Abstract
We conducted an umbrella evaluation to quantitatively synthesize previous systematic reviews and meta-analyses, thereby collating evidence on the association between vitamin D and core symptoms in people with autism in anticipation of informing clinical vitamin D supplementation. Based on the pre-established protocol, we ended up with 9 studies. Based on rigorous analysis, we found that vitamin D deficiency early in life is a risk factor for the development of ASD and that vitamin D supplementation improves the core symptoms of ASD. Our study concludes that vitamin D supplementation is beneficial for individuals with autism, that vitamin D deficiency early in embryonic life increases the risk of ASD, and that our study supports the idea that prevention begins with vitamin D supplementation early in life. At the same time, we must recognize that our current conclusions support the benefits of vitamin D supplementation for children with ASD, but we are unable to determine the causal relationship, ie, how vitamin D works in ASD, and we need more basic research to explore the mechanisms involved.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterized by challenges in social communication and interaction, entailing two defining features: repetitive behaviors and restricted interests.Citation1 Individuals with ASD frequently present with behavioral challenges such as aggression, resistance to changes in routine, and deficits in conventional conversational skills. They also often suffer from social anxiety, attentional deficits, hyperactivity, sleep disruptions, and gastrointestinal disturbances.Citation2,Citation3 These complex challenges significantly impede their ability to attain educational achievements comparable to their peers and to develop the skills necessary for independent living.Citation4–6
Current epidemiological data indicate a significant increase in ASD prevalence, now affecting roughly 1 in 59 children.Citation7–9 This escalation in ASD cases has been linked to a complex interplay of genetic and environmental factors.Citation10–12 Studies have found that individuals with ASD possess lower levels of vitamin D compared to their typically developing counterparts.Citation13–15 Although the precise etiology of ASD remains elusive, both environmental and genetic contributors are acknowledged to influence its development.Citation16–18 Research suggests that genetic variations associated with nutrition and metabolism, including mitochondrial dysfunction and oxidative stress, are common among those with ASD.Citation19 Consequently, vitamin and mineral supplementation has been proposed as a means to modulate these underlying physiological disruptions.Citation19,Citation20
Vitamin D, an essential fat-soluble nutrient, also serves as a hormone precursor.Citation21 It is produced in the skin following ultraviolet light exposure and associates with vitamin D-binding protein for transport in the bloodstream. In the liver, the nutrient undergoes enzymatic conversion to 25-hydroxyvitamin D (1,25(OH)2D) by the enzyme 25-hydroxylase. In the kidneys by 1α-hydroxylase generates the biologically active form, 1.25-dihydroxyvitamin D (1,25(OH)2D),Citation22 which can modulate a variety of target genes through interaction with the vitamin D receptor (VDR), affecting brain function.Citation23,Citation24 While 25(OH)D is the predominant circulating form of vitamin D, both the VDR and 1α-hydroxylase are widely present in brain tissue.Citation25 Nevertheless, Vitamin D3 (VD3) is highly susceptible to environmental stressors, rendering it vulnerable to oxidation which diminishes its physiological advantages and functions.Citation26 It is characterized by limited water solubility and suboptimal oral bioavailability, the latter approximating 44.8%.Citation27 As a lipid-soluble compound, Vitamin D absorption primarily occurs in the small intestine, where its uptake is bolstered by both active and passive mechanisms associated with fatty acid and fat transport.Citation28 Given its affordability and favorable benefit-to-risk profile, Vitamin D supplementation has emerged as a particularly promising strategy for managing autism.
Vitamin D, recognized as a crucial neurosteroid hormone,Citation29,Citation30 is essential for neurodevelopment and modulates critical functions in the nervous system, including neuronal growth, synaptic transmission, and oxidative stress regulation.Citation31,Citation32 Numerous studies suggest an association between 25-hydroxyvitamin D (25(OH)D) levels and ASD.Citation33–36 Small-scale epidemiological studies indicate that children with ASD often have significantly lower 25(OH)D levels compared to neurotypical peers,Citation37–40 a finding supported by a retrospective review of 616 samples.Citation41 However, results from clinical controlled studies are mixed. A 2020 meta-analysis described that vitamin D supplementation may worsen hyperactivity symptoms in ASD,Citation42 while another meta-analysis from the same year found potential symptom improvements following vitamin D supplementation.Citation43–45 Additionally, early childhood vitamin D supplementation could reduce ASD risk in younger siblings.Citation46,Citation47 Rodent studies suggest that high-dose vitamin D may have protective and therapeutic effects, particularly as a preventative measure.Citation48,Citation49
Research also indicates shorter outdoor activity durations among children with ASD compared to controls during their second year, potentially reducing ultraviolet light exposure and subsequent vitamin D synthesis.Citation50 Links between vitamin D metabolism, genetic variations in the vitamin D receptor, and ASD have been identified.Citation51,Citation52 Despite ongoing debates over the specifics of the relationship between 25(OH)D levels and ASD, given the inconsistent research findings and the public health urgency to clarify the role of vitamin D supplementation in ASD management, rigorous evaluation of its actual benefits is paramount. This necessitates comprehensive methodologies, including umbrella reviews, meta-analyses, and systematic reviews targeting vitamin D use in individuals with ASD, to render a more precise determination of its clinical efficacy.
Materials and Methods
Additional searches were conducted within the identified articles’ references. Titles and abstracts were independently screened by two reviewers (Yuwei Jiang and Wenjun Dang), who excluded irrelevant studies and thoroughly evaluated the remaining for eligibility. Disagreements were resolved by a consensus Discussion with a third reviewer (Hong Nie).
Protocol
This study follows the preferred reporting items recommended by the the Umbrella Review Methodology Working Group and considers systematic reviews and meta-analysis (PRISMA).
Criteria for Considering Reviews for the Overview
Literature Search
PubMed, the Cochrane Database of Systematic Reviews, EMBASE, Sinomed, CNKI, wanfang data, and VIP were searched electronically for studies published from inception until December 31, 2023. The reference lists of included papers were also reviewed to identify any further relevant reviews for inclusion. In addition, manual searches were also performed by tracking citations from the reference lists of all included reviews and relevant reviews in autism intervention, as well as by contacting authors of included reviews. The terms used for the search were “autism”, “vitamin D”, and “systematic review OR meta analysis” separated by the Boolean operator AND. In order to understand the linking role of vitamin D for autism, we insisted on selecting RCT studies in which the intervention was vitamin D only.
Review Selection
We chose systematic reviews with or without meta-analysis. The reason for this is that we searched the relevant literature databases and there have been many systematic evaluations and meta-analyses for summaries of autism and vitamin D. And while the research team of the project has published systematic evaluations of vitamin D and Omega-3 in the previous phase of the project, its findings show that vitamin D supplementation has a positive effect on the behavior of people with ASD. We were eager to find out whether vitamin D had a significant effect on other major aspects of ASD patients. For the supplementation of vitamin D for autism, there is no umbrella evaluation in the existing research method, which can include more studies at one time, and we are more obsessed with finding out the differences between different systematic evaluations, and under the umbrella evaluation of direct evidence, we can better reflect the effect of vitamin D supplementation for autism, and make better guidance for the next research.
