Abstract
Introduction
The Shiley™ Flexible adult tracheostomy tube with TaperGuard™ cuff has been designed through its geometry, materials, diameter, and wall thickness to minimize micro-aspiration of fluids past the cuff and to provide an effective air seal in the trachea while also minimizing the risk of excessive contact pressure on the tracheal mucosa. The cuff also has a deflated profile that may allow for easier insertion through the stoma site. This unique design is known as the TaperGuard™ cuff. The purpose of the observational, in vitro study reported here was to compare the TaperGuard™ taper-shaped cuff to a conventional high-volume low-pressure cylindrical-shaped cuff (Shiley™ Disposable Inner Cannula Tracheostomy Tube [DCT]) with respect to applied tracheal wall pressure, air and fluid sealing efficacy, and insertion force.
Methods
Three sizes of tracheostomy tubes with the two cuff types were placed in appropriately sized tracheal models and lateral wall pressure was measured via pressure-sensing elements on the inner surface. Fluid sealing performance was assessed by inflating the cuffs within the tracheal models (25 cmH2O), instilling water above the cuff, and measuring fluid leakage past the cuff. To measure air leak, tubes were attached to a test lung and ventilator, and leak was calculated by subtracting the average exhaled tidal volume from the average delivered tidal volume. A tensile test machine was used to measure insertion force for each tube with the cuff deflated to simulate clinical insertion through a stoma site.
Results
The average pressure exerted on the lateral wall of the model trachea was lower for the taper-shaped cuff than for the cylindrical cuff under all test conditions (P<0.05). The taper-shaped cuff also demonstrated a more even, lower pressure distribution along the lateral wall of the model trachea. The average air and fluid seal performance with the taper-shaped cuff was significantly improved, when compared to the cylindrical-shaped cuff, for each tube size tested (P<0.05). The insertion force for the taper-shaped cuff was ~40% less than that for the cylindrical-shaped cuff.
Conclusion
In a model trachea, the Shiley™ Flexible Adult tracheostomy tube with TaperGuard™ cuff, when compared to the Shiley™ Disposable Inner Cannula Tracheostomy tube with cylindrical cuff, exerted a lower average lateral wall pressure and a more evenly distributed pressure. In addition, it provided more effective fluid and air seals and required less force to insert.
Introduction
High-volume low-pressure (HVLP) cuffs, on both endotracheal and tracheostomy tubes, have been the predominant cuff type used to create a seal between the tube shaft and the tracheal wall of patients for many years. The primary feature of a HVLP cuff is a large diameter and a large residual volume such that the cuff resting diameter is larger than the patient’s tracheal diameter. The intra-cuff pressure of an un-stretched HVLP cuff correlates closely with the tracheal wall pressure, which is not the case for low-volume cuffs.Citation1,Citation2 In order to ensure that the wall of the cuff is not stretched during use, the cuff resting diameter must be greater than the tracheal diameter. Another important characteristic of HVLP cuffs is the thin compliant wall material that, when inflated, adapts and conforms easily to the topography of the trachea wall. This cuff technology was developed several decades ago and in vivo experiments as well as clinical studies have demonstrated the effectiveness of HVLP cuffs at creating low-pressure seals in the trachea.Citation3 Research has also demonstrated that, when inflated, HVLP cuffs, which are traditionally cylindrical shaped, form longitudinal folds with micro-channels that allow for the direct passage of air and fluid past the cuff.Citation4–Citation6
While the intra-cuff pressure of un-stretched HVLP cuffs correlates closely with the pressure applied to the tracheal wall, patient complications can still occur when excessive pressure is exerted on the tracheal wall that may damage the tracheal mucosa. These complications can be attributed to over inflation of the cuff or, as demonstrated in this study, to poor cuff design and the use of excessively rigid cuff materials. In theory, a HVLP cuff constructed of an infinitely flexible material will conform perfectly to the wall of the trachea, creating pressure on the tracheal wall that equals the intra-cuff pressure. In practice, however, cuff material is not infinitely flexible and, therefore, there will be areas of contact and noncontact between the wall of the trachea and the cuff because the intra-cuff pressure is not sufficient to overcome the rigidity of the cuff. This intermittent contact will create areas of higher and lower contact pressure.Citation7 Traditional tracheostomy tubes, including the Shiley™ Disposable Inner Cannula tracheostomy (DCT) tube, have a cylindrical-shaped HVLP cuff ().
