographic guidanceAm Heart J19961316106710758644583
- QinFPanettaTFVascular stentsMooreWSAhnSSEndovascular SurgeryPhiladelphia, PASaunders201189105
- BoynePJFree grafting of traumatically displaced or resected mandibular condylesJ Oral Maxillofac Surg19894732282322921657
- CantoreGPDelfiniRMariottiniASantoroACasconePAnterior displacement of the mandible for better exposure of the distal segment of the extracranial carotid arteryActa Neurochir (Wien)1987861–256603618307
- FisherDFClagettGPParkerJIMandibular subluxation for high carotid exposureJ Vasc Surg1984167277336492304
- GutermanLRFesslerRDHopkinsLNCervical carotid revascularizationNeurosurg Clin N Am20001113948 viii10565869
- WhiteCJRameeSRCollinsTJJenkinsJSReillyJPPatelRACarotid artery stenting: patient, lesion, and procedural characteristics that increase procedural complicationsCatheter Cardiovasc Interv201382571572623630062
- NaylorARBoliaAAbbottRJRandomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trialJ Vasc Surg19982823263349719328
- Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trialLancet200135792701729173711403808
- MasJLChatellierGBeyssenBEVA-3S InvestigatorsEndarterectomy versus stenting in patients with symptomatic severe carotid stenosisN Engl J Med2006355161660167117050890
- RinglebPAAllenbergJBrückmannHSPACE Collaborative Group30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trialLancet200636895431239124717027729
- YadavJSWholeyMHKuntzREStenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy InvestigatorsProtected carotid-artery stenting versus endarterectomy in high-risk patientsN Engl J Med2004351151493150115470212
- MasJLTrinquartLLeysDEVA-3S investigatorsEndarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trialLancet Neurol200871088589218774745
- EcksteinHHRinglebPAllenbergJRResults of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trialLancet Neurol200871089390218774746
- EderleJDobsonJFeatherstoneRLInternational Carotid Stenting Study investigatorsCarotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trialLancet2010375971998599720189239
- BonatiLHJongenLMHallerSICSS-MRI study groupNew ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS)Lancet Neurol20109435336220189458
- LalBKMeschiaJFBrottTGCREST-2: guiding treatments for asymptomatic carotid diseaseEndovascular Today201397376
- VorwerkDGüntherRWStent placement in iliac arterial lesions: three years of clinical experience with the WallstentCardiovasc Intervent Radiol19921552852901423388
- ZollikoferCLAntonucciFPfyfferMArterial stent placement with use of the Wallstent: midterm results of clinical experienceRadiology199117924494562014291
- WilliamsJSMechanical devices used for carotid stentingTech Vasc Interv Radiol200032102113
- TadrosROSpyrisCTVouyoukaAGComparing the embolic potential of open and closed cell stents during carotid angioplasty and stentingJ Vasc Surg2012561899522386144
- GurbelPACallahanKPMalininAISerebruanyVLGillisJCould stent design affect platelet activation? Results of the Platelet Activation in STenting (PAST) StudyJ Invasive Cardiol2002141058458912368510
- HongMKMintzGSLeeCWIncidence, mechanism, predictors, and long-term prognosis of late stent malapposition after bare-metal stent implantationCirculation2004109788188614967732
- StoeckelDBonsignoreCDudaSA survey of stent designsMinim Invasive Ther Allied Technol200211413714716754063
- PierceDSRoseroEBModrallJGOpen-cell versus closed-cell stent design differences in blood flow velocities after carotid stentingJ Vasc Surg2009493602606 discussion 60619268763
- TimaranCHRoseroEBHigueraAIlarrazaAModrallJGClagettGPRandomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolizationJ Vasc Surg201154513101316. e1 discussion 131621723064
- GrunwaldIQReithWKarpKComparison of stent free cell area and cerebral lesions after unprotected carotid artery stent placementEur J Vasc Endovasc Surg2012431101422078854
- BosiersMde DonatoGDelooseKDoes free cell area influence the outcome in carotid artery stenting?Eur J Vasc Endovasc Surg2007332135141 discussion 142–14317097897
