Abstract
Purpose
Occasional meal-related bloating, gassiness, and discomfort can affect the quality of life of healthy individuals without a diagnosis of gastrointestinal conditions. This study explored the efficacy and tolerability of a digestive enzyme and herbal dietary supplement in alleviating these symptoms.
Subjects and Methods
Adults aged 18–45 years (n = 25) with daily post-meal bloating but no pre-existing condition were enrolled. After obtaining informed consent, the fasted participants were randomized to consume either placebo or test product and consume a test meal. Waist circumference was measured at baseline and at 30- and 90-minutes post meal. Participants rated the severity of bloating, distension, gas, and indigestion on a 4-point scale. Following a 1-week washout, the participants repeated the procedure using the alternate product.
Results
Participants (n = 20) experienced 58% less abdominal distension at 30 minutes (0.93 cm vs 1.50 cm, P = 0.04) and 68% less at 90 minutes with the test product compared to the placebo (0.94 cm vs 2.12 cm, P = 0.007). Overall, 80% of participants reduced distension with the test product. No significant differences were noted between questions assessing momentary bloating, distended stomach, gas, or indigestion. There was a trend to agree that the test product was effective for overall feelings of bloating, stomach discomfort and distension, and indigestion, with 65% of participants reporting less stomach discomfort and 55% reporting feeling less gassy than normal with the test product. No product-related adverse events or discomfort were reported.
Conclusion
Digestive enzymes and herbal dietary supplements effectively reduced post-meal abdominal distension in healthy subjects, without adverse effects.
Introduction
Gastrointestinal symptoms, such as gas, discomfort, bloating, and distention, are not only ailments in individuals diagnosed with functional gastrointestinal diseases or syndromes (ie, irritable bowel syndrome). Bloating and abdominal distension in particular are widely reported, ranging from 16%,Citation1 14%,Citation2 and 8.9% to 19% (distension and bloating, respectively)Citation3 in samples representative of the general US population.
The etiology of these GI complaints is complicated, with multiple contributing factors that have not been fully elucidated. Some report bloating and distension with flatulence or burping, some not. While no unified pathophysiologic mechanism can be applied to all subjects, some etiologies include increased intraluminal content (from excess gas, air, water, fecal material), alterations in gut microbiota, abnormal gastrointestinal motility, diet or food intolerance, pelvic floor dysfunction, underlying pathologies, visceral hypersensitivity or a paradoxical abdominophrenic response called abdominophrenic dyssnergia, which is characterized by diaphragm contractions and descent and relaxation of anterior abdominal wall muscles, leading to abdominal distension, in contrast to the normal response to increased intraluminal gas.Citation4–7 Studies of subjects presenting with bloating have documented both increased intestinal gas accumulation and abnormal gas transit, with some patients feeling bloated with actual distension, while others have no distension.Citation8
It has been reported that 50% of subjects who reported bloating (but no IBD diagnosis) had a reduction in their daily activities.Citation1 The widespread prevalence and impact on quality of life may have contributed to the expansion of dietary supplement products marketed to reduce bloating. The digestive health market, which includes digestive enzymes, probiotics, herbs, and others, for the United States alone has been estimated to be as high as $99 billion in 2023.Citation9
For meal-related GI distress, various individual and blends of digestive enzymes have shown efficacy in reducing intestinal gas produced by fermentation of undigested food. For example, the enzyme alpha-galactosidase acts by breaking down nonabsorbable oligosaccharides in the gastrointestinal tract and is effective in reducing breath hydrogen and preventing gas formation in the colon.Citation10,Citation11 It has been shown to be an effective and well-tolerated treatment for gas-related symptoms in pediatric patients taking consistently,Citation12 healthy adults with a single meal of beans,Citation11 and adults with IBS who took with a low FODMAP but high galactooligosaccharide diet.Citation13 Gluten-digesting enzymes, including peptidase and proteases, reduced symptoms including bloating and discomfort in a sample of individuals with nonceliac gluten sensitivity.Citation14 When taken before a fatty meal, lipase supplementation reduced feelings of stomach fullness.Citation15
This study aimed to evaluate the effectiveness and tolerability of a dietary supplement containing a blend of 18 digestive enzymes and three herbal ingredients to relieve meal-related distension and bloating in healthy adults.