Figure 1 (A) Shiley™ Disposable Cannula tracheostomy (DCT) tube with cylindrical cuff (left) and Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff (right). (B) Shiley™ DCT tube cylindrical cuff (left) and TaperGuard™ cuff (right).
Significant advancements have been made in cuff design to overcome deficiencies in the performance of traditional HVLP cuffs. The Shiley™ Flexible Adult tracheostomy tube has a taper-shaped TaperGuard™ cuff which is constructed from a thinner, more compliant, and lower friction material than that used for the predecessor Shiley™ tracheostomy tube line, with a geometry designed to seal more effectively. A lower friction surface may also promote a more effective seal because friction between the cuff and tracheal wall might lead to the formation of folds in the cuff.Citation7
Studies of the TaperGuard™ cuff in endotracheal tubes have demonstrated that the smaller-volume TaperGuard™ cuff, with its reduced tracheal contract area, can effectively seal the trachea.Citation8,Citation9 However, assessment of the pressures at which this effective seal is achieved in tracheostomy tubes has not been undertaken. In addition, comparison between tracheostomy tubes having the DCT tube cylindrical-shaped and TaperGuard™ taper-shaped cuffs has not been made. Therefore, bench tests were performed to compare the new Shiley™ Flexible Adult tracheostomy tube with the TaperGuard™ cuff to the Shiley™ DCT tube with cylindrical cuff with respect to lateral wall pressure, air seal performance, fluid seal performance, and insertion force.
Methods
Lateral wall assessment
The lateral wall pressure test was performed to compare the average pressure exerted by each cuff type at its point of contact with the lateral wall of a model trachea. In addition, the pressure profile exerted by each cuff type against the tracheal model wall was also examined.
The smallest (6.5 mm), midrange (7.5 mm), and largest (10.0 mm) internal-diameter (ID) models of the adult tracheostomy tubes were tested to ensure that the extreme, as well as the most commonly used, sizes were included. The Shiley™ DCT tubes, which use the Jackson sizing system, were matched to the Shiley™ Flexible tubes which use the International Organization for Standardization sizing system. lists the dimensions of the tubes and the sizes of the model tracheas in which they were tested.
Table 1 Dimensions of tracheostomy tubes and model tracheas
Polycarbonate model tracheas with pressure sensors mounted on the inner surface were used. Three model tracheas were constructed for use with each tube size having internal diameters of 18.0 mm, 20.5 mm, and 22.5 mm. The pressure-sensing strips mounted on the model tracheas contained multiple individual elements that generated an electrical response in proportion to the pressure applied to that element. This pressure-mapping system contained the electronic hardware necessary to amplify the signal from the array which was then transmitted to a computer for further processing and analysis by Chameleon TVR™ software version 1.6.2.0 (Pressure Profile Systems, Los Angeles, CA, USA). Each tube was inserted into the corresponding model trachea and the average pressure was measured at contact points between the cuff and model trachea along the cuff profile. The tracheal wall pressure was measured for each size tracheostomy tube as cuff inflation was increased in a stepwise fashion from 0 to 10, 20, and 30 cmH2O pressure. Each test was performed on ten samples of each product type. The test method used a rigid model trachea which did not expand, allowing the pressure applied by the cuff to be captured without variability in the rate and degree to which a flexible model may respond to the force applied, thus adding noise into the measurement system. In addition, current “state of the art” low-pressure sensors do not expand, therefore, it was not practical to perform this test using a soft, flexible model trachea.
The average pressure exerted by each cuff type was quantified and the pressure distribution was qualitatively assessed and graphically represented using a high-resolution pressure-sensing array. This assessment was performed on the 7.5 mm Shiley™ Flexible tracheostomy tube, with the TaperGuard™ cuff and a size 6 Shiley™ DCT tube with cylindrical cuff. This design enabled the examination of the smaller contact band of the taper-shaped cuff compared to the larger contact area of the HVLP cuff. All experiments were conducted according to an internally created cuff-pressure-mapping protocol.