Our review encompassed English and Chinese meta-analyses/systematic reviews (MAs/SRs) that evaluated vitamin D supplementation’s efficacy for individuals with Autism Spectrum Disorder (ASD).
Primary outcomes: vitamin D levels, 25(OH)D3 level.
Secondary outcomes: assessments from the Childhood Autism Rating Scale (CARS) scores, the Autism Behavior Checklist (ABC), Social Responsiveness Scale (SRS), Autism Treatment Evaluation Checklist (ATEC) et al.
Exclusion Criteria: Excluded from this review were: non-systematic reviews, case studies, animal studies, conference abstracts, articles without full-text access or valid data, duplicates, and non-English/Chinese publications.
Data Extraction and Management
We recorded complete information about citations, date of publication, study type, database, quality assessment, sample size, countries, over all study size, and measurement tool assessed.
In order to ensure consistency in the inclusion of study populations for late comparisons, included studies focused on children diagnosed with ASD according to the criteria specified by the DSM-IV or DSM-V of the American Psychiatric Association, or the ICD-10 of the World Health Organization. In the selected studies, participants in the treatment group received vitamin D supplementation, without restrictions on dosage or duration of treatment.
Methodological Quality Assessment of Included Reviews
Two independent reviewers (Yuwei Jiang and Wenjun Dang) extracted data, including details such as authorship, publication year, origin, design, sample size, participant ages, diagnostic criteria, interventions, outcomes, and quality indicators like PRISMA, AMSTAR2, and GRADE. Inconsistencies were reconciled by consulting a third reviewer (Hong Nie).The quality of included studies was independently assessed by two researchers (Yuwei Jiang and Wenjun Dang), with consensus validations conducted thereafter. Discrepancies were settled through discussion or third-party adjudication (Xiangying Kong).
Assessment Methodological quality was assessed utilizing the AMSTAR2 tool, comprising 16 discrete items. Notably, items 2, 4, 7, 9, 11, 13, and 15 were identified as critical for the quality appraisal.
The comprehensiveness of the reporting quality in included studies was gauged through a meticulous review of the PRISMA checklist, which encompasses 27 criteria. In the discipline of meta-analyses/systematic reviews, a fully reported criterion was awarded 1 point, a partially reported one received 0.5 points, and an unreported criterion scored 0 points, culminating in a total possible score of 27. A score of 15 or less signified considerable informational inadequacies in the report, a score between 15 and 21 pointed to some deficiencies, and a score between 21 and 27 suggested a high level of reporting completeness.
The quality of evidence from Meta-Analyses/Systematic Reviews (MAs/SRs) was appraised using the GRADE system, which considers study limitations, inconsistency, indirectness, imprecision, and publication bias. The evidence from randomized controlled trials (RCTs) was rated based on these criteria: absent downgrades across the five domains indicated high-quality evidence; a single downgrade in any domain signified moderate quality; two downgrades denoted low quality; and three or more downgrades in any domain qualified the evidence as very low quality.
Results
Literature Search and Selection
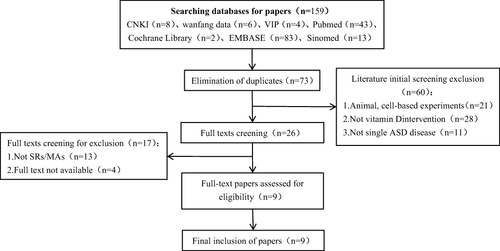
The search yielded 159 articles, of which 73 were duplicates and subsequently removed, resulting in 86 unique articles. During the preliminary screening, 60 articles were excluded due to reasons including animal studies, interventions not related to vitamin D, or studies of multiple disorders rather than solely ASD, leaving 26 articles for further review. Upon full-text evaluation, 17 articles were eliminated because full texts were inaccessible or they did not qualify as meta-analyses/systematic reviews (MAs/SRs), ultimately including 9 articles that met the inclusion criteria for the umbrella evaluation. Study selection flowchart illustrates the systematic literature selection process.
Characteristics of the Included
Studies of the nine selected articles, six were in English and three in Chinese, published from 2011 to 2023. A surge in publications was observed post-2020, encompassing a range of 3–34 individual studies. The body of work included both experimental and observational designs, with six experimental studies totaling 4663 participants and three observational studies comprising 30,136 participants. Participant ages varied widely from 0 to 23 years. In terms of methodological assessment, four studies were evaluated using the Cochrane risk-of-bias tool, three via the Newcastle-Ottawa Scale (NOS), and one according to the STROBE guidelines. One study underwent a dual evaluation employing both the Cochrane tool and MINORS criteria. Detailed information can be found in .
Table 1 Summary of Study Details
Quality Assessment of Included Studies
Upon assessing the methodological quality of the six randomized controlled trials (RCTs) incorporated in this study, it was found that two RCTs exhibited low methodological quality, whereas four were categorized as very low. Key factors affecting these assessments included: (1) One studyCitation53 was registered prior to commencement but lacked detailed reconciliation with the original protocol, and the remaining four had no registration records; (2) Certain studiesCitation43,Citation54,Citation56 failed to articulate the rationale behind utilizing RCTs; (3) Some reportsCitation43,Citation53–55 omitted discussion on the impact of study-specific risk of bias on the collection or interpretation of meta-analytic evidence; (4) Several reportsCitation53,Citation54 did not address bias risk when interpreting individual study outcomes; (5) Some analysesCitation53,Citation54 inadequately accounted for heterogeneity within study findings; (6) Multiple studiesCitation43,Citation53,Citation54,Citation56 neglected to evaluate how the risk of bias within included RCTs might bias the overall meta-analytic conclusions; (7) Some publicationsCitation54,Citation56 lacked declarations on conflicts of interest or funding sources, raising concerns about potential selective publication bias. Detailed information can be found in .
Table 2 AMSTAR 2 Quality Assessment Scale
Assessment of Reporting Quality
Reporting quality was evaluated using the PRISMA checklist. Scores of the studies analyzed spanned from 21 to 23, with two studies each obtaining scores of 21, 22, and 23, respectively. Deficiencies identified included: (1) An absence of described methods for countering bias due to data omission in the synthesis process, potentially contributing to reporting bias; (2) A lack of detailed risk of bias due to missing results for each combined outcome, increasing the possibility of reporting bias; (3) The majority of studies failed to couch their results within the broader evidence context in the evidence summary, which complicates comparisons with similar MAs/SRs and may lead to imprecise result interpretation and reporting bias; (4) Omissions in the detailing of study protocol registrations, which impedes readers’ ability to discern between pre-planned and reported information, heightening the risk of reporting bias.Detailed information can be found in .