Materials and Methods
Design
This was a single-center, double-blind, randomized, placebo-controlled, crossover clinical study conducted in an ambulatory setting between July and December 2022.
This study was approved by the Argus IRB and was registered at Clinicaltrials.gov (NCT05520411) and conducted in accordance with the principle of the Declaration of Helsinki.
Subjects
Eligible participants were recruited from Citruslab’s proprietary app-based network of volunteers residing in the Los Angeles metro area who responded to an advertisement for the study on the app. The study subjects were male or females aged 18–45 years with self-reported bloating or abdominal distension at least once per day after a meal. Exclusion criteria included those who were pregnant or breastfeeding, currently taking any prescribed medication to treat gut diseases, fluid balance, and currently suffering from any preexisting conditions including diagnosis of IBS, chronic constipation, gastroesophageal reflux disease (GERD), Crohn’s disease, ulcerative colitis, celiac disease, liver disease, kidney disease, heart failure, and lactose allergy or intolerance. Additional exclusion was for behavioral or health habits that may lead to or exacerbate bloating, including exclusion of current smokers, those with any food allergies, wearers of dentures, use of an antibiotic in the previous 3 months, taking an iron supplement of >65 mg in the last week, and any condition that would prevent them from consuming the test products or test meal.
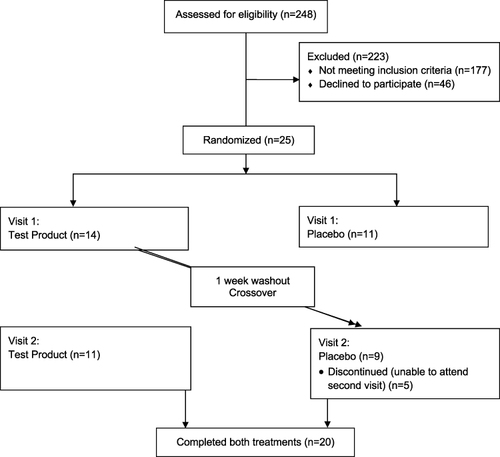
Participants completed a baseline prescreen survey, and 25 subjects were recruited and consented, with 5 lost to follow-up between visits 1 and 2 ().
Intervention
Participants visited the study site on two separate occasions in a fasted state and were randomized via computerized randomization to receive either placebo or test supplement at the first visit. Surveys and waist circumference were measured at baseline and 30 and 90 minutes after consuming the test meal. The participants were instructed to consume the test product or placebo immediately before consuming the test meal. Both participants and staff implementing the intervention were blinded to patient allocation.
The test meal consisted of two slices of a large cheese pizza from a local restaurant.
The test product consisted of a commercially available dietary supplement containing a proprietary blend of 18 digestive enzymes (a blend of proteases, lactase, lipase, amylases, cellulases) and herbs (ginger root, fennel seed, peppermint leaf). The placebo was a similar-looking capsule of maltodextrin starch.
Assessment
Waist circumference was performed by a trained laboratory staff member and measured with a measuring tape flat against the skin at the level of the umbilicus parallel to the ground around the subject. The subjects were asked to breathe normally, and measurements were taken at exhalation. The measurements were repeated twice, and the average value in centimeters was reported. Difference scores were calculated with positive values indicating a decrease in waist circumference from baseline to the measurement point and negative values indicating an increase in waist circumference.
Survey questionnaires asked participants to rate the momentary severity of bloating, distended stomach, gas, and indigestion on a 4-point scale (severe, moderate, mild, or absent). At the 90-minute time point, the participants also rated their agreement with statements using a Likert-type scale.
Following a washout period of at least 1 week, participants returned and completed the study procedures with the product they had not yet used (either test product or placebo) (see ).
Both subjects and investigators administering the procedures were blinded to the study allocation.