Air seal assessment
The purpose of this test was to determine the efficacy of the tracheostomy tube cuffs at creating a seal with the trachea to prevent air leakage past the cuff to the atmosphere. Tracheostomy tubes were connected to a ventilator via a breathing circuit to simulate the cyclic air flow applied to the cuff during routine use. Tracheostomy tubes of each size were placed into a rigid acrylic tracheal model attached to a Dual Adult Test Lung test lung (Michigan Instruments, Grand Rapids, MI, USA). The tracheostomy tube cuff was inflated and the tube connected to a Puritan Bennett 760 ventilator (Covidien, Carlsbad, CA, USA) via a breathing circuit. Ventilator settings were as follows: pressure control, inspiratory pressure 15 cmH2O; respiratory rate, 20 breaths per minute; inspiratory to expiratory time ratio, 1:2; rise time, 70 ms; positive end-expiratory pressure, 5 cmH2O; sensitivity, 15.2 L/min; and oxygen, 21%. The cuff was inflated to 25 cmH2O via a cuff inflation system attached to a digital pressure manometer which allowed for continuous cuff pressure monitoring and adjustment. The compliance of the test lung was adjusted to obtain an exhaled tidal volume of 330–338 mL for 6.5 mm tubes and 495–505 mL for other tube sizes. Tidal volume ranges were based on the average predicted body weight of the patients in which tubes are used. It is recommended that adult females and males should receive, at least initially, tracheostomy tubes with outer diameters of 10 mm and 11 mm, respectively.Citation10 Therefore, a 55 kg female would receive a 6.5 mm tube and, when ventilated at 6–8 mL/kg, have an expected tidal volume of ~330 mL (eg, a 75 kg male ventilated at 6–8 mL/kg would have a tidal volume of ~500 mL). Thirty samples of each tube type were tested. The system was stabilized for 60 seconds, after which time five breaths were recorded. The average exhaled volume was subtracted from the average delivered volume as measured by the ventilator. The delta between average exhaled volume and average delivered volume is the loss in air past the cuff.
Fluid seal assessment
The purpose of this test was to determine the efficacy of the tracheostomy tube cuffs to create a seal with the trachea to prevent the passage of fluid past the cuff. A tracheostomy tube was inserted into an acrylic trachea and the cuff inflated to 25 cmH2O while the tracheal model was maintained in a 37°C–39°C water bath for 15–30 minutes. A distilled water reservoir at 37°C–39°C was maintained 2.0–2.4 cm above the proximal cuff via a siphon tube for 10 minutes. Any water able to leak past the cuff was collected in a beaker under the model trachea. The weight of the water was used to calculate the leak.
Ease of insertion assessment
The insertion-force bench test assessed the maximum force required to insert a deflated cuffed tracheostomy tube through an artificial stoma opening. The artificial stoma consisted of a synthetic latex sheet 0.49 mm thick with an incision 30 mm long. The sheet was held taut within a support fixture. An Instron® Tensile Test Machine (Instron®, Norwood, MA, USA) was used to measure and record the insertion force.
Statistical analysis
Two-sample t-tests were performed to detect differences between cuff types in terms of lateral wall pressure, fluid and air leak, and mean insertion force for each tube size. Data are summarized as mean and standard deviation.
Results
Lateral wall pressure
The relationship between the intra-cuff pressure and the pressure applied by the cuff to the model trachea wall is shown for each tracheostomy tube size in .
Figure 2 Mean pressure exerted on tracheal wall plotted against intra-cuff pressure. (A) Small tube size, (B) midrange tube size, (C) large tube size.
Abbreviations: DCT, Disposable Cannula tracheostomy; TG, TaperGuard™.
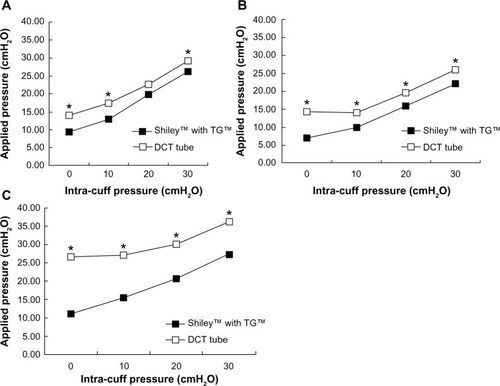
For each tube size, the average pressure exerted on the lateral wall of the model trachea was lower for the Shiley™ Flexible tube with TaperGuard™ cuff than for the Shiley™ DCT tube with the cylindrical-shaped cuff. As tube size increased, so did the difference between the cuff types in terms of pressure applied to the tracheal wall, with applied pressure differences reaching ~10 cmH2O at each intra-cuff pressure for the largest tracheostomy tube sizes ().