Table 3 PRISMA 2020 Checklist
GRADE Assessment of Evidence Quality
In this analysis, 35 outcome measures were evaluated using the GRADE framework. For RCT-derived evidence, assessed outcomes included levels of 25-hydroxyvitamin D (25(OH)D), Social Responsiveness Scale (SRS) scores, and subscales and total scores from the Autism Behavior Checklist (ABC), Gilliam Autism Rating Scale (GARS), Childhood Autism Rating Scale (CARS), and Autism Treatment Evaluation Checklist (ATEC). Observational studies solely addressed the association between vitamin D and ASD. GRADE’s quality classification yielded 20 outcomes as low quality and 15 as very low quality, attributable to: (1) Limitations in study methodologies, notably due to the moderate to low quality of primary RCTs, with many neglecting to delineate blinding, randomization, and allocation concealment processes; (2) Inconsistency, exemplified by moderate to high heterogeneity in data synthesis, possibly related to the inclusion of many low-quality studies and disparate confidence intervals leading to significant I2 values upon result amalgamation; (3) A lack of precision in original literature, often due to small sample sizes and wide confidence intervals in study findings.Detailed information can be found in .
Table 4 GRADE Evidence Quality Rating Results
Discussion
Factors Influencing the Effectiveness of Vitamin D Supplementation on ASD Incidence
The efficacy of vitamin D supplementation in managing ASD can be affected by numerous factors, such as dosage, treatment duration, timing of measurement, and achieved serum 25(OH)D concentrations.Citation59 Referring to related studies, for vitamin D supplementation studies, factors such as malabsorption, liver disease, and kidney disease are excluded before enrollment in normal children, because diseases in these populations can affect vitamin D absorption, distribution, and metabolism. However, in the RCT studies of autism, disease factors affecting vitamin D were not actually excluded. This exclusion criterion is the same in the criteria of the review of the relationship between vitamin D dose and serum levels.Citation60 The type of vitamin D supplementation is also an influencing factor on serum effects, but this one distinction is not reflected in many studies. Types of vitamin D include vitamin D2 and vitamin D3, but vitamin D2 and vitamin D3 do not have the same molecular structure, and thus are converted to 25(OH)D at different rates of breakdown. However, a query of relevant studies did not reveal a difference between vitamin D2 and vitamin D3 supplementation in autistic patients, which could be a next step in research. A search of the literature found that for normal children, a single large dose of supplementation to improve the effect of serum 25(OH)D in patients, vitamin D3 is significantly better than vitamin D2.
Age is also an important factor, although the studies included in this review were all conducted on children under the age of 18 to better understand the benefits of vitamin D supplementation on neurodevelopment. However, many studies did not conduct detailed age analyses. Referring to the included literature for further analysis, children under the age of 18 were divided into several different time periods, with only Kerley et alCitation61 studies stratifying by age (<6 and 6 years), and the treatment effects of vitamin D also showed that those <3 years of age had a more significant improvement. Therefore, it is suggested that vitamin D supplementation should be given during infancy or pregnancy. Based on this result, this review also hypothesizes that there may be a window of opportunity during the early stages of life in which vitamin D supplementation would be more beneficial for establishing neural connections, and we hope that future experimental studies can explore the optimal supplementation period.
In RCTs two adjusted the vitamin D dosage according to patient weight,Citation62 while another applied a uniform high dose of 50,000 IU weekly.Citation63 The American Endocrine Society recommends maintaining serum 25(OH)D levels between 100–150 nmol/L (40–60 ng/mL),Citation64,Citation65 yet specific dosing guidelines for children with ASD are still to be established. It is postulated that higher doses may be required to reach desired serum 25(OH)D levels in ASD cases. Kerley et alCitation66 observed that 11.11% of their subjects experienced no serum 25(OH)D increase post-supplementation, suggesting that ASD subtypes with concurrent gastrointestinal issues could impede vitamin D absorption and highlighting the necessity for tailored research in determining efficacious dosing. Additionally, for ASD patients with significant vitamin D absorption or metabolism difficulties, calcitriol could serve as an alternative treatment. Rigorous monitoring of serum 25(OH)D levels before, during, and post-intervention is essential to optimize dosage regimens. Unfortunately, many prospective studies are limited by a solitary measurement of vitamin D levels, inadequately portraying the overall vitamin D status across different developmental phases. Moreover, substantial variability within observational studies may arise from a range of demographic characteristics.
Furthermore, it can be seen from the included RCT literature, as shown in , the supplementation doses vary. We strive to find a dose that is suitable for more geographical populations, where Saad et al studyCitation67 is a RCT conducted in Egypt, which clearly indicates that 300 IU vitamin D3/kg/day of vitamin D is generally well tolerated by the ASD children. After supplementation for 4 months, the symptoms of ASD improved significantly, proving the effectiveness and tolerability of high-dose vitamin D for ASD children. We also need to consider that Saad et al studyCitation67 was conducted in the Egyptian region, where different levels of sunlight can affect the children’s vitamin D content. In RCT studies, more rigorous involvement needs to take into account factors such as sunlight, latitude and longitude, and season. Among them, the most rigorous study is Mazahery et al,Citation45 which considered different seasons’ serum 25(OH)D concentrations in different regions when conducting the study. At the same time, Mazahery et alCitation45 study considering the different nutritional deficiencies of ASD patients before the study, conducted a preliminary management strategy, including vitamin D, iron, and vitamin B12. This is also something that can be further referenced in future RCT studies. Fernell et al studyCitation68 included individuals with autism spectrum disorder (ASD) and their siblings, compared to which, the common linkage factors and genetic factors of ASD are closer, which is conducive to obtaining better research results.
Table 5 Detailed Information of Literatures Included in Systematic Reviews (RCT Studies)
Table 6 Detailed Information of Literatures Included in Systematic Reviews (Case-Control Studies)
Table 7 Detailed Information of Literatures Included in Systematic Reviews (Cohort Studies)
Table 8 Detailed Information of Literatures Included in Systematic Reviews (Cross Sectional Studies)
The Relationship Between Vitamin D and ASD
Vitamin D and Brain Development
Vitamin D is vital to early brain development,Citation102 particularly in synaptic formation, potentially leading to neurochemical alterations and changes in neuronal function.Citation103 Research indicates that vitamin D underpins various aspects of brain homeostasis and neurodevelopment, such as neuronal migration and growth, excitatory and inhibitory neurotransmission, production of neurotrophic factors, and cytokine regulation.Citation104–107 Dysregulation in any of these domains can directly affect brain development. Specifically, vitamin D’s role in neurotrophic factor expression is instrumental in modulating hippocampal development and cognition, as evidenced by the significant influence of the vitamin D receptor (VDR) and 1-alpha hydroxylase in the hippocampus.Citation108
Experimental models have demonstrated that vitamin D deficiency during gestation may result in pronounced brain structural abnormalities and altered neurodevelopmental markers postnatally,Citation109 potentially impairing memory and cognitive function in children.Citation110–113 Consequently, low vitamin D levels during pregnancy have been linked with an increased risk of ASD,Citation93 corroborated by the findings of multiple meta-analyses. Additional research underscores a positive association between maternal hypovitaminosis D and reduced head circumference in neonates,Citation114 a common characteristic in autism. Moreover, genetic variations in the VDR gene have been closely associated with ASD.Citation85 Study by Schmidt et alCitation115 highlighted a significant connection between the GC AA genotype/A allele of the VDR gene and ASD prevalence through comparative analysis between children with ASD and typically developing peers.