Statistical Methods
Descriptive statistics, including mean and standard deviation for continuous and categorical variables, were summarized. Analyses were completed for the intent-to-treat sample. Independent sample t-tests were used to examine differences in scores between the placebo and test product groups. Data were examined for normality distribution with a visual inspection of histograms and Shapiro-Wilk test. To normalize the abdominal distension measures (the primary outcome variable), difference scores were created for comparisons across the groups. Paired sample t-tests were conducted to examine the differences in the scores calculated at the same time points during the two visits. The survey responses were assessed using dependent-sample t-tests on paired samples. Statistical significance was set at 0.05, and JASP software version 0.16.2 was used for all analyses. Power calculation was not conducted given the pilot nature of this trial to identify short-term effects and effects size.
Results
Twenty participants completed both visits. Five participants dropped out due to scheduling conflicts to attend the follow-up visit. One participant outlier was removed from the waist circumference analysis because the difference scores were greater than four standard deviations of what would be expected.
Participants experienced 58% less abdominal distension as assessed by the waist circumference measure 30 minutes after taking the test product with a meal compared to the placebo (0.93 cm vs 1.50 cm difference) and 68% less than 90 minutes after taking the test product with a meal compared to the placebo (0.94 cm vs 2.12 cm difference) with a meal. The difference was greater at 90 minutes with a statistically significant difference at both 30 minutes post-meal (P = 0.04) and 90 minutes post-meal (P = 0.007 (see ). Overall, 80% of the participants had less distension with the test product than with placebo.
Table 1 Difference Score Results from Waist Circumference Measures
No significant differences were noted between the mean Likert-type responses to momentary bloating, distended stomach, gas, and indigestion. However, there was a trend for less severe bloating and indigestion at both 30- and 90-minutes post meal for the test product compared to the placebo (see ).
Table 2 Means and Standard Deviations of Common Gastrointestinal Issues After the Test Meal
Overall, participants reported that they preferred to continue taking the test product versus the placebo (P = 0.04) and felt that it helped them digest better (P = 0.07). There was a trend to strongly agree that the test product was effective for overall feelings of bloat, stomach discomfort, distension, and indigestion, with 65% of participants reporting less stomach discomfort than normal with the test product and 55% reporting feeling less gassy than normal with the test product (see ).
Table 3 Descriptive Data from the Likert Scale Questions
No adverse events or discomfort associated with the product were reported by participants.
Discussion
This study evaluated the effects of a test product and a placebo administered with a test meal on abdominal distension and subjective assessments of related symptoms. Overall, the test product was effective at reducing the severity of abdominal distension compared to the placebo. Specifically, participants experienced significantly less distension at 30 minutes, with the test product condition (−0.63 cm) performing better than the placebo condition (−1.50 cm), with continued effectiveness at 90 minutes after taking the test product (−0.67 cm) compared to the placebo (−2.12 cm). These findings were statistically significant and revealed noteworthy changes in reduced distension when the test product was used.
When asked to rate the severity of their bloating, distention, gas, and indigestion at 30 and 90 minutes after their meal, participants reported less severe bloating (at 30- and 90-minutes post-meal), less severe distention at 30 minutes post-meal, less gas at 90 minutes post-meal, and less indigestion (at 30- and 90-minutes post-meal). However, no significant differences were found between the test conditions for any self-report measures. As this study examined a test product with a single meal occasion, it is possible that extended use would result in a compounded effect and more substantial long-term improvement.
Practice guidance for the management of bloating and indigestion includes a variety of agents including antispasmodics, secretagogues, dietary modification, probiotics, prokinetic agents, neuromodulators, biofeedback, and complementary and alternative approaches.Citation5 As an alternative approach, digestive enzyme supplementation appears to be effective in reducing intestinal gas and bloating symptoms via breaking down of various constituents that may be nondigestible with supplementation of alpha-galactosidase (and the commercially available product “Beano”) to treat oligosaccharide intolerance being well-studied and shown to reduce flatulenceCitation10,Citation11 A commercially available multi-enzyme proprietary blend (protease, amylase, lipase, cellulase and lactase) Poolzyme® Multi (Giellepi S.p.A., Milan, Italy) was evaluated in subjects with functional dyspepsia and shown to improve quality of life and sleep quality, but the authors did not disclose any specific benefits to bloating or abdominal distention symptoms.Citation16 The efficacy of a dual layer tablet Combizym (Daiichi-Sankyo Europe, Germany) that contains an extract of Aspergillus oryzae with cellulase, protease, amylase and pancreatin has been evaluated in several clinical trials, including one with 151 patients that reported after 2 weeks post meal three times a day supplementation there was a significant improvement in abdominal distension, belching, and abdominal pain.Citation17 Various digestive enzymes are routinely prescribed or recommended as part of the management of digestive and malabsorption disorders, including lactose intolerance and pancreatic insufficiency.Citation18 A 2018 systematic review explored enzyme therapy treatment in “patients who may benefit from enzyme therapy”, including “those with meal-associated dyspepsia (eg, abdominal distension, belching, abdominal pain, abdominal distention and epigastric burning)” and concluded that the data overall support benefits of enzyme therapy for post-prandial complaints including bloating and abdominal distension.Citation19
In addition to the blend of digestive enzymes, the herbal ingredients fennel seed, ginger root, and peppermint leaf may contribute to the beneficial effects of distension and discomfort.