Graphical representations of the pressure array exerted by the 7.5 mm Shiley™ Flexible tracheostomy tube with the TaperGuard™ cuff and a size 6 Shiley™ DCT with cylindrical cuff on the lateral wall of the model trachea are shown in , with the cuffs inflated to increasing intra-cuff pressures from 0 to 30 cmH2O. As stated earlier, it is widely accepted that, for un-stretched HVLP cuffs, the intra-cuff pressure will correlate closely with tracheal wall pressure. This was found to be true when the quantitative analysis was performed, as shown in . However, deviations from the expected 1:1 relationship between the intra-cuff pressure and the pressure applied to the tracheal wall are also apparent. The graphical representation in demonstrates that, given that materials are not infinitely flexible, there will be points of contact and noncontact between the cuff and the model trachea.
Figure 3 Pressure exerted on the model trachea with increasing amounts of intra-cuff pressure ([A] 0 cmH2O; [B] 20 cmH2O; [C] 30 cmH2O) applied to the Shiley™ Disposable Cannula tracheostomy (DCT) tube cuff (left) and the Shiley™ Flexible TaperGuard™ (TG) cuff (right).
![Figure 3 Pressure exerted on the model trachea with increasing amounts of intra-cuff pressure ([A] 0 cmH2O; [B] 20 cmH2O; [C] 30 cmH2O) applied to the Shiley™ Disposable Cannula tracheostomy (DCT) tube cuff (left) and the Shiley™ Flexible TaperGuard™ (TG) cuff (right).](/cms/asset/1c305b12-5a68-4f40-b202-892f142d2cfc/dmde_a_76960_f0003_c.jpg)
Air seal
The average volume of air leak past the two cuff types is listed in . Results demonstrated that the air leak was significantly lower for the Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff than for the Shiley™ DCT tube with cylindrical cuff when both cuffs were inflated to an intra-cuff pressure of 25 cmH2O.
Table 2 Cuff performance data
Fluid seal
The average volume of water leak past each cuff type is shown in . Results demonstrate that the Shiley™ Flexible tube with TaperGuard™ cuff had significantly less fluid leakage past the cuff than the Shiley™ DCT tube with cylindrical cuff for all tube sizes tested.
Insertion force
The average insertion force for each tube type is listed in . The force required to insert the Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff was 40% less than that needed to insert the Shiley™ DCT tube with cylindrical cuff. Insertion force was significantly lower with the TaperGuard™ cuff for each tube size tested.
Discussion
The cuff on a tracheostomy tube seals the tracheal lumen, permits the maintenance of airway pressure and tidal volume during mechanical ventilation, and guards against aspiration. A known hazard of the inflated cuff is tracheal damage caused by pressure transmitted to the tracheal wall that exceeds mucosal perfusion pressure.Citation11 Current standard practice includes the maintenance of intra-cuff pressure between 20 and 30 cmH2O, which will seal the airway to prevent aspiration while minimizing damage to the trachea. The intra-cuff pressure range used in the study was 0 to 30 cmH2O, which is considered clinically relevant.Citation12
In the current study, for each tube size tested, the average pressure exerted on the lateral wall of the model trachea was lower for the Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff than for the Shiley™ DCT tube with the cylindrical cuff. This is likely due to the DCT cuff being larger in diameter than the tracheal model, which is one of the core design features universal to all HVLP cuffs. This oversized cuff, when constrained within the patient’s trachea, will develop folds and channels. These folds and channels create localized contact points. The TaperGuard™ cuff has been designed through its geometry, materials, diameter, and wall thickness to minimize folds and channels while providing more flexibility to the cuff material. This combination allows for increased surface-to-surface contact between the cuff and the wall of the trachea, resulting in a more even distribution of forces and a reduction in pressure spikes. The high-pressure spikes elevate the average lateral wall pressure values for the cylindrical-shaped cuffs.
Intra-cuff pressure did not equal lateral wall pressure at every contact point and, moreover, the measured lateral wall pressure was higher than the intra-cuff pressure at many points of contact. At an intra-cuff pressure of zero, pressure was transmitted to the lateral wall of the model trachea because the deflated cuff profile could be bigger than the diameter of the model trachea. The TaperGuard™ cuff has a lower cuff profile when deflated than the cylindrical-shaped cuff. When HVLP cuffs are inflated to atmospheric pressure, the cuff diameter can be 120%–150% of the internal tracheal diameter.Citation13 Even when an HVLP cuff is deflated to a sub-atmospheric pressure and the cuff fully deflates, the surface topography of the cuff may be such that portions of the cuff may still make contact with the tracheal wall and transmit pressure, as was observed during testing.