Immune Response
There exists a complex interplay between the immune and nervous systems, with significant implications for brain development and functionality.Citation116 Immune activation is increasingly recognized as a potential risk factor forASD, and vitamin D deficiency might perturb the immune responses in individuals with ASD.Citation117,Citation118 There is potential for vitamin D supplementation to mitigate ASD-related behavioral disturbances stemming from such immune activation. Vitamin D3 plays a multifaceted role in immune function, including the activation and regulation of T cells, B cells, and synaptic cells;Citation119 it also promotes upregulation of dendritic cells and an increase in interleukin-10 levels.Citation120 Notably, altered T cell activity may affect the adaptive immune responses in children with ASD.Citation121 Elevated levels of autoimmune markers—specifically, interferon-gamma, interleukin-1 beta, interleukin-6, and interleukin-12Citation122,Citation123—have been observed in ASD patients, with studies establishing a strong positive association between these cytokine levels and ASD symptom severity.Citation55 Furthermore, animal models suggest that sustained vitamin D insufficiency can activate microglia and provoke an inflammatory response.Citation124 Corroborating these findings, clinical research indicates that vitamin D supplementation can enhance social and communicative abilities in patients with ASD and exert an anti-inflammatory effect.Citation125
Oxidative Stress
Reactive oxygen species (ROS) play a pivotal role in the etiology and progression of ASD, as well as in the manifestation of its severity.Citation126,Citation127 A hallmark of ASD could be oxidative stress, with heightened levels in the brain causing developmental damage and resulting in ASD-related symptoms.Citation128 Vitamin D is notable for its antioxidant capabilities, which include suppressing nitric oxide synthase synthesis, elevating glutathione levels—an intrinsic neuroprotectant—and attenuating neuroglial activation and subsequent neuroinflammation.Citation128 The antioxidant glutathione is vital for the survival of neurons in early development, and vitamin D’s ability to boost intracellular glutathione is crucial for the brain’s detoxification mechanisms.Citation129 Moreover, vitamin D influences the expression of glial cell line-derived neurotrophic factor (GDNF), essential for dopaminergic neuron viability, and a deficiency in vitamin D may disrupt dopaminergic signaling.Citation130 Clinical observations have revealed that lymphocytes and granulocytes in ASD patients express mitochondrial H2O2 at significantly higher levels than those in healthy children,Citation131 suggesting increased vulnerability to oxidative stress in the brains of individuals with ASD.Citation132
The Javadfar et al studyCitation44 will statistically analyze the levels of serum IL-6 and vitamin D supplementation, but the study shows that supplementation with vitamin D did not result in a statistically significant decrease in the serum IL-6 levels of children with ASD. IL-6 is an important indicator of chronic inflammation and an independent predictor of ASD risk. The anti-inflammatory effects of vitamin D may include reducing active oxygen (ROS) levels by increasing cell glutathione, lowering ROS, monocyte chemotactic protein-1, and IL-8 secretion.
Serotonin
Serotonin (5-HT), synthesized from tryptophan via hydroxylation by tryptophan hydroxylase (TPH) and subsequent decarboxylation, is a critical neurotransmitter that emerges early in embryonic development and significantly shapes brain structure and neurotransmitter system development, including that of dopamine. The release of 5-HT and its receptors at synapses mediates numerous physiological functions and modulates neural transmission.Citation133
Vitamin D upregulates the expression of enzymes such as tyrosine hydroxylase (TH)—involved in dopamine synthesis—and tryptophan hydroxylase 2 (TPH2), thereby enhancing 5-HTP production in the brain. Serotonin’s function extends to the regulation of emotions and social decision-making processes. Additionally, vitamin D suppresses the transcription of TPH1 in peripheral tissues, which may result in elevated central and reduced peripheral serotonin levels. Serotonin is a recognized biomarker of ASD, characterized by lower cerebral levels and elevated peripheral concentrations in affected individuals,Citation134,Citation135 with potential correlations to clinical severity in children with ASD.Citation136 Most of the body’s serotonin is produced in the enterochromaffin cells of the intestines, while a small fraction is neuronally synthesized within the gut. Studies have identified dysregulation of the serotonergic system in ASD patients,Citation137,Citation138 with markedly lower cerebral serotonin levels compared to the general population.Citation139 Animal studies have revealed that diminished serotonin during critical periods of brain development can lead to structural anomalies,Citation140 such as reduced synaptic density, whereas high perinatal serotonin consumption can precipitate ASD-like behaviors.Citation141 This evidence indicates that abnormal serotonin levels may disrupt normal brain development and are intimately linked to the pathogenesis of ASD.
Studies have speculated that adequate vitamin D may be necessary for the activation of the serotonin-synthesizing gene tryptophan hydroxylase 2 (TPH2). Vitamin D-mediated serotonin production is essential for generating serotonergic signaling during neurodevelopment, which shapes the developing brain, and throughout adulthood, it plays a key role in regulating multiple brain functions, including social behavior. In addition, adequate vitamin D hormone levels inhibit the expression of tryptophan hydroxylase 1 (TPH1), which is important for reducing gastrointestinal inflammation, increasing bone density, and maintaining autoimmunity.
Conclusion
This study analyzes a spectrum of research, the aggregated data suggest that vitamin D deficiency during early life may contribute to the risk of developing ASD, while experimental findings reveal that vitamin D supplementation might improve core ASD symptoms. Further research should explore whether high-dose vitamin D supplementation in early pregnancy could serve as a preventative measure against ASD.In essence, this investigation represents a foundational comprehensive review of vitamin D supplementation’s efficacy in individuals with ASD. Focusing on the fact that we comprehensively reviewed and systematically collated the included studies for systematic evaluation, the collated results support the conclusions of this study. We also found a definite recommendation regarding the amount of vitamin D supplementation for children with ASD, but we also suggest the feasibility of taking into account informed consent, potential risks associated with supplementation, and individualized therapeutic approaches in RCT studies, given the limitations of RCT studies, focusing on the characteristics of different morbidity populations and geographic regions.The meta-analysis supports the notion that vitamin D supplementation can be beneficial in ASD management and that a deficiency in early life could augment the risk of ASD. These findings support the proactive initiation of vitamin D supplementation as a potential preventative intervention for ASD. At the same time, we should also realize that vitamin D supplementation is only one part of the therapeutic management of children with ASD, and that more research is needed to explore all aspects of life that can help children with ASD. We come from the front lines of clinical practice, and we look forward to more ways to help children with ASD.