- HartJPPeetersPVerbistJDelooseKBosiersMDo device characteristics impact outcome in carotid artery stenting?J Vasc Surg2006444725730 discussion 730–73117011998
- SchillingerMGschwendtnerMReimersBDoes carotid stent cell design matter?Stroke200839390590918239162
- AuricchioFContiMFerraroMRealiAEvaluation of carotid stent scaffolding through patient-specific finite element analysisInt J Numer Method Biomed Eng201228101043105523027634
- KabinejadianFCuiFZhangZHoPLeoHLA novel carotid covered stent design: in vitro evaluation of performance and influence on the blood flow regime at the carotid artery bifurcationAnn Biomed Eng20134191990200223842696
- GreilOKleinschmidtTWeissWFlow velocities after carotid artery stenting: impact of stent design. A fluid dynamics study in a carotid artery model with laser Doppler anemometryCardiovasc Intervent Radiol2005281667615602638
- Müller-HülsbeckSJahnkeTStolzmannPPaulsenFWenkeRHellerMA new concept for covered stent protected carotid angioplasty: an ex vivo studyRofo2003175121634163814661133
- SchillingerMDickPWiestGCovered versus bare self-expanding stents for endovascular treatment of carotid artery stenosis: a stopped randomized trialJ Endovasc Ther200613331231916784318
- SzólicsASztrihaLKSzikraPSzólicsMPalkóAVörösEThe use of covered stents for the endovascular treatment of extracranial internal carotid artery stenosis: a prospective study with a 5-year follow-upEur Radiol20102071772177620033177
- FarhatniaYTanAMotiwalaACousinsBGSeifalianAMEvolution of covered stents in the contemporary era: clinical application, materials and manufacturing strategies using nanotechnologyBiotechnol Adv201331552454223305892
- SiddiquiAHChenPRIntracranial collateral anastomoses: relevance to endovascular proceduresNeurosurg Clin N Am200920327929619778700
- BrownKEUsmanAKibbeMRCarotid stenting using tapered and nontapered stents: associated neurological complications and restenosis ratesAnn Vasc Surg200923443944519128933
- ChangDWSchubartPJVeithFJZarinsCKA new approach to carotid angioplasty and stenting with transcervical occlusion and protective shunting: Why it may be a better carotid artery interventionJ Vasc Surg2004395994100215111851
- LarrazabalRKlurfanPSarmaDGunnarssonTSurgical exposure of the carotid artery for endovascular interventional proceduresActa Neurochir (Wien)2010152353754419806304
- SamaniegoEADabusGRajuRTsoukasAILinfanteIIntracranial angioplasty and stenting through direct carotid punctureJ Neuroimaging201323220721022082129
- DumontTMMokinMWachMMUnderstanding risk factors for perioperative ischemic events with carotid stenting: is patient age over 80 years or is unfavorable arch anatomy to blame?J Neurointerv Surg20146321922423685755
- LamRCLinSCDeRubertisBHynecekRKentKCFariesPLThe impact of increasing age on anatomic factors affecting carotid angioplasty and stentingJ Vasc Surg200745587588017466784
- LinSCTrocciolaSMRheeJAnalysis of anatomic factors and age in patients undergoing carotid angioplasty and stentingAnn Vasc Surg200519679880416200468
- ChakhtouraEYHobsonRW2ndGoldsteinJIn-stent restenosis after carotid angioplasty-stenting: incidence and managementJ Vasc Surg2001332220225 discussion 225–22611174771
- BendokBRRoubinGSKatzenBTCutting balloon to treat carotid in-stent stenosis: technical noteJ Invasive Cardiol200315422723212668854
- HeckDResults of cutting balloon angioplasty for carotid artery in-stent restenosis in six patients: description of the technique, long-term outcomes, and review of the literatureJ Neurointerv Surg200911485021994106
- ShahQAGeorgiadisALSuriMFRodriguezGJQureshiAICutting balloon angioplasty for carotid in-stent restenosis: case reports and review of the literatureJ Neuroimaging200818442843218333838
- SetacciFSirignanoPde DonatoGCarotid highly-calcified de novo stenosis and cutting-balloon angioplasty: a tool to prevent haemodynamic depression?J Cardiovasc Surg (Torino)2009503357364