The pressure distribution of the cuff on the lateral tracheal wall was not uniform or homogeneous for either cuff type, as depicted by the intermittent appearance of red- and green-tipped spikes that indicate higher pressure points (). The TaperGuard™ cuff has a more homogeneous and lower pressure distribution than the DCT cuff.
As in this study, Li Bassi et alCitation7 also observed points at which the pressure exerted on the lateral tracheal wall was higher than the intra-cuff pressure and suggested that this may be due to the formation of folds pressing on the trachea. They proposed that the portion of the tracheal wall that abuts the cuff fold may be exposed to higher transmitted pressure due to tangential force exerted on a smaller contact area.Citation7 This is common to all HVLP cuffs and is the result of the device design such that the cuff inflated to atmospheric pressure has a larger diameter than the internal tracheal diameter.
The TaperGuard™ cuff provides a more effective seal of the tracheaCitation9 with a smaller tracheal contact area.Citation7 The TaperGuard™ cuff forms a small band where the inflated cuff has a diameter equal to the trachea at a point between the oversized proximal and undersized distal portions of the cuff. This band of contact reduces the number of micro-channels and the associated passage of air and fluid leaking past the cuff.Citation4 The leakage of secretions or gastric contents through these channels and into the lungs increases the risk for complications including ventilator-associated pneumonia.Citation14 An incomplete seal resulting in gas leakage can result in a failure to maintain positive end-expiratory pressure and hypoventilation.Citation15 In a bench study using a model of the trachea, Madjdpour et alCitation4 demonstrated that an endotracheal tube with a taper-shaped cuff made from polyvinyl chloride (PVC) significantly improved air sealing compared to standard cylindrical-shaped cuffs. The cylindrical-shaped PVC cuff did not effectively seal the trachea, even at the high end of clinically accepted intra-cuff pressure (ie, 30 cmH2O), when measured by both sevoflurane concentration passing around the cuff and the ratio of expired tidal volume to inspired tidal volume. In a clinical study, when the two cuff types were compared during short-term use on surgical patients, the taper-shaped cuff demonstrated better protection against aspiration.Citation16
Changing the patient’s cuffed tracheostomy tube may cause irritation to the stoma due to the bulk of the deflated cuff material. The Shiley™ Flexible tracheostomy tube with its taper-shaped cuff, made from thinner material and having a smaller volume than the Shiley™ DCT tube cuff, requires less force when inserted into the simulated patient’s stoma () and, therefore, may cause less trauma to the patient.
Another enhancement to the Shiley™ Flexible tracheostomy tube is a transparent, soft PVC flange designed to conform to the patient’s clavicle. The central portion of the flange has symmetrical windows and is offset to help reduce contact with the patient’s skin. These changes in flange characteristics may improve the comfort of patients with tracheostomy tubes, many of whom have these devices in place for extended periods of time. The flange of the Shiley™ Flexible tracheostomy tube is constructed from a new non-di-(2-ethylhexyl)-phthalate (DEHP) PVC formulation. PVC is softened through the use of plasticizers of which DEHP is the most commonly used in tracheostomy tubes and many other medical devices. Health concerns related to the release of DEHP into biological fluids and tissues have been raised by the US Food and Drug Administration;Citation17 the Ministry of Health, Labor and Welfare, Health, Canada;Citation18 and the European Commission.Citation19 As a precaution, DEHP has been removed from the Shiley™ Flexible tracheostomy tube and replaced with a citrate and dioctyl terephthalate-based plasticizer. Citric acid, a metabolite of plants and animals, and dioctyl terephthalate are widely used in food packaging, medical products, soft toys for children, and cosmetics and are not known to have any ill-effects on human health.Citation20
Study limitations
The trachea model used in the bench testing was a rigid circular tube, while an actual trachea is non-circular and somewhat distensible. Therefore, the study conditions do not necessarily reflect what might happen in a clinical situation. In addition, the products were tested at approximately room temperature, while, when in clinical use, tracheostomy tubes are used at body temperature, which could impact device performance.