Study Limitations
While the reviewed studies converge on the theme of vitamin D supplementation in ASD, they diverge in aspects such as additional treatments, dosage levels, and treatment durations, complicating the consistency of the findings. Additionally, the long-term effects of vitamin D therapy on ASD are yet to be determined, Clinical trials are needed to validate the findings. Moreover, the umbrella evaluation approach used in this study was characterized by heterogeneity in study design, potential sources of bias, and generalization of findings to different populations. Although we have made efforts to search for the original literature in order to explore more studies to avoid heterogeneity, we found that the original literature included different types of studies, which on the one hand, broadened the richness of the study, and at the same time, allowed us to learn that The original paper first itself does have more heterogeneity.
Ethics Approval and Consent to Participate
Institutional Review Board Statement: Ethical review and approval were not required for this systematic review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Thank you for the countless nights spent trying to learn to fight for yourself. And we can plough deeper into the field of autism, we hope we can help more autistic children with our little efforts.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Additional information
Funding
References
- Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(Suppl 1):S55–S65. doi:10.21037/tp.2019.09.09
- World Health Organization. ICD-11: International Classification of Diseases. World Health Organization; 2019.
- Genovese A, Ellerbeck K. Autism spectrum disorder: a review of behavioral and psychiatric challenges across the lifespan. SN Compr Clin. 2022;4:217. doi:10.1007/s42399-022-01302-1
- Lord C, Elsabbagh M, Baird G, et al. Autism spectrum disorder. Lancet. 2018;392(10146):508–520. doi:10.1016/S0140-6736(18)31129-2
- Lai MC, Kassee C, Besney R, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):819–829. doi:10.1016/S2215-0366(19)30289-5
- Kereszturi E. Diversity and classification of genetic variations in autism spectrum disorder. Int J Mol Sci. 2023;24(23):16768. doi:10.3390/ijms242316768
- Christensen D, Baio J, van Naarden Braun K, et al. Correction and republication: prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Morb Mortal Wkly Rep. 2018;67(45):1279. doi:10.15585/mmwr.mm6745a7
- Mottron L, Bzdok D. Autism spectrum heterogeneity: fact or artifact? Mol Psychiatry. 2020;25(12):3178–3185. doi:10.1038/s41380-020-0748-y
- Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15(5):778–790. doi:10.1002/aur.2696
- Hertz-Picciotto I, Croen LA, Hansen R, et al. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125. doi:10.1289/ehp.8483
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi:10.1001/archgenpsychiatry.2011.76
- Bolte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–1297. doi:10.1007/s00018-018-2988-4
- Meguid NA, Hashish AF, Anwar M, et al. Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. J Altern Complement Med. 2010;16(6):641–645. doi:10.1089/acm.2009.0349
- Arastoo AA, Khojastehkia H, Rahimi Z, et al. Evaluation of serum 25-Hydroxy vitamin D levels in children with autism Spectrum disorder. Ital J Pediatr. 2018;44(1):150. doi:10.1186/s13052-018-0587-5
- Schmidt RJ, Niu Q, Eyles DW, et al. Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case-control study. Autism Res. 2019;12(6):976–988. doi:10.1002/aur.2118
- Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22(4):476–485. doi:10.1097/EDE.0b013e31821d0e30
- Kim JY, Son MJ, Son CY, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600. doi:10.1016/S2215-0366(19)30181-6
- Wang Z, Ding R, Wang J. The association between vitamin D status and autism spectrum disorder (ASD): a systematic review and meta-analysis. Nutrients. 2020;13(1):86. doi:10.3390/nu13010086
- Adams JB, Audhya T, McDonough-Means S, et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011;11:111. doi:10.1186/1471-2431-11-111
- Adams JB, Audhya T, Geis E, et al. Comprehensive nutritional and dietary intervention for autism spectrum disorder-a randomized, controlled 12-month trial. Nutrients. 2018;10(3):369. doi:10.3390/nu10030369
- Liu Z, Huang S, Yuan X, et al. The role of vitamin D deficiency in the development of paediatric diseases. Ann Med. 2023;55(1):127–135. doi:10.1080/07853890.2022.2154381
- Dominguez LJ, Farruggia M, Veronese N, et al. Vitamin D sources, metabolism, and deficiency: available compounds and guidelines for its treatment. Metabolites. 2021;11(4):255. doi:10.3390/metabo11040255
- Bennour I, Haroun N, Sicard F, et al. Vitamin D and obesity/adiposity-A brief overview of recent studies. Nutrients. 2022;14(10):2049. doi:10.3390/nu14102049
- Luan W, Hammond LA, Cotter E, et al. Developmental vitamin D (DVD) deficiency reduces nurr1 and TH expression in post-mitotic dopamine neurons in rat mesencephalon. Mol Neurobiol. 2018;55(3):2443–2453. doi:10.1007/s12035-017-0497-3
- Bennour I, Haroun N, Sicard F, et al. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes Rev. 2022;23(8):e13453. doi:10.1111/obr.13453
- Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem. 2012;60(3):836–843. doi:10.1021/jf204194z
- Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59:14–19.