The efficacy of herbal medicines for the treatment of functional gastrointestinal disorders was subjected to a review and meta-analysis by Tan et al in 2020.Citation20 Subgroup analysis revealed that herbal medicines were superior to placebo in symptom alleviation for IBS, functional diarrhea, and constipation. In particular, peppermint oil has been shown to provide beneficial physiological effects, including normalization of GI motility, reduced hypersensitivity, reduced inflammation, and suppression of excess gastric secretions).Citation21
The results show that the test production inclusion of a blend of digestive enzymes that break down all macronutrients (and lactose), as well as the herbal blend, may be an effective aid for reducing distention and mild improvement in GI symptoms. The comprehensive nature of the supplement likely contributed to its effects. It is possible that a longer duration of evaluation after the test meal would result in a more pronounced effect on the severity ratings and distension. There are limited studies on the effect of dietary supplements on improving bothersome GI symptoms in healthy subjects who suffer from occasional distress without a clinical diagnosis. The within-subject design is a strength that removes possible intra-individual variations that may occur. While it may be considered a limitation, the single-use design of the study does highlight potential benefits to single use in subjects who experience occasional distress without longer term supplementation. There may be cumulative benefits when used for longer duration or the effects may differ depending on the variability of normal food intake. Self-diagnosis of functional gastrointestinal distress is also a limitation given the subjective nature of the inclusion criteria. Future studies on the test product should evaluate any extended or cumulative effects using a larger sample size, as well as invitro digestion analyses to understand the extent of nutrient breakdown by the enzymes.
Conclusion
A dietary supplement including a blend of digestive enzymes and herbal ingredients was effective and well tolerated when provided with a meal, in reducing post-meal abdominal distension compared to a placebo. While not statistically significant, there was a trend to provide mild benefit to other gas-related symptoms better than a placebo in healthy adults suffering from occasional bloating and discomfort after meals.
Ethics Approval and Informed Consent
This study was approved by the Argus Institutional Review Board. Informed consent was obtained from all participants. This trial was registered at Clinicaltrials.gov (NCT05520411).
Disclosure
The author was an employee of HUM Nutrition, Inc. at the time this research was conducted.
Acknowledgments
Special appreciation to the team at Citruslabs for their contributions and collaborations. The abstract of this paper was presented at the American Society for Nutrition 2023 as a poster presentation. The poster’s abstract was published in July 2023 in Current Developments in Nutrition: [https://cdn.nutrition.org/article/S2475-2991(23)26022-9/fulltext] and was uploaded to Research Square as a preprint: [https://www.researchsquare.com/article/rs-3416887/v1].
Data Sharing Statement
Deidentified data are available upon written Email request to the author.
Additional information
Funding
References
- Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000;45(6):1166–1171. doi:10.1023/a:1005554103531
- Oh JE, Chey WD, Spiegel B. Abdominal bloating in the United States: results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023;21(9):2370–7.doi:10.1016/j.cgh.2022.10.031
- Jiang X, Locke GR, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57(6):756–763. doi:10.1136/gut.2007.142810
- Villoria A, Azpiroz F, Burri E, Cisternas D, Soldevilla A, Malagelada J-R. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Official J Am Coll Gastroenterol. 2011;106(5):815–819. doi:10.1038/ajg.2010.408
- Lacy BE, Cangemi D, Vazquez-Roque M. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021;19(2):219–231. e1. doi:10.1016/j.cgh.2020.03.056
- Mari A, Abu Backer F, Mahamid M, et al. Bloating and abdominal distension: clinical approach and management. Advan Therapy. 2019;36(5):1075–1084. doi:10.1007/s12325-019-00924-7
- Foley A, Burgell R, Barrett JS, Gibson PR. Management strategies for abdominal bloating and distension. Gastroenterol Hepatol. 2014;10(9):561–571.