Conclusion
The Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff offers several improvements compared to the Shiley™ DCT tube with a cylindrical-shaped cuff, including a tapered shape and thinner cuff. The results of this study demonstrate that, in a model trachea, the Shiley™ Flexible tracheostomy tube with TaperGuard™ cuff, when compared to the Shiley™ DCT tube with cylindrical cuff, exerted a lower average lateral wall pressure and a more evenly distributed pressure. In addition, it provided more effective fluid and air seals and required less force to insert.
Disclosure
Frances Haury, Seamus Maguire, and Korinne Jew all work for Covidien, the manufacturer of the Shiley™ tracheostomy tubes tested and discussed in this paper. The authors report no other conflicts of interest in this work.
References
- BernetVDullenkopfACannizzaroVStutzKWeissMAn in vitro study of the compliance of paediatric tracheal tube cuffs and tracheal wall pressureAnaesthesia2006611097898316978314
- SpiegelJEEndotracheal tube cuffs: design and functionAnesthesiology News20103685158
- CarrollRGMcGinnisGEGrenvikAPerformance characteristics of tracheal cuffsInt Anesthesiol Clin19741231111414609916
- MadjdpourCMauchJDaveMHSpielmannNWeissMComparison of air-sealing characteristics of tapered- vs cylindrical-shaped high-volume, low-pressure tube cuffsActa Anaesthesiol Scand201156223023522091784
- PavlinEGVanNimweganDHornbeinTFFailure of a high-compliance low-pressure cuff to prevent aspirationAnesthesiology19754222162191115375
- YoungPJRollinsonMDownwardGHendersonSLeakage of fluid past the tracheal tube cuff in a benchtop modelBr J Anaesth19977855575629175972
- Li BassiGRanzaniOTMartiJDAn in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressuresCrit Care Med201341251852623263575
- ShiotsukaJLeforATSanuiMNagataOHoriguchiASasabuchiYA quantitative evaluation of fluid leakage around a polyvinyl chloride tapered endotracheal tube cuff using an in-vitro modelHSR Proc Intensive Care Cardiovasc Anesth20124316917523439857
- ZanellaAScaravilliVIsgròSFluid leakage across tracheal tube cuff, effect of different cuff material, shape, and positive expiratory pressure: a bench-top studyIntensive Care Med201137234334721152894
- HessDRTracheostomy tubes and related appliancesRespir Care200550449751015807912
- SeegobinRDvan HasseltGLEndotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffsBr Med J (Clin Res Ed)19842886422965968
- BhardwajNPediatric cuffed endotracheal tubesJ Anaesthesiol Clin Pharmacol20132921131823492803
- DullenkopfAGerberAWeissMFluid leakage past tracheal tube cuffs: evaluation of the new Microcuff endotracheal tubeIntensive Care Med200329101849185312923620
- MethenyNAClouseREChangYHStewartBJOliverDAKollefMHTracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factorsCrit Care Med20063441007101516484901
- TobinMJJubranALaghiFFighting the ventilatorTobinMJPrinciples and Practice of Mechanical Ventilation3rd edChicago, ILMcGraw Hill201312371258
- D’HaeseJDe KeukeleireTRemoryIVan RompaeyKUmbrainVPoelaertJAssessment of intraoperative microaspiration: does a modified cuff shape improve sealing?Acta Anaesthesiol Scand201357787388023556486
- US Food and Drug Administration (FDA) Center for Devices and Radiological HealthSafety Assessment of Di(2-ethylhexyl)phthalate (DEHP) Released from PVC Medical DevicesRockville, MDFDA Center for Devices and Radiological Health2001 Available from: http://www.fda.gov/downloads/MedicalDevices/…/UCM080457.pdfAccessed March 5, 2015
- di(2-ethylhexyl)phthalate (DEHP): Health Canada Expert Advisory Panel on DEHP in medical devices [web page on the Internet]. Final report. mindfully.org2002 Available from: http://www.mindfully.org/Plastic/DEHP-Health-Canada11jan02.htmAccessed February 9, 2015
- European CommissionScientific Committee on Medicinal Products and Medical Devices (SCMPMD)Opinion on Medical Devices Containing DEHP Plasticised PVC; Neonates and Other Groups Possibly at Risk from DEHP ToxicityEuropean Commission SCMPMD2002Brussels, Belgium
- BabichMAVersar Inc., Syracuse Research CorporationReview of Exposure and Toxicity Data for Phthalate SubstitutesBethesda, MDUS Consumer Product Safety Commission2010 Available from: http://www.cpsc.gov/PageFiles/126546/phthalsub.pdfAccessed March 5, 2015