- Goncalves A, Roi S, Nowicki M, et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155–160. doi:10.1016/j.foodchem.2014.09.021
- Bivona G, Gambino CM, Iacolino G, et al. Vitamin D and the nervous system. Neurol Res. 2019;41(9):827–835. doi:10.1080/01616412.2019.1622872
- Saidi L, Hammou H, Sicard F, et al. Maternal vitamin D deficiency and brain functions: a never-ending story. Food Funct. 2023;14(14):6290–6301. doi:10.1039/D3FO00166K
- Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–1541. doi:10.2215/CJN.01160308
- Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3(8):784–794. doi:10.1039/c2fo10262e
- Wang J, Huang H, Liu C, et al. Research progress on the role of vitamin D in autism spectrum disorder. Front Behav Neurosci. 2022;16:859151. doi:10.3389/fnbeh.2022.859151
- Arija V, Esteban-Figuerola P, Morales-Hidalgo P, et al. Nutrient intake and adequacy in children with autism spectrum disorder: EPINED epidemiological study. Autism. 2023;27(2):371–388. doi:10.1177/13623613221098237
- Esnafoglu E, Subasi B. Association of low 25-OH-vitamin D levels and peripheral inflammatory markers in patients with autism spectrum disorder: vitamin D and inflammation in Autism. Psychiatry Res. 2022;316:114735. doi:10.1016/j.psychres.2022.114735
- Shan L, Dong H, Wang T, et al. Screen time, age and sunshine duration rather than outdoor activity time are related to nutritional vitamin D status in children with ASD. Front Pediatr. 2021;9:806981. doi:10.3389/fped.2021.806981
- Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: relation to autoimmunity. J Neuroinflammation. 2012;9:201. doi:10.1186/1742-2094-9-201
- Altun H, Kurutaş EB, Şahin N, et al. The levels of vitamin D, Vitamin D receptor, homocysteine and complex B vitamin in children with autism spectrum disorders. Clin Psychopharmacol Neurosci. 2018;16(4):383–390. doi:10.9758/cpn.2018.16.4.383
- Neumeyer AM, Gates A, Ferrone C, et al. Bone density in peripubertal boys with autism spectrum disorders. J Autism Dev Disord. 2013;43(7):1623–1629. doi:10.1007/s10803-012-1709-3
- Tostes MH, Polonini HC, Gattaz WF, et al. Low serum levels of 25-hydroxyvitamin D (25-OHD) in children with autism. Trends Psych Psych. 2012;34(3):161–163. doi:10.1590/S2237-60892012000300008
- Bener A, Khattab A, Bhugra D, et al. Iron and vitamin D levels among autism spectrum disorders children. Ann Afr Med. 2017;16(4):186–191. doi:10.4103/aam.aam_17_17
- Li B, Xu Y, Zhang X, et al. The effect of vitamin D supplementation in treatment of children with autism spectrum disorder: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci. 2022;25(4):835–845.
- Song L, Luo X, Jiang Q. Vitamin D supplementation is beneficial for children with autism spectrum disorder: a meta-analysis. Clin Psychopharmacol Neurosci. 2020;2(18):203–213.
- Javadfar Z, Abdollahzad H, Moludi J, et al. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: a randomized clinical trial. Nutrition. 2020;79-80:110986.
- Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9–16.
- Stubbs G, Henley K, Green J. Autism: will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses. 2016;88:74–78. doi:10.1016/j.mehy.2016.01.015
- Miller M, Musser ED, Young GS, et al. Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatr. 2019;173(2):147–152. doi:10.1001/jamapediatrics.2018.4076
- Alfawaz HA, Bhat RS, Al-Ayadhi L, et al. Protective and restorative potency of Vitamin D on persistent biochemical autistic features induced in propionic acid-intoxicated rat pups. BMC Complement Altern Med. 2014;14:416. doi:10.1186/1472-6882-14-416
- Ali A, Vasileva S, Langguth M, et al. Developmental vitamin D deficiency produces behavioral phenotypes of relevance to autism in an animal model. Nutrients. 2019;11(5):1187. doi:10.3390/nu11051187
- Liu D, Zhan JY, Shao J. Environmental risk factors for autism spectrum disorders in children. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(11):1147–1153.
- Bahrami A, Sadeghnia HR, Tabatabaeizadeh S-A, et al. Genetic and epigenetic factors influencing vitamin D status. J Cell Physiol. 2018;233(5):4033–4043. doi:10.1002/jcp.26216
- Trifonova EA, Klimenko AI, Mustafin ZS, et al. The mTOR signaling pathway activity and vitamin D availability control the expression of most autism predisposition genes. Int J Mol Sci. 2019;20(24):6332. doi:10.3390/ijms20246332
- Zhang M, Wu Y, Lu Z, et al. Effects of vitamin D supplementation on children with autism spectrum disorder: a systematic review and meta-analysis. Clin Psychopharmacol Neurosci. 2023;21(2):240–251. doi:10.9758/cpn.2023.21.2.240
- Liuyi CSXZ. Meta-analysis on the efficacy and safety of vitamin D therapy on children with autism spectrum disorder. Chin J Pharmacoepidemiol. 2021;12(30):801–805.
- Wang T, Shan L, Du L, et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2016;25(4):341–350.
- Li C. Correlation Between Vitamin D and Autism Spectrum Disorder in Children. Hainan Medical University; 2022.
- Gallardo-Carrasco MC, Jiménez-Barbero JA, Bravo-Pastor MDM, et al. Serum Vitamin D, folate and fatty acid levels in children with autism spectrum disorders: a systematic review and meta-analysis. J Autism Dev Disord. 2022;52(11):4708–4721. doi:10.1007/s10803-021-05335-8
- Ying WYGD. Meta-analysis of relationship between vitamin D and autism spectrum disorder in children. J Pediatr Pharm. 2021;7(27):1–7.
- Islam MZ, Bhuiyan NH, Akhtaruzzaman M, et al. Vitamin D deficiency in Bangladesh: a review of prevalence, causes and recommendations for mitigation. Asia Pac J Clin Nutr. 2022;31(2):167–180. doi:10.6133/apjcn.202206_31(2).0002
- Dunlop E, Kiely ME, James AP, et al. Vitamin d food fortification and biofortification increases serum 25-hydroxyvitamin D concentrations in adults and children: an updated and extended systematic review and meta-analysis of randomized controlled trials. J Nutr. 2021;151(9):2622–2635. doi:10.1093/jn/nxab180
- Kerley CP, Power C, Gallagher L, Coghlan D. Lack of effect of vitamin D3 supplementation in autism: a 20-week, placebo-controlled RCT. Arch dischildhood. 2017;11(102):1030–1036. doi:10.1136/archdischild-2017-312783
- Moradi H, Sohrabi M, Taheri H, et al. Comparison of the effects of perceptual-motor exercises, vitamin D supplementation and the combination of these interventions on decreasing stereotypical behavior in children with autism disorder. Int J Dev Disabil. 2018;66(2):122–132.
- Ansari S. Effects of vitamin D and/or aquatic exercise on IL-1β and IL-1RA serum levels and behavior of children with autism spectrum disorder. Stud Med Sci. 2020;31:690–699.
- Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70(4):750–759. doi:10.1016/j.mehy.2007.08.016
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74(11):1498–1513. doi:10.1038/s41430-020-0558-y
- Kerley CP, Elnazir B, Greally P, et al. Blunted serum 25(OH)D response to vitamin D(3) supplementation in children with autism. Nutr Neurosci. 2020;23(7):537–542. doi:10.1080/1028415X.2018.1529342
- Al SKAA. Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. J Child Psychol Psychiatry. 2018;1(59):20–29.
- Fernell E, Bejerot S, Westerlund J, et al. Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol Autism. 2015;6:3. doi:10.1186/2040-2392-6-3
- Nan L. A clinical study of vitamins A and D in the treatment of children with autism spectrum disorders. Clin Res. 2021;29(4):55–57.
- Huang J. Effectiveness of VitD3 combined with ESDM intervention in the treatment of children with autism spectrum disorders. J Prev Med Chin People’s Liberat. 2020;38(10):144–146+150.
- Han S. Vitamin D3 combined with the Denver model of early intervention in the treatment of autism spectrum disorders in young children. China Rural Health. 2020;12(18):3.