- Azpiroz F. Intestinal gas dynamics: mechanisms and clinical relevance. Gut. 2005;54(7):893–895. doi:10.1136/gut.2004.048868
- Digestive health product market is estimated to be US$ 99.01 Billion by 2032 with a CAGR of 8.4% during the forecast period 2032 - By PMI. Available from: https://www.globenewswire.com/fr/news-release/2022/09/29/2525227/0/en/Digestive-Health-Product-Market-is-estimated-to-be-US-99-01-Billion-by-2032-with-A-CAGR-of-8-4-during-the-forecast-period-2032-By-PMI.html. Accessed January 10, 2023.
- Solomons N, Vasquez A, Grazioso C. Orally-ingested, microbial alpha-galactosidases produce effective in vivo, intraintestinal digestion of the Bean oligosaccharide, raffinose. Gastroenterology. 1991;100:A251.
- Di Stefano M, Miceli E, Gotti S, Missanelli A, Mazzocchi S, Corazza GR. The effect of oral α-galactosidase on intestinal gas production and gas-related symptoms. Dig Dis Sci. 2007;52(1):78–83. doi:10.1007/s10620-006-9296-9
- Di Nardo G, Oliva S, Ferrari F, et al. Efficacy and tolerability of α-galactosidase in treating gas-related symptoms in children: a randomized, double-blind, placebo controlled trial. BMC Gastroenterol. 2013;13(1):142. doi:10.1186/1471-230X-13-142
- Tuck CJ, Taylor KM, Gibson PR, Barrett JS, Muir JG. Increasing symptoms in irritable bowel symptoms with ingestion of galacto-oligosaccharides are mitigated by α-galactosidase treatment. Official J Am Coll Gastroenterol. 2018;113(1):124–134. doi:10.1038/ajg.2017.245
- Ido H, Matsubara H, Kuroda M, et al. Combination of gluten-digesting enzymes improved symptoms of non-celiac gluten sensitivity: a randomized single-blind, placebo-controlled crossover study. Clin Transl Gastroenterol. 2018;9(9):181. doi:10.1038/s41424-018-0052-1
- Levine ME, Koch SY, Koch KL. Lipase supplementation before a high-fat meal reduces perceptions of fullness in healthy subjects. Gut Liver. 2015;9(4):464–469. doi:10.5009/gnl14005
- Ullah H, Di Minno A, Piccinocchi R, et al. Efficacy of digestive enzyme supplementation in functional dyspepsia: a monocentric, randomized, double-blind, placebo-controlled, clinical trial. Biomed Pharmacother. 2023;169:115858. doi:10.1016/j.biopha.2023.115858
- Ran ZH, Yuan YZ, Li ZS, et al. The efficacy of Combizym in the treatment of Chinese patients with dyspepsia: a multicenter, randomized, placebo-controlled and cross-over study. J Digest Dis. 2009;10(1):41–48. doi:10.1111/j.1751-2980.2008.00361.x
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G. Digestive enzyme supplementation in gastrointestinal diseases. Current Drug Metabolism. 2016;17(2):187–193. doi:10.2174/138920021702160114150137
- Graham DY, Ketwaroo GA, Money ME, Opekun AR. Enzyme therapy for functional bowel disease‐like post‐prandial distress. J Digest Dis. 2018;19(11):650–656. doi:10.1111/1751-2980.12655
- Tan N, Gwee KA, Tack J, et al. Herbal medicine in the treatment of functional gastrointestinal disorders: a systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35(4):544–556. doi:10.1111/jgh.14905
- Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48(6):505–512. doi:10.1097/MCG.0b013e3182a88357