- MontorsiPGalliSRavagnaniPMDrug-eluting balloon for treatment of in-stent restenosis after carotid artery stenting: preliminary reportJ Endovasc Ther201219673474223210870
- SangiorgiGRomagnoliEBiondi-ZoccaiGCommentary: drug-eluting balloons for carotid in-stent restenosis: can this technology deliver the goods?J Endovasc Ther201219674374823210871
- RitterMATheismannKSchmiedelMRingelsteinEBDittrichRVascularization of carotid plaque in recently symptomatic patients is associated with the occurrence of transcranial microembolic signalsEur J Neurol20132081218122123163829
- GrayWAHopkinsLNYadavSARCHeR Trial CollaboratorsProtected carotid stenting in high-surgical-risk patients: the ARCHeR resultsJ Vasc Surg200644225826816890850
- IyerSSWhiteCJHopkinsLNBEACH InvestigatorsCarotid artery revascularization in high-surgical-risk patients using the Carotid WALLSTENT and FilterWire EX/EZ: 1-year outcomes in the BEACH Pivotal GroupJ Am Coll Cardiol200851442743418222352
- MatsumuraJSGrayWChaturvediSYamanouchiDPengLVertaPResults of carotid artery stenting with distal embolic protection with improved systems: Protected Carotid Artery Stenting in Patients at High Risk for Carotid Endarterectomy (PROTECT) trialJ Vasc Surg2012554968976. e522236885
- AngeliniAReimersBDella BarberaMCerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debrisStroke200233245646111823652
- HenryMAmorMHenryICarotid stenting with cerebral protection: first clinical experience using the PercuSurge GuardWire systemJ Endovasc Surg19996432133110893133
- ReimersBCorvajaNMoshiriSCerebral protection with filter devices during carotid artery stentingCirculation20011041121511435330
- SchlüterMTüblerTSteffensJCMatheyDGSchoferJFocal ischemia of the brain after neuroprotected carotid artery stentingJ Am Coll Cardiol20034261007101313678921
- VosJAvan den BergJCErnstSMCarotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patientsRadiology2005234249349915616120
- TouzéETrinquartLChatellierGMasJLSystematic review of the perioperative risks of stroke or death after carotid angioplasty and stentingStroke20094012e683e69319892997
- EskandariMKNajjarSFMatsumuraJSKibbeMRMoraschMDTechnical limitations of carotid filter embolic protection devicesAnn Vasc Surg200721440340717368834
- LianXLiuWLiMRisk factors and complications associated with difficult retrieval of embolic protection devices in carotid artery stentingCardiovasc Intervent Radiol2012351434821387123
- TallaritaTRabinsteinAACloftHAre distal protection devices ‘protective’ during carotid angioplasty and stenting?Stroke20114271962196621566230
- KimYWKangDHHwangJHParkJHwangYHKimYSRescue strategy for acute carotid stent thrombosis during carotid stenting with distal filter protection using forced arterial suction thrombectomy with a reperfusion catheter of the Penumbra System: a technical noteActa Neurochir (Wien)201315581583158823689967
- CasserlyIPAbou-CheblAFathiRBSlow-flow phenomenon during carotid artery intervention with embolic protection devices: predictors and clinical outcomeJ Am Coll Cardiol20054681466147216226169
- GrayWAYadavJSVertaPThe CAPTURE registry: results of carotid stenting with embolic protection in the post approval settingCatheter Cardiovasc Interv200769334134817171654
- StingeleRBergerJAlfkeKSPACE investigatorsClinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE studyLancet Neurol20087321622218242141
- ZahnRIschingerTHochadelMArbeitsgemeinschaft Leitende Kardiologische KrankenhausärzteCarotid artery stenting in octogenarians: results from the ALKK Carotid Artery Stent (CAS) RegistryEur Heart J200728337037517158826
- MetzgerDCEmbolic protection in carotid artery stenting: new optionsTech Vasc Interv Radiol2011142869421550511
- IyerVde DonatoGDelooseKThe type of embolic protection does not influence the outcome in carotid artery stentingJ Vasc Surg200746225125617664102
- ThéronJCosgroveRMelansonDEthierREmbolization with temporary balloon occlusion of the internal carotid or vertebral arteriesNeuroradiology19862832462533725012