- Jun-Yan FENG, Li -H-H, Shan L, et al. Clinical effect of vitamin D3 combined with the early start Denver model in the treatment of autism spectrum disorder in toddlers. Chin J Contemp Pediatr. 2019;21(4):337–341. doi:10.7499/j.issn.1008-8830.2019.04.007
- Feng J, Shan L, Du L, et al. Clinical improvement following vitamin D3 supplementation in autism spectrum disorder. Nutr Neurosci. 2016;20:284–290. doi:10.1080/1028415X.2015.1123847
- Duan X. A Preliminary Study of Vitamin D in the Treatment of Autism Spectrum Disorders in Children. Jilin University; 2015.
- Azzam HM. Autism and vitamin D: an intervention study. Middle East Curr Psych. 2015;1(22):9–14.
- Windham GC, Pearl M, Anderson MC, et al. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: a case-control study in California. Autism Res. 2019;12(6):989–998. doi:10.1002/aur.2092
- Lee BK, Eyles DW, Magnusson C, et al. Developmental vitamin D and autism spectrum disorders: findings from the Stockholm youth cohort. Mol Psychiatry. 2021;26(5):1578–1588. doi:10.1038/s41380-019-0578-y
- Bicikova M, Máčová L, Ostatníková D, et al. Vitamin D in autistic children and healthy controls. Physiol Res. 2019;68(2):317–320. doi:10.33549/physiolres.933902
- Yan M. Changes and significance of serum 25 hydroxyvitamin D and folate levels in children with autism spectrum disorders. Shandong Med J. 2019;59(10):75–77.
- Basheer S, Natarajan A, van Amelsvoort T, et al. Vitamin D status of children with autism spectrum disorder: case-control study from India. Asian J Psychiatr. 2017;30:200–201. doi:10.1016/j.ajp.2017.10.031
- Cieslinska A, Kostyra E, Chwała B, et al. Vitamin D receptor gene polymorphisms associated with childhood autism. Brain Sci. 2017;7(9):115. doi:10.3390/brainsci7090115
- Desoky T, Hassan MH, Fayed H, et al. Biochemical assessments of thyroid profile, serum 25-hydroxycholecalciferol and cluster of differentiation 5 expression levels among children with autism. Neuropsychiatr Dis Treat. 2017;13:2397–2403. doi:10.2147/NDT.S146152
- Chen J, Xin K, Wei J, et al. Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J Psychosom Res. 2016;89:98–101. doi:10.1016/j.jpsychores.2016.08.013
- Saad K, Abdel-rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346–351. doi:10.1179/1476830515Y.0000000019
- Coskun S, Şimşek Ş, Camkurt MA, et al. Association of polymorphisms in the vitamin D receptor gene and serum 25-hydroxyvitamin D levels in children with autism spectrum disorder. Gene. 2016;588(2):109–114. doi:10.1016/j.gene.2016.05.004
- Guler S, Yesil G, Ozdil M, et al. Sleep disturbances and serum vitamin D levels in children with autism spectrum disorder. Int J Clin Exp Med. 2016;9:14691–14697.
- Hashemzadeh M, Moharreri F. Comparative study of vitamin D levels in children with autism spectrum disorder and normal children: a case-control study. J Fundam Mental Health. 2015;2015:(17):197–201.
- Du L, Shan L, Wang B, et al. Serum levels of 25-hydroxyvitamin D in children with autism spectrum disorders. Chin J Contemp Pediatr. 2015;17:68–71.
- Ugur C, Gurkan K. Vitamin D levels in children with autism spectrum disorders. Klinik Psikofarmakoloji Bulteni. 2014;24:S117–S118.
- Gong ZL. Serum 25-hydroxy vitamin D levels in Chinese children with autism spectrum disorders. Neuroreport. 2014;1(25):23–27.
- Adams JB, Audhya T, McDonough-Means S, et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab. 2011;8(1):34. doi:10.1186/1743-7075-8-34
- Ali Y, Anderson LN, Smile S, et al. Prospective cohort study of vitamin D and autism spectrum disorder diagnoses in early childhood. Autism. 2019;23(3):584–593. doi:10.1177/1362361318756787
- Vinkhuyzen A, Eyles DW, Burne THJ, et al. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry. 2018;23(2):240–246. doi:10.1038/mp.2016.213
- Wu DM, Wen X, Han X-R, et al. Relationship between neonatal vitamin D at birth and risk of autism spectrum disorders: the NBSIB study. J Bone Miner Res. 2018;33(3):458–466. doi:10.1002/jbmr.3326
- Chtourou M, Naifar M, Grayaa S, et al. Vitamin d status in TUNISIAN children with autism spectrum disorders. Clin. Chim. Acta. 2019;493:S619–S620. doi:10.1016/j.cca.2019.03.1296
- El-Ansary A, Cannell JJ, Bjørklund G, et al. In the search for reliable biomarkers for the early diagnosis of autism spectrum disorder: the role of vitamin D. Metab Brain Dis. 2018;33(3):917–931. doi:10.1007/s11011-018-0199-1
- Alzghoul L, AL-Eitan LN, Aladawi M, et al. The association between serum vitamin D3 levels and autism among Jordanian boys. J Autism Dev Disord. 2020;50(9):3149–3154. doi:10.1007/s10803-019-04017-w
- Yuhan D, Bin W. Correlation between serum 25-hydroxyvitamin D level and core symptoms of autism spectrum disorder in children. Chin J Pediatr. 2017;12(55):916–919.
- Garipardic M, Doğan M, Bala KA, et al. Association of attention deficit hyperactivity disorder and autism spectrum disorders with mean platelet volume and vitamin D. Med Sci Monit. 2017;23:1378–1384. doi:10.12659/MSM.899976
- Fahmy SF, Sabri NA, El Hamamsy MH, et al. Vitamin D intake and sun exposure in autistic children. Int J Pharm Sci Res. 2016;2016:(7):1043–1049.
- Bala KA, Doğan M, Kaba S, et al. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs). J Pediatr Endocrinol Metab. 2016;29(9):1077–1082. doi:10.1515/jpem-2015-0473
- Mirarchi A, Albi E, Beccari T, et al. Microglia and brain disorders: the role of vitamin D and its receptor. Int J Mol Sci. 2023;24(15):11892. doi:10.3390/ijms241511892
- Cui X, Gooch H, Petty A, et al. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 2017;453:131–143. doi:10.1016/j.mce.2017.05.035
- Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi:10.1016/j.yfrne.2012.07.001
- Harms LR, Burne THJ, Eyles DW, et al. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab. 2011;25(4):657–669. doi:10.1016/j.beem.2011.05.009
- Lang F, Ma K, Leibrock CB. 1,25(OH)(2)D(3) in brain function and neuropsychiatric disease. Neurosignals. 2019;27(1):40–49.
- Wassif GA, Alrehely MS, Alharbi DM, et al. The impact of vitamin D on neuropsychiatric disorders. Cureus. 2023;15(10):e47716. doi:10.7759/cureus.47716
- Laughlin GA, Kritz-Silverstein D, Bergstrom J, et al. Vitamin D insufficiency and cognitive function trajectories in older adults: the Rancho Bernardo study. J Alzheimers Dis. 2017;58(3):871–883. doi:10.3233/JAD-161295
- Grecksch G, Rüthrich H, Höllt V, et al. Transient prenatal vitamin D deficiency is associated with changes of synaptic plasticity in the dentate gyrus in adult rats. Psychoneuroendocrinology. 2009;34(1):S258–64. doi:10.1016/j.psyneuen.2009.07.004
- Lopez-Vicente M, Sunyer J, Lertxundi N, et al. Maternal circulating Vitamin D(3) levels during pregnancy and behaviour across childhood. Sci Rep. 2019;9(1):14792. doi:10.1038/s41598-019-51325-3
- Berg AO, Jørgensen KN, Nerhus M, et al. Vitamin D levels, brain volume, and genetic architecture in patients with psychosis. PLoS One. 2018;13(8):e0200250. doi:10.1371/journal.pone.0200250
- Cui X, Eyles DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. 2022;14(20):4353. doi:10.3390/nu14204353
- Matta SM, Hill-Yardin EL, Crack PJ. The influence of neuroinflammation in autism spectrum disorder. Brain Behav Immun. 2019;79:75–90. doi:10.1016/j.bbi.2019.04.037
- Miliku K, Vinkhuyzen A, Blanken LM, et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am J Clin Nutr. 2016;103(6):1514–1522. doi:10.3945/ajcn.115.123752
- Schmidt RJ, Hansen RL, Hartiala J, et al. Selected vitamin D metabolic gene variants and risk for autism spectrum disorder in the CHARGE Study. Early Hum Dev. 2015;91(8):483–489. doi:10.1016/j.earlhumdev.2015.05.008
- Samsam M, Ahangari R, Naser SA. Pathophysiology of autism spectrum disorders: revisiting gastrointestinal involvement and immune imbalance. World J Gastroenterol. 2014;20(29):9942–9951. doi:10.3748/wjg.v20.i29.9942
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi:10.1016/j.bbi.2010.08.003
- Jyonouchi H, Geng L, Streck DL, et al. Immunological characterization and transcription profiling of peripheral blood (PB) monocytes in children with autism spectrum disorders (ASD) and specific polysaccharide antibody deficiency (SPAD): case study. J Neuroinflammation. 2012;9:4. doi:10.1186/1742-2094-9-4
- Jones AP, Tulic MK, Rueter K, et al. Vitamin D and allergic disease: sunlight at the end of the tunnel? Nutrients. 2012;4(1):13–28. doi:10.3390/nu4010013
- Liu Y, Ding C, Xu R, et al. Effects of vitamin D supplementation during pregnancy on offspring health at birth: a meta-analysis of randomized controlled trails. Clin Nutr. 2022;41(7):1532–1540. doi:10.1016/j.clnu.2022.05.011
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25(5):840–849. doi:10.1016/j.bbi.2010.09.002
- Shen L, Feng C, Zhang K, et al. Proteomics study of peripheral blood mononuclear cells (PBMCs) in autistic children. Front Cell Neurosci. 2019;13:105. doi:10.3389/fncel.2019.00105
- El AD, El Ashry H, Hodeib H, et al. Vitamin D in children with inflammatory bowel disease: a randomized controlled clinical trial. J Clin Gastroenterol. 2021;55(9):815–820. doi:10.1097/MCG.0000000000001443
- Morgese MG, Schiavone S, Maffione AB, et al. Depressive-like phenotype evoked by lifelong nutritional omega-3 deficiency in female rats: crosstalk among kynurenine, Toll-like receptors and amyloid beta oligomers. Brain Behav Immun. 2020;87:444–454. doi:10.1016/j.bbi.2020.01.015
- Mazahery H, Conlon CA, Beck KL, et al. Inflammation (IL-1beta) modifies the effect of vitamin D and omega-3 long chain polyunsaturated fatty acids on core symptoms of autism spectrum disorder-an exploratory pilot study(double dagger). Nutrients. 2020;12(3):661. doi:10.3390/nu12030661
- Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4(5):519–522. doi:10.3892/br.2016.630
- Kumar A, Ratan RR. Oxidative stress and huntington’s disease: the good, the bad, and the ugly. J Huntingtons Dis. 2016;5(3):217–237. doi:10.3233/JHD-160205
- DeLuca HF. Vitamin D: historical overview. Vitam Horm. 2016;100:1–20.
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17(4):389–401. doi:10.1038/mp.2011.165
- Lin CI. 1,25(OH)(2)D(3) alleviates abeta(25-35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y cells. Int J Mol Sci. 2020;21(12):1.
- Rose S, Niyazov DM, Rossignol DA, et al. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagn Ther. 2018;22(5):571–593. doi:10.1007/s40291-018-0352-x
- Mokhtari-Zaer A, Hosseini M, Salmani H, et al. Vitamin D(3) attenuates lipopolysaccharide-induced cognitive impairment in rats by inhibiting inflammation and oxidative stress. Life Sci. 2020;253:117703. doi:10.1016/j.lfs.2020.117703
- Adams D, Simpson K, Keen D. Exploring anxiety at home, school, and in the community through self-report from children on the autism spectrum. Autism Res. 2020;13(1):603–614. doi:10.1002/aur.2246
- Wang B, Dong H, Li H, et al. A probable way vitamin D affects autism spectrum disorder: the nitric oxide signaling pathway. Front Psychiatry. 2022;13:908895. doi:10.3389/fpsyt.2022.908895
- Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29(6):2207–2222. doi:10.1096/fj.14-268342
- Guo M, Zhu J, Yang T, et al. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): a pilot study. Brain Res Bull. 2018;137:35–40. doi:10.1016/j.brainresbull.2017.11.001
- Zafeiriou DI, Ververi A, Vargiami E. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 2009;7(2):150–157. doi:10.2174/157015909788848848
- Janusonis S, Anderson GM, Shifrovich I, et al. Ontogeny of brain and blood serotonin levels in 5-HT receptor knockout mice: potential relevance to the neurobiology of autism. J Neurochem. 2006;99(3):1019–1031. doi:10.1111/j.1471-4159.2006.04150.x
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(6):919–929. doi:10.1016/j.euroneuro.2014.02.004
- Mazer C, Muneyyirci J, Taheny K, et al. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760(1–2):68–73. doi:10.1016/s0006-8993(97)00297-7
- Hohmann CF, Walker EM, Boylan CB, et al. Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Res. 2007;1139:163–177. doi:10.1016/j.brainres.2006.12.095