- WholeyMHFinolEWholeyMScottiCMVerdinelliIHow effective are distal embolic protection filters in carotid stenting? Clinical and benchtop testing resultsAm J Cardiol200596Suppl 7A77H
- PowellRJAlessiCNolanBComparison of embolization protection device-specific technical difficulties during carotid artery stentingJ Vasc Surg2006441566116828426
- D’ErcoleLQuarettiPCionfoliNPatient dose during carotid artery stenting with embolic-protection devices: evaluation with radiochromic films and related diagnostic reference levels according to factors influencing the procedureCardiovasc Intervent Radiol201336232032923150118
- ParodiJCFerreiraLMSicardGLa MuraRFernandezSCerebral protection during carotid stenting using flow reversalJ Vasc Surg200541341642215838474
- AnselGMHopkinsLNJaffMRInvestigators for the ARMOUR Pivotal TrialSafety and effectiveness of the INVATEC MO.MA proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trialCatheter Cardiovasc Interv20107611820222019
- BersinRMStabileEAnselGMA meta-analysis of proximal occlusion device outcomes in carotid artery stentingCatheter Cardiovasc Interv20128071072107822454248
- MontorsiPCaputiLGalliSMicroembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protectionJ Am Coll Cardiol201158161656166321982309
- NikasDReithWSchmidtAProspective, multicenter European study of the GORE flow reversal system for providing neuroprotection during carotid artery stentingCatheter Cardiovasc Interv20128071060106822644906
- StabileERubinoPMontorsiPClamping intolerance during proximal protected carotid artery stentingCatheter Cardiovasc Interv2013821606123494846
- PinterLRiboMLohCSafety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF StudyJ Vasc Surg20115451317132321658889
- GoodeSDHoggardNMacdonaldSEvansDHClevelandTJGainesPAAssessment of reverse flow as a means of cerebral protection during carotid artery stent placement with diffusion-weighted and transcranial Doppler imagingJ Vasc Interv Radiol201324452853323462063
- Castro-AfonsoLHAbudLGRoloJGFlow reversal versus filter protection: a pilot carotid artery stenting randomized trialCirc Cardiovasc Interv20136555255924084627
- BrewsterLPBeaulieuRCorriereMACarotid revascularization outcomes comparing distal filters, flow reversal, and endarterectomyJ Vasc Surg201154410001004 discussion 1004–100521871772
- ZahnRIschingerTMarkBArbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK)Embolic protection devices for carotid artery stenting: is there a difference between filter and distal occlusive devices?J Am Coll Cardiol200545111769177415936604
- StabileEEspositoGOperator’s experience is the most efficient embolic protection device for carotid artery stentingCirc Cardiovasc Interv20136549649724129932
- AuricchioFContiMFerraraAMorgantiSRealiAPatient-specific finite element analysis of carotid artery stenting: a focus on vessel modelingInt J Numer Method Biomed Eng201329664566423729192
- DzierwaKPieniazekPTekieliLCarotid artery stenting according to the “tailored CAS” algorithm performed in the very elderly patients: the thirty day outcomeCatheter Cardiovasc Interv201382568168823825008
- MathiesenEBBønaaKHJoakimsenOEcholucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromsø studyCirculation2001103172171217511331258
- ClairDGHopkinsLNMehtaMEMPiRE Clinical Study InvestigatorsNeuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE Clinical StudyCatheter Cardiovasc Interv201177342042920853365
- ClarkDJLessioSO’DonoghueMSchainfeldRRosenfieldKSafety and utility of intravascular ultrasound-guided carotid artery stentingCatheter Cardiovasc Interv200463335536215505835
- WehmanJCHolmesDRJrEckerRDIntravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stentingCatheter Cardiovasc Interv200668685385717086527
- FuchsMHeiderPPelisekJPoppertHEcksteinHHEx vivo characterization of carotid plaques by intravascular ultrasonography and virtual histology: concordance with real plaque pathomorphologyJ Cardiovasc Surg (Torino) Epub12102